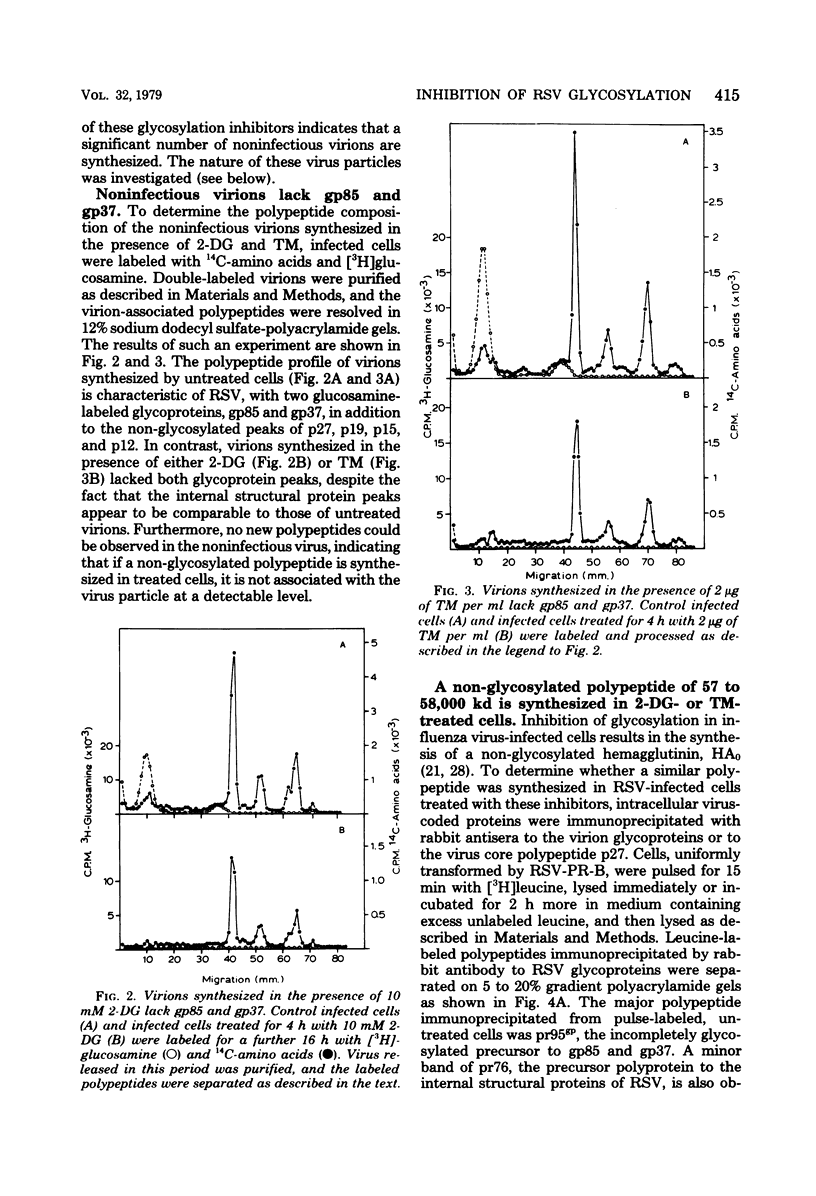
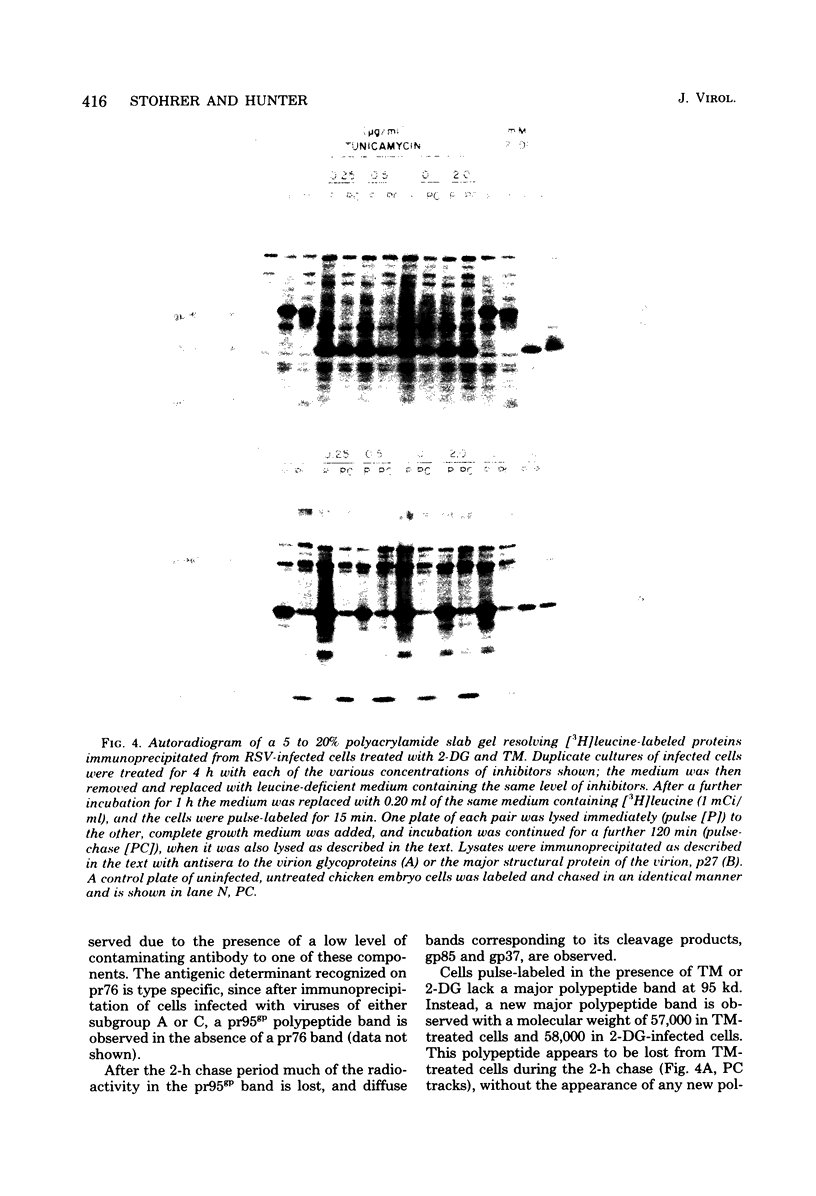
Abstract
Two inhibitors of glycosylation, 2-deoxyglucose and tunicamycin, depressed the synthesis of infectious Rous sarcoma virus greater than 100-fold. Under the same conditions only a two- to threefold decrease in the production of virus particles was observed. The noninfectious particles had a lower density (1.145 g/ml) in isopycnic sucrose gradients and lacked the two virion glycoproteins, gp85 and gp37, found on infectious virions. The four internal structural proteins of the virus, p27, p19, p15, and p12, appeared to be assembled normally into the noninfectious virus. Polypeptides related to the Rous sarcoma virus glycoproteins were immunoprecipitated from pulse-labeled Rous sarcoma virus (Prague strain, subgroup B)-transformed cells. pr95gp, the polyprotein precursor to gp85 and gp37, was the major protein precipitated from untreated cells. PR95GP, THE POLYPROTEIN PRECURSOR TO GP85 AND GP37, WAS THE MAJOR PROTEIN PRECIPITATED FROM UNTREATED CELLS. This was absent in both tunicamycin- and 2-deoxyglucose-treatec ells, and a new polypeptide of molecular weight 57,000 to 58,000 was the major species precipitated. In tunicamycin-treated cells this product was unstable and was degraded during a 2-h chase; in 2-deoxyglucose-treated cells, on the other hand, the polypeptide appeared to be more stable and underwent partial glycosylation. The synthesis and processing of pr76, the polyprotein precursor to the internal structural proteins of the virion, occurred normally in both treated and untreated cells. It is concluded that the unglycosylated env gene product is a polypeptide of molecular weight 57,000 to 58,000.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- England J. M., Bolognesi D. P., Dietzschold B., Halpern M. S. Evidence that a precursor glycoprotein is cleaved to yield the major glycoprotein of avian tumor virus. J Virol. 1977 Feb;21(2):810–814. doi: 10.1128/jvi.21.2.810-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Hunter E., Vogt P. K. Inhibition of avian sarcoma virus replication by glucosamine: a specific effect on the synthesis and processing of viral proteins. Virology. 1976 Jun;71(2):402–411. doi: 10.1016/0042-6822(76)90368-8. [DOI] [PubMed] [Google Scholar]
- Hayman M. Synthesis and processing of avian sarcoma virus glycoproteins. Virology. 1978 Apr;85(2):475–486. doi: 10.1016/0042-6822(78)90454-3. [DOI] [PubMed] [Google Scholar]
- Hunter E. Biological techniques for avian sarcoma viruses. Methods Enzymol. 1979;58:379–393. doi: 10.1016/s0076-6879(79)58153-1. [DOI] [PubMed] [Google Scholar]
- Hunter E., Friis R. R., Vogt P. K. Inhibition of avian sarcoma virus replication by glucosamine. Virology. 1974 Apr;58(2):449–456. doi: 10.1016/0042-6822(74)90079-8. [DOI] [PubMed] [Google Scholar]
- Hunter E., Hayman M. J., Rongey R. W., Vogt P. K. An avian sarcoma virus mutant that is temperature sensitive for virion assembly. Virology. 1976 Jan;69(1):35–49. doi: 10.1016/0042-6822(76)90192-6. [DOI] [PubMed] [Google Scholar]
- Ishizaki R., Vogt P. K. Immunological relationships among envelope antigens of avian tumor viruses. Virology. 1966 Nov;30(3):375–387. doi: 10.1016/0042-6822(66)90116-4. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Schmidt M. F., Scholtissek C. Effect of 2-deoxy-D-glucose on the multiplication of Semliki Forest virus and the reversal of the block by mannose. Virology. 1973 Jul;54(1):179–189. doi: 10.1016/0042-6822(73)90127-x. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler D. O., Rudigier J. F., Bischoff E., Decker K. F. The trapping of uridine phosphates by D-galactosamine. D-glucosamine, and 2-deoxy-D-galactose. A study on the mechanism of galactosamine hepatitis. Eur J Biochem. 1970 Dec;17(2):246–253. doi: 10.1111/j.1432-1033.1970.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. The generation of the two envelope glycoproteins of Rous sarcoma virus from a common precursor polypeptide. Virology. 1978 Mar;85(1):63–74. doi: 10.1016/0042-6822(78)90411-7. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Impaired intracellular migration and altered solubility of nonglycosylated glycoproteins of vesicular stomatitis virus and Sindbis virus. J Biol Chem. 1977 Dec 25;252(24):9018–9023. [PubMed] [Google Scholar]
- Lewandowski L. J., Smith R. E., Bolognesi D. P., Halpern M. S. Viral glycoprotein synthesis under conditions of glucosamine block in cells transformed by avian sarcoma viruses. Virology. 1975 Aug;66(2):347–355. doi: 10.1016/0042-6822(75)90208-1. [DOI] [PubMed] [Google Scholar]
- Moelling K., Hayami M. Analysis of precursors to the envelope glycoproteins of avian RNA tumor viruses in chicken and quail cells. J Virol. 1977 Jun;22(3):598–607. doi: 10.1128/jvi.22.3.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Prochownik E. V., Panem S., Kirsten W. H. Biological and physical modifications of a murine oncornavirus by 2-deoxy-D-glucose. J Virol. 1975 Jun;15(6):1323–1331. doi: 10.1128/jvi.15.6.1323-1331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D., Compans R. W. Identification of the spike proteins of Rous sarcoma virus. Virology. 1971 Nov;46(2):485–489. doi: 10.1016/0042-6822(71)90049-3. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schwarz R. T., Ludwig H. Fluorosugars inhibit biological properties of different enveloped viruses. J Virol. 1976 Jun;18(3):819–823. doi: 10.1128/jvi.18.3.819-823.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schwarz R. T., Scholtissek C. Nucleoside-diphosphate derivatives of 2-deoxy-D-glucose in animal cells. Eur J Biochem. 1974 Nov 1;49(1):237–247. doi: 10.1111/j.1432-1033.1974.tb03828.x. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Detection of an unstable RNA in chick fibroblasts after reduction of the UTP pool by glucosamine. Eur J Biochem. 1971 Dec;24(2):358–365. doi: 10.1111/j.1432-1033.1971.tb19694.x. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Klenk H. D. Inhibition of glycosylation of the influenza virus hemagglutinin. J Virol. 1974 Nov;14(5):1023–1034. doi: 10.1128/jvi.14.5.1023-1034.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shealy D. J., Rueckert R. R. Proteins of Rous-associated virus 61, an avian retrovirus: common precursor for glycoproteins gp85 and gp35 and use of pactamycin to map translational order of proteins in the gag, pol, and env genes. J Virol. 1978 May;26(2):380–388. doi: 10.1128/jvi.26.2.380-388.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozawa H., Bauer H., Graf T., Gelderblom H. Strain-specific antigen of the avian leukosis sarcoma virus group. I. Isolation and immunological characterization. Virology. 1970 Mar;40(3):530–539. doi: 10.1016/0042-6822(70)90196-0. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Envelope classification of avian RNA tumor viruses. Bibl Haematol. 1970;(36):153–167. doi: 10.1159/000391704. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]




