Abstract
Background Cardiovascular disease (CVD) mortality has more than halved in England since the 1980s, but there are few data on small-area trends. We estimated CVD mortality by ward in 5-year intervals between 1982 and 2006, and examined trends in relation to starting mortality, region and community deprivation.
Methods We analysed CVD death rates using a Bayesian spatial technique for all 7932 English electoral wards in consecutive 5-year intervals between 1982 and 2006, separately for men and women aged 30–64 years and ≥65 years.
Results Age-standardized CVD mortality declined in the majority of wards, but increased in 186 wards for women aged ≥65 years. The decline was larger where starting mortality had been higher. When grouped by deprivation quintile, absolute inequality between most- and least-deprived wards narrowed over time in those aged 30–64 years, but increased in older adults; relative inequalities worsened in all four age–sex groups. Wards with high CVD mortality in 2002–06 fell into two groups: those in and around large metropolitan cities in northern England that started with high mortality in 1982–86 and could not ‘catch up’, despite impressive declines, and those that started with average or low mortality in the 1980s but ‘fell behind’ because of small mortality reductions.
Conclusions Improving population health and reducing health inequalities should be treated as related policy and measurement goals. Ongoing analysis of mortality by small area is essential to monitor local effects on health and health inequalities of the public health and healthcare systems.
Keywords: Cardiovascular diseases, epidemiology, population health, small-area analysis, Bayesian spatial analysis, health inequality
Introduction
Mortality from cardiovascular disease (CVD) has more than halved since the 1980s in England; CVD, nonetheless, remains the leading cause of death. CVD mortality differentials are an important determinant of inequalities in all-cause mortality between regions and by individual and community socio-economic status (SES).1–4 The impressive national decline in CVD mortality inevitably raises the question of whether the advantageous trends have benefited all communities equally and of how trends may vary by initial CVD mortality, region and community SES.
Previous research has documented inequalities in CVD mortality by SES and by larger administrative units such as region or district.1–6 Regional studies however mask variations in mortality levels and trends among local communities. This limits our ability to prioritize, plan for and evaluate public health and healthcare interventions that are implemented and managed locally. Evidence-based planning and evaluation with a local focus is particularly important for CVD because the coverage and effectiveness of preventive interventions and treatments may vary locally, based on factors like number and training of general practitioners, access to cardiac interventionists and specialist stroke services and emergency response times.7–9 To overcome this gap, we estimated electoral ward-level CVD mortality between 1982 and 2006. We also used these estimates to examine how past trends have affected CVD mortality inequalities. The methods and empirical findings also demonstrate how routine mortality data can be used for informing local CVD trends and CVD mortality inequalities.
Methods
We analysed trends in age-standardized CVD mortality, separately for men and women aged 30–64 years and ≥65 years for all 7932 English Standard Table Wards in five consecutive periods: 1982–86, 1987–91, 1992–96, 1997–2001 and 2002–06. We also examined differences in CVD mortality across wards and differentials among wards grouped by deprivation quintile.
Analysis units
English electoral wards are administrative units with an average population of 3420 persons. To deal with changing administrative boundaries over time, in 2003 the Office for National Statistics (ONS) produced a set of ‘Standard Table wards’ (ST wards) based on wards used for the 2001 census tables.10 Wards with <1000 residents or 400 households were merged into receiving wards to ensure confidentiality of the data released in the Census Standard Tables. We used ST wards as our units of analysis over time (referred to simply as ‘ward’ hereafter).
Data sources
Data were from national databases, including the census and mortality statistics, held by the Small Area Health Statistics Unit. Population data by age and sex were available for ST wards for 2001 and all subsequent years.11 Data were available for Enumeration Districts (ED) in census years 1981 and 1991. ED populations were proportionally divided among all postcodes in each ED and aggregated to ward level. For other years, population data were available at district (Local Authority) level.12 We used census data in 1981, 1991 and 2001 to estimate the proportion of district population that fell into each ward within a district, by sex and age group; the proportion for inter-censal years was calculated using linear interpolation. We then used the ratio of ward-to-district population, by sex and age group, to redistribute district population into wards.
CVD deaths (ICD-9 codes 390–459 in 1982–2000 and ICD-10 codes I00–I99 in 2001–06) were extracted by age, sex, year and postcode and aggregated to ST wards. We used the Index of Multiple Deprivation (IMD) 2007 to measure community SES in the most recent analysis period. IMD is a measure of community SES, which incorporates 38 indicators of income, employment, health, education, housing and services, living environment/infrastructure and crime. We excluded the health domain from our calculations and reweighted the remaining domains because CVD mortality is itself a health variable.13 Following previous analyses,14 scores for each IMD domain, which were available at the Lower Super Output Area level, were transformed to ward-level domain scores by population weighting. For analysis of mortality in relation to deprivation, we grouped wards by modified IMD quintile because IMD is a non-linear measure of community SES.15,16 We used the same quintile grouping throughout the analysis period because we wanted to assess the change in inequality in the same group of wards over time.
Statistical methods
We estimated ward-level CVD mortality for the aforementioned 5-year analysis periods. All analyses were done separately for men and women for each period, and at ages 30–64 years and ≥65 years.
Simple CVD death rate estimates in small areas such as wards may be unstable owing to small numbers of deaths. To overcome this issue, we first pooled ward CVD deaths and populations in each 5-year period. We then used a Bayesian spatial model to estimate CVD mortality by ward, sex and age group for each period. The model, known as the Besag, York and Mollie (BYM) model, is described in detail elsewhere.17,18 In brief, the number of deaths in each ward–period–sex–age group was specified using a Poisson model. The Poisson model estimates mortality in each ward, relative to that expected if all wards had the same death rate as the national rate. The estimated ratios can then be converted into mortality rates through simple multiplication by the national death rate for the corresponding age group, sex and analysis period. This process also age-standardizes mortality to the national age structure for the corresponding age–sex group. In the calculations of change in mortality over time, we conducted all analyses relative to the national population for the corresponding age group and sex during the 25 years of analysis (when comparing men and women in Table 1, we age-standardized to the combined population for men and women for the corresponding age group).
Table 1.
Age-standardized CVD death rates (per 100 000) by sex, age group and ward deprivation quintile
| 1982–86 | 1987–91 | 1992–96 | 1997–2001 | 2002–06 | Absolute reduction between 1982–86 and 2002–06 | Percentage reduction between 1982–86 and 2002–06 (%) | |
|---|---|---|---|---|---|---|---|
| Men aged 30–64 years | |||||||
| Least deprived | 214 | 164 | 127 | 96 | 72 | 142 | 66 |
| Q2 | 242 | 193 | 146 | 111 | 85 | 157 | 65 |
| Q3 | 265 | 209 | 165 | 127 | 102 | 163 | 62 |
| Q4 | 297 | 244 | 194 | 156 | 125 | 172 | 58 |
| Most deprived | 357 | 309 | 261 | 216 | 180 | 177 | 50 |
| Q5–Q1 difference | 143 | 145 | 134 | 120 | 108 | 35 | |
| Q5/Q1 ratio | 1.67 | 1.88 | 2.06 | 2.25 | 2.5 | ||
| Women aged 30–64 years | |||||||
| Least deprived | 69 | 54 | 42 | 34 | 26 | 43 | 62 |
| Q2 | 80 | 64 | 51 | 41 | 31 | 49 | 61 |
| Q3 | 90 | 72 | 59 | 48 | 37 | 53 | 59 |
| Q4 | 109 | 89 | 74 | 60 | 47 | 62 | 57 |
| Most deprived | 140 | 123 | 105 | 90 | 71 | 69 | 49 |
| Q5–Q1 difference | 71 | 69 | 63 | 56 | 45 | 26 | |
| Q5/Q1 ratio | 2.03 | 2.28 | 2.5 | 2.65 | 2.73 | ||
| Men aged ≥65 years | |||||||
| Least deprived | 3334 | 2927 | 2586 | 2104 | 1630 | 1704 | 51 |
| Q2 | 3482 | 3085 | 2700 | 2208 | 1759 | 1723 | 49 |
| Q3 | 3562 | 3160 | 2820 | 2317 | 1840 | 1722 | 48 |
| Q4 | 3718 | 3294 | 2977 | 2468 | 1991 | 1727 | 46 |
| Most deprived | 3845 | 3497 | 3185 | 2752 | 2265 | 1580 | 41 |
| Q5–Q1 difference | 511 | 570 | 599 | 648 | 635 | −124 | |
| Q5/Q1 ratio | 1.15 | 1.19 | 1.23 | 1.31 | 1.39 | ||
| Women aged ≥65 years | |||||||
| Least deprived | 2848 | 2506 | 2242 | 1892 | 1582 | 1266 | 44 |
| Q2 | 2958 | 2617 | 2321 | 1963 | 1675 | 1283 | 43 |
| Q3 | 2959 | 2639 | 2369 | 2021 | 1727 | 1232 | 42 |
| Q4 | 3114 | 2754 | 2469 | 2122 | 1809 | 1305 | 42 |
| Most deprived | 3163 | 2855 | 2585 | 2258 | 1985 | 1178 | 37 |
| Q5–Q1 difference | 315 | 349 | 343 | 366 | 403 | −88 | |
| Q5/Q1 ratio | 1.11 | 1.14 | 1.15 | 1.19 | 1.25 |
In the BYM model, the estimated mortality in each ward is influenced by its own data, as well as by those of its neighbours (defined as wards that share a boundary), borrowing information to balance between (overly) unstable within-ward estimates and (overly) simplified aggregate large-area estimates that mask small-area variation. To achieve this balance, the estimation partitions the variance of mortality across wards into a spatial component (specified using a Gaussian conditional autoregressive prior distribution) and a ward-specific component (specified using a Normal prior distribution). We also included the ONS’ urban and rural classification19 and Government Office Region as covariates in the models, which improved model specification according to the Deviance Information Criterion.20 Because each deprivation quintile contains nearly 1600 wards, death rates for quintiles are not affected by small numbers of deaths and were estimated directly using the numbers of deaths and population.
The model was fitted in open-source software R version 2.13.0 using the package Integrated Nested Laplace Approximation, which provides a computationally efficient approximation to Markov-Chain Monte Carlo algorithm. We report the posterior mean of CVD mortality by age group and sex for each ward and the posterior probabilities that the estimated CVD mortality is above the age- and sex-specific national death rate.
Results
Between 2002 and 2006, CVD accounted for 38 and 37% of all deaths among men and women ≥30 years of age, respectively. Nationally, age-standardized CVD mortality was higher among men than women: 120 vs 45 per 100 000 in young and middle-age adults and 2234 vs 1590 per 100 000 among those aged ≥65 years. Men had higher mortality than women in >99% of wards for ages <65 years. In older ages, women had higher CVD mortality than men in one out of five wards. Below, we describe the geographical patterns and inequalities in ward CVD mortality in the most recent period, present the trends since the 1980s and discuss how and the inequalities have changed as a result of these trends.
Ward-level CVD mortality and inequalities in the 2000s
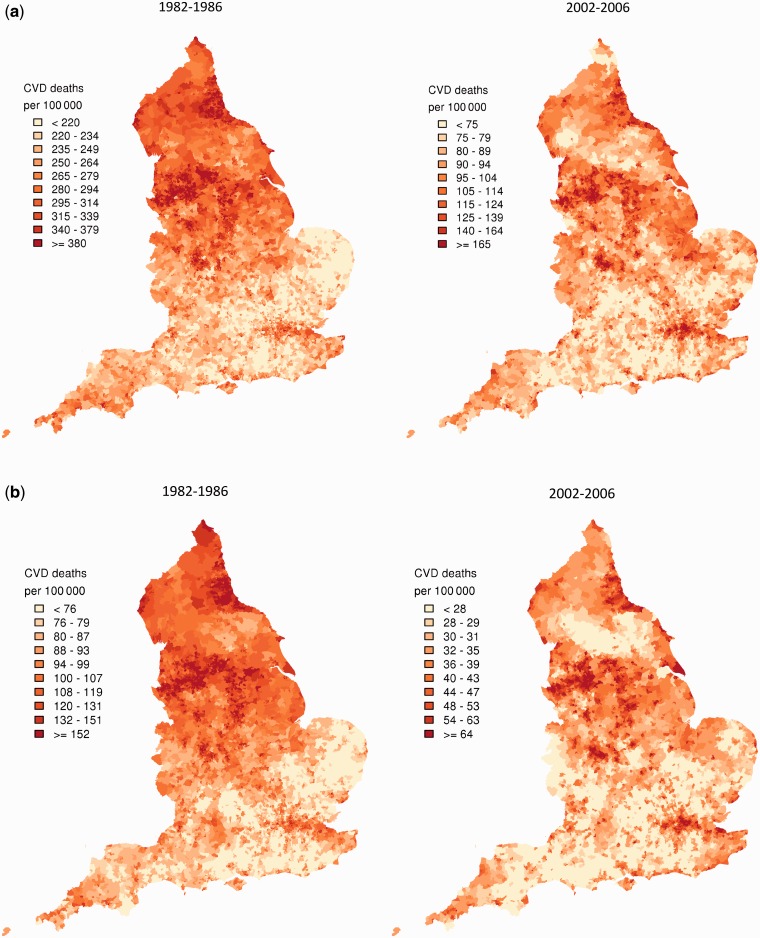
In 2002–06, there was a nearly 4-fold difference between the 1st and 99th percentiles of CVD mortality at ages 30–64 years among English wards (Figure 1a and b, right hand panels; Supplementary Tables S1 and S2, available as Supplementary data at IJE online). CVD mortality was highest in northwestern England, especially for wards in and around metropolitan areas of Liverpool, Manchester, Nottingham, Burnley and Blackpool; in parts of Yorkshire, Leeds and Bradford; in and around Birmingham and in deprived boroughs of London, including Newham, Hackney, Haringey, Tower Hamlets and Waltham Forest. There was also high CVD mortality along the northeastern coastline and, for men, in patches along the southern coastline, e.g. in Plymouth. Except in London, CVD mortality in young and middle-age adults was generally low in southern England, with Surrey, East Dorset and Cotswolds having some of the lowest death rates. Parts of Yorkshire and the Lake District also formed a northern low-mortality band. Despite the relatively small numbers of deaths per ward in young and middle-age adults, there was high confidence that the estimated high and low mortality rates deviated from the national average, e.g. with posterior probabilities >0.80 or <0.20 (Supplementary Figure S2a and b, available as Supplementary data at IJE online).21
Figure 1.
Posterior mean of CVD mortality in 1982–86 and 2002–06 from the Bayesian spatial model, by ward in (a) men aged 30–64 years; (b) women aged 30–64 years; (c) men aged ≥65 years; and (d) women aged ≥65 years. See Supplementary Tables S1–S4 for numerical information by ward. See Supplementary Figure S1 for a map of England that identifies specific regions. See Supplementary Figure S2 for posterior probabilities corresponding to this figure. Each shade in the legend corresponds to a decile of wards in the analysis and includes 793 or 794 wards
For ages 65 and above, the 1st–99th percentiles of ward-level CVD mortality had an almost 2-fold difference (Figure 1c and d, right-hand panels; Supplementary Tables S3 and S4, available as Supplementary data at IJE online). High- and low-mortality wards were spatially more heterogeneous in older ages than in ages <65 years, especially for women. CVD mortality was high in some of the same northern wards as for those aged <65 years; it was also high in additional areas in the northeast and northwest (both sexes) and in the West Midlands, near the Welsh border and in some rural wards (women only). Low-CVD-mortality wards were widely dispersed throughout the country for older adults, especially for women. Southern and southwestern wards had low mortality at these ages as they did for <65 years. In contrast to younger adults, London wards did not feature prominently as high-risk areas in older ages; rather, some London wards were among those with the lowest older-adult CVD mortality in the country.
Trends in ward-level CVD mortality and inequalities
Nationally, CVD mortality declined by about two-thirds for men and women aged 30–64 years between 1982 and 2006 and by over one-half for those aged ≥65 years. There were larger absolute (but not relative) reductions among men, leading to a convergence of male and female CVD mortality. CVD mortality declined in the vast majority of wards during these 25 years, though it increased in 186 wards for older women and in a handful of wards for other age–sex groups.
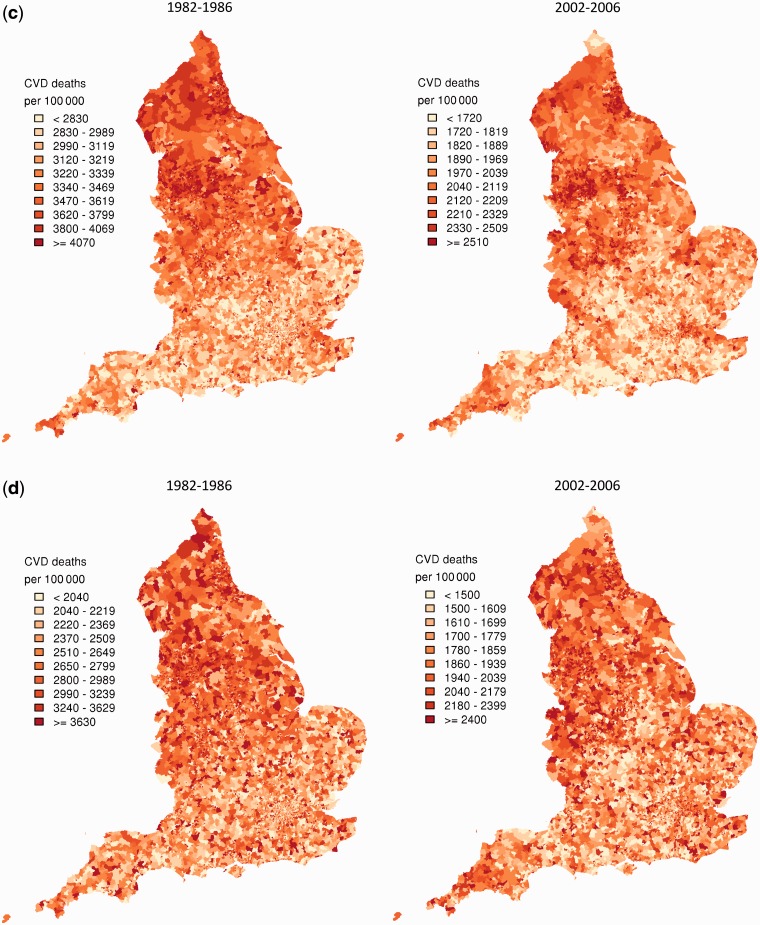
For ages 30–64 years, the reduction in CVD mortality varied substantially across wards, declining 4.5 times more in the 1% best-performing wards (i.e. those with the largest improvement) than in the worst-performing 1% for men, and seven times more for women (Figure 2a and b). Importantly, CVD mortality declined more in wards with higher starting death rates in 1982–86, such as those in the northeast and northwest, Yorkshire and the West Midlands (Figure 2a and b). The smallest declines were in some London wards that had relatively low or average mortality in the 1980s. These wards fell behind over these two and a half decades, and by 2002–06 had some of the highest CVD mortality in the country (Figure 1a and b right-hand panels). CVD mortality decline was also relatively small in some wards in southern and eastern England, although these areas had such low mortality in the mid-1980s that they maintained their low-mortality positions. As a result of these trends, the standard deviation of CVD mortality across wards declined from 63 deaths per 100 000 in 1982–86 to 37 in 2002–06 for men and from 31 to 15 for women aged 30–64 years. Despite shrinking absolute inequality across wards, wards largely retained their relative positions; correlations between CVD mortality in 1982–86 and 2002–06 were 0.65 for men and 0.71 for women.
Figure 2.
Change in ward CVD mortality between 1982–86 and 2002–06 from the Bayesian spatial model, in (a) men aged 30–64 years; (b) women aged 30–64 years; (c) men aged ≥65 years; and (d) women aged ≥65 years. Each shade in the legend corresponds to a decile of wards in the analysis and includes 793 or 794 wards
In those aged ≥65 years, CVD mortality declined nearly five times more in the best-performing 1% of wards than in the worst-performing 1% for men; there was a 10-fold variation for women (Figure 2c and d). CVD mortality decline at older ages was also strongly linked to mortality in the early 1980s, with the correlation between mortality in 1982–86 and its decline 0.84 for men and 0.91 for women. High-mortality-large-decline wards were located in northern England and in small pockets of the south and southeast for men, but were dispersed throughout the country for women. Wards with the lowest CVD mortality in older adults in 2002–06 were those which started off with average or lower-than-average mortality in the early 1980s and sustained average (rather than spectacular) declines; these wards were located in southern England and in affluent parts of London like Westminster, Kensington and Chelsea and Kingston-upon-Thames. The standard deviation of ward-level CVD mortality declined from 508 to 311 deaths per 100 000 in older men and 785 to 380 in older women. The relative positions of wards changed more for older adults than those aged <65 years; correlations between ward-level CVD mortality in 1982–86 and 2002–06 were 0.45 for men and 0.28 for women.
CVD mortality in relation to ward deprivation
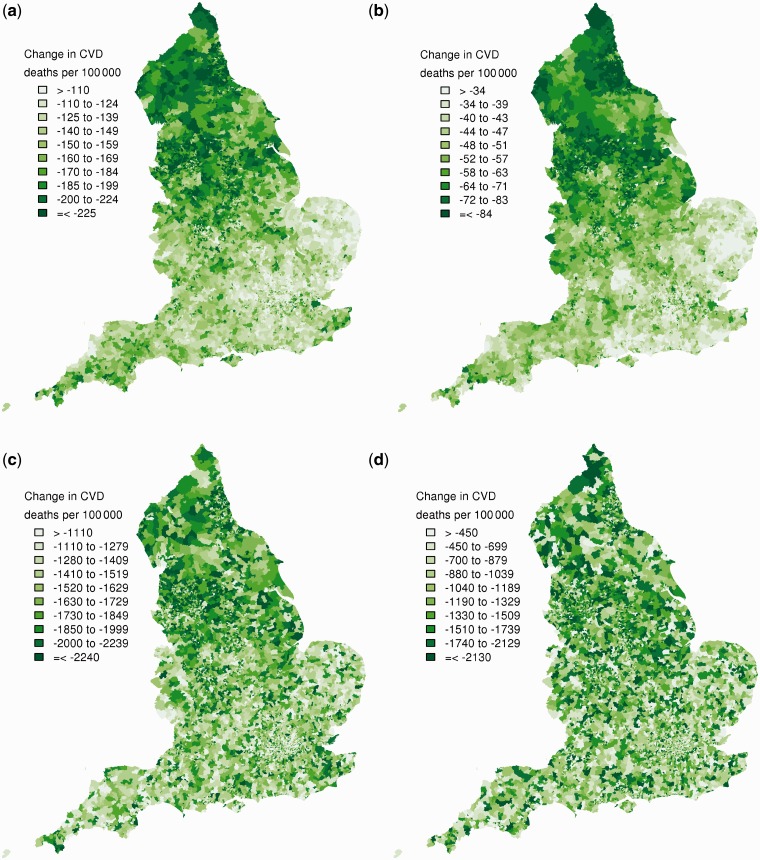
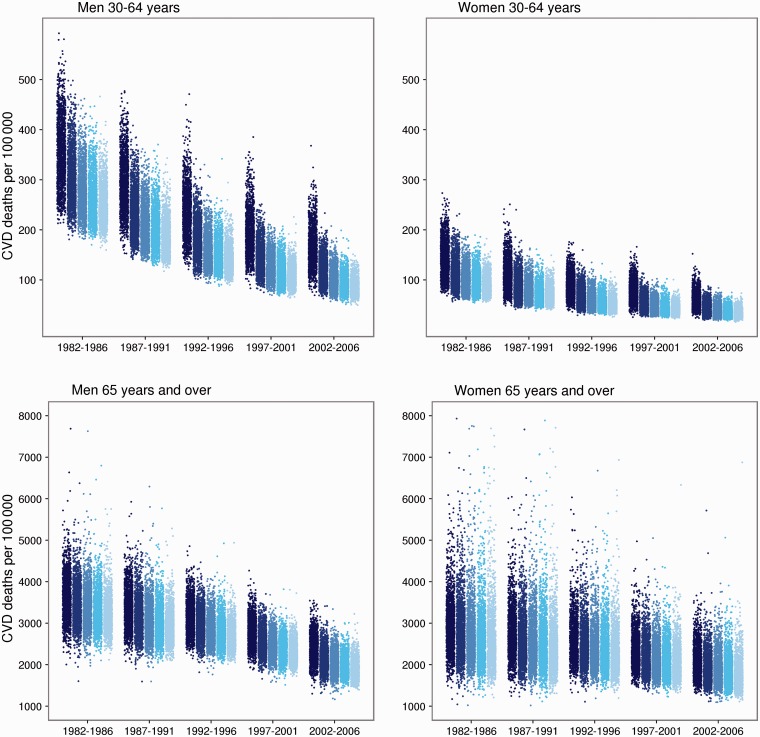
CVD mortality in the mid 2000s was strongly associated with community deprivation among young and middle-age adults seen in both the correlation between modified IMD and mortality (Supplementary Figure S3, available as Supplementary data at IJE online) and in the effect of adjustment on the geographical patterns of CVD mortality (Figure 3). Specifically, after adjusting for Region and urbanicity, additional adjustment for deprivation led to a > 80% reduction in spatial variation of CVD mortality in those aged <65 years (Figure 3a and b). The association of ward CVD mortality with deprivation was less marked at older ages, with the spatial variation declining by 58% in men and 42% in women after adjustment for deprivation. Correlation coefficients between modified IMD and CVD morality were 0.56 for men and 0.32 for women aged ≥65 years (Supplementary Figure S3, available as Supplementary data at IJE online). Further, mortality varied more within each deprivation quintile than the difference between the most- and least-deprived quintiles (Figure 4).
Figure 3.
Posterior standardized mortality ratio of CVD mortality 2002–2006 from the Bayesian spatial model without adjustment (left-hand panels), adjusted for urbanicity and Government Office Region (middle panels), and with additional adjustment for modified IMD quintile (right-hand panels) for (a) men aged 30–64 years; (b) women aged 30–64 years; (c) men aged ≥65 years; and (d) women aged ≥65 years. Each tone in the legend corresponds to a decile of wards in the unadjusted panels
Figure 4.
Cardiovascular (CVD) mortality by ward arranged by quintiles of ward deprivation. Each dot represents the posterior mean of CVD mortality for one ward. The darkest shade shows the most-deprived quintile and the lightest the least-deprived quintile
CVD mortality declined between the 1980s and 2000s in all deprivation quintiles. For groups <65 years of age, the absolute difference between CVD mortality in the most- and least-deprived wards decreased by 24% (men) and 37% (women) (Table 1). On the other hand, relative inequalities between the most- and least-deprived quintiles increased from 1.7 to 2.5 for men and from 2.0 to 2.7 for women because there were smaller ‘relative’ reductions in mortality in the most-deprived wards than the least-deprived ones. In older adults, both relative and absolute deprivation-based inequalities increased steadily over time. This occurred because mortality decline in the most-deprived quintile was smaller than in the least-deprived quintile.
Discussion
We found that the downward CVD mortality trends in England in recent decades benefited nearly all communities, except for a small number of wards where CVD mortality actually increased, especially among older women. The largest absolute reductions in CVD mortality since the early 1980s occurred in wards that started with the highest mortality. As a result of these trends, the overall distribution of CVD mortality across wards narrowed over time in all four age–sex groups. Mirroring this narrowing, absolute inequality between the most- and least-deprived wards (quintiles) declined for young and middle-age adults; at older ages, absolute inequality across deprivation quintiles increased. An important aim of the public health and healthcare system is to achieve an absolute mortality reduction and relieve the CVD burden, especially in deprived and high-burden communities.22,23 In England, this aim has been partially met through reductions in absolute inequalities across wards in young and middle-age adults, but older adults in deprived wards have been left behind. Furthermore, relative inequalities worsened in all four age–sex groups, more so in young and middle ages, pointing to persistent environmental, social and health system injustice.24
Our study has strengths and limitations. Our Bayesian model used spatial relationships and covariates to make stable small-area estimates and track trends and inequalities––thus overcoming the limitations of regional analysis, which mask small-area variation, and those of crude small-area estimates that are unstable owing to measurement error. We also reported the uncertainty of our estimates, which helps identify wards with truly high or low mortality. Our analysis was done by sex, not only for premature mortality but also for mortality at older ages where most CVD deaths occur. This allowed us to identify important similarities and differences in mortality levels and trends across sex and age groups in relation to both geography and deprivation, and to document the substantial variation within each deprivation level. We used a consistent classification of ward-deprivation quintile over time, which allowed tracking a consistent group of wards; this may however underestimate historical inequalities if some wards have switched quintiles and if changes in deprivation quintile are associated with change in health outcomes.25 The alternative of using period-specific deprivation quintiles would not have been affected by this issue, but the wards in each quintile would have differed between periods, restricting comparability. There is, nonetheless, high correlation between current and historical measures of deprivation in England; for example, at the district level, correlations between modified IMD 2007 score and Carstairs deprivation score in 1981, 1991 and 2001 are 0.92, 0.94 and 0.95, respectively. Finally, if health is associated with absolute level of deprivation, vs with relative ranking of wards, then a time series of comparable deprivation measures might be used to investigate trends.26
England changed from using ICD9 to using ICD10 to classify cause of death in 2001; this may affect our estimated trends. Bridge-coding studies demonstrate that the change has had only a small effect on the number of CVD deaths, amounting to a 3–4% increase.27 The direction of this change means that the reductions in CVD mortality, and possibly of the inequalities, may be larger than estimated by us. Because censuses are done every 10 years, district and ward populations in inter-censal years need to be estimated indirectly, and are hence measured with error. The different approaches to estimating small-area population, and their corresponding advantages and disadvantages, are subjects of ongoing research.28 In crude estimates of ward death rate, population measurement error would tend to lead to larger variance than expected had population been measured directly every year.29,30 The Bayesian spatial model may help remove some of this error, and the extreme outliers, by ‘borrowing strength’ across wards.31 In addition to the error associated with distributing the district population across wards, the annual district population figures, estimated by the ONS based on births, deaths and age- and sex-specific in- and out-migration are subject to error especially because migration is not precisely known. Finally, population-level estimates like ours that use death registration by place of residence cannot determine whether the observed change in mortality over time is due to changes in the health of individuals vs due to changes in population composition as a result of migration.32–34 Previous longitudinal and simulation studies have found that internal migration may have a role in small-area trends and inequalities but does not explain the extent of inequalities, indicating the importance of local social, public health and health system factors.35–38 In addition to internal migration, first-generation migrants from South Asia to the UK, but not those from elsewhere, may have higher CVD mortality than those born in the UK.39 Even if a role for migration is established in poor trends and outcomes in some areas, out-migration of healthy groups or in-migration of those with worse health status deserves policy response.40
To our knowledge, our study is the first analysis of trends over time in CVD mortality at small-area level in England, and at both older and younger ages. Previous ward-level analyses in the 1980s and 1990s1,3,4 were restricted to <65 years of age at one point in time with results aggregated by region or deprivation quintile. They found that associations with deprivation accounted for >50% of the variability among wards for mortality of those <65 years of age, consistent with our findings at those ages. These previous studies did not analyse trends and (owing to aggregation) did not provide small-area estimates. A further study found declines in absolute inequalities in age-standardized CVD mortality, aggregated by deprivation quintile, but did not conduct ward- or age-specific analyses,41 and thus the increasing absolute inequality at older ages noted here went unreported. Other reports have focused on inequalities in all-cause or coronary heart disease mortality at the region, district or parliamentary constituency level, for ages <65–75 years.6,42–44 These studies found increasing relative inequalities among geographical areas43,44 and among places classified according to SES,6,42 as we did for CVD mortality, but did not examine absolute inequality or mortality at older ages where the main burden of CVD mortality lies. Trends in all-cause mortality by region have also been analysed, demonstrating a northern disadvantage,44 but the analyses did not extend to small-area level. As a result, within-region variations and local patterns of low and high mortality, which are particularly important in older ages, went undetected. Few of the previous analyses had examined trends in both absolute and relative inequalities.
Little is known on how small-area mortality levels and differentials track over time, and how ward mortality distributions and inequalities may be modified by good or poor public health measures and by local health system performance, a particularly important metric for evaluating decentralized and local programmes and policies. Our results demonstrate that the currently worst-off wards fell into two groups: those primarily around large metropolitan cities in northern England that started with disproportionately high mortality and simply could not ‘catch up’, despite impressive mortality declines, and a second group that started with average or relatively low mortality but performed poorly on mortality reduction and ‘fell behind’. These included some deprived wards of London and wards along the northeastern coast.
The decline in CVD mortality in high-income countries has been attributed to a combination of changes in risk factors at the population level, better primary prevention and better treatment.45,46 In parallel, explanations for health inequalities implicate psychosocial factors, community physical environment, health behaviours and healthcare access and quality.47–50 Clearly, the roles of these factors are not independent, as deprivation, stress and poor working and living environments may themselves be causes of hazardous health behaviours or lower-quality healthcare. This also means that further improvements in CVD mortality, especially in the high-mortality wards, should rely on social and economic measures, as well as dietary, lifestyle and healthcare interventions; either of these actions alone may be insufficient in terms of reduction in inequality and may not be possible to implement in isolation.24,51
Measuring local outcomes as a component of health and health equity is especially important in today’s England and Europe for a number of reasons. First, the economic downturn, rising unemployment and the austerity measures may have disproportionately large effects in deprived areas such as northern England where economic recovery has historically been delayed and fragile after each recession. This could slow down or diminish health gains in these areas, especially among young and middle-aged adults. Second, England’s health system, like those of some other industrialized countries, is heading towards further fragmentation with multiple private sector contractors to both commission and provide health services and devolution of public health responsibilities to local governments.52–54 These new arrangements are likely to be accompanied by tighter budgets and harsher consequences for health providers who seemingly ‘overspend’, compared with arrangements under which healthcare spending by individual general practitioners (GPs) was cushioned by a larger pool, such as the Primary Care Trust in England.54,55 In such a public health and health system environment, rigorous comparative analysis of small-area health interventions and outcomes, in relation to historical trends, will be essential to ensure that all of England’s communities receive proven interventions and are not left behind.
Supplementary Data
Funding
Small Area Health Statistics Unit (SAHSU), which is funded by the Health Protection Agency (HPA) in England as part of the Medical Research Council—Health Protection Agency (MRC-HPA) Centre for Environment and Health at Imperial College London [MRC G0801056]. Wellcome Trust Clinical PhD Fellowship [grant number 092853/Z/10/Z] (to P.A.). National Institute for Health Research (NIHR) Biomedical Research Centre at Imperial College Healthcare NHS Trust and Imperial College (to P.E.). P.E. is an NIHR Senior Investigator [grant number PI0427930]. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest: None declared.
Key Messages.
CVD mortality declined in the vast majority of wards between 1982–86 and 2002–06, but increased in 186 wards for women aged ≥65 years.
CVD mortality decline was larger in wards where starting mortality had been higher, leading to a narrowing of the distribution of CVD mortality across wards in all age–sex groups.
Absolute inequality between most- and least-deprived quintiles of wards narrowed over time for those aged <65 years, but increased in older adults. Relative inequalities became larger in all age and sex groups.
Wards with high CVD mortality in 2002–06 fell into two groups: those primarily around large metropolitan cities in northern England that started with disproportionately high mortality in 1982–86 and could not ‘catch up’, despite impressive declines, and those that started with average or low mortality in the 1980s but ‘fell behind’ owing to small reductions.
Supplementary Material
References
- 1.Law MR, Morris JK. Why is mortality higher in poorer areas and in more northern areas of England and Wales? J Epidemiol Community Health. 1998;52:344–52. doi: 10.1136/jech.52.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uren Z, Fitzpatrick J, Reid A, Goldblatt P. Geographic variation in mortality by social class and alternative social classifications. In: Griffiths C, Fitzpatrick J, editors. Geographic Variations in Health. London: The Stationery Office; 2001. pp. 339–58. [Google Scholar]
- 3.Uren Z, Fitzpatrick J. Analysis of mortality by deprivation and cause of death. In: Griffiths C, Fitzpatrick J, editors. Geographic Variations in Health. London: The Stationery Office; 2001. [Google Scholar]
- 4.Eames M, Ben-Shlomo Y, Marmot MG. Social deprivation and premature mortality: regional comparison across England. BMJ. 1993;307:1097–102. doi: 10.1136/bmj.307.6912.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarborough P, Peto V, Bhatnagar P, et al. Stroke Statistics 2009. Oxford: British Heart Foundation Statistics Database; 2009. [Google Scholar]
- 6.Scarborough P, Bhatnagar P, Wickramasinghe K, Smolina K, Mitchell C, Rayner M. Coronary Heart Disease Statistics. 2010 edn. Oxford: British Heart Foundation Statistics Database; 2010. [Google Scholar]
- 7.Soljak M, Samarasundera E, Indulkar T, Walford H, Majeed A. Variations in cardiovascular disease under-diagnosis in England: national cross-sectional spatial analysis. BMC Cardiovasc Disord. 2011;11:12. doi: 10.1186/1471-2261-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The MINAP Steering Group. Myocardial Ischaemia National Audit Project: How the NHS Cares for Patients with Heart Attack. Tenth Public Report. London: National Institute for Cardiovascular Outcomes Research; 2011. [Google Scholar]
- 9.Unit Trialists’ Collaboration Stroke. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2007;4:CD000197. doi: 10.1002/14651858.CD000197.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Office of National Statistics. Statistical Wards, CAS Wards and ST Wards. http://www.ons.gov.uk/ons/guide-method/geography/beginner-s-guide/administrative/england/electoral-wards-divisions/statistical-wards-cas-wards-and-st-wards/index.html (11 July 2012, date last accessed)
- 11.Bates A. Methodology used for producing ONS’s small area population estimates. Popul Trends. 2006;125:30–36. [PubMed] [Google Scholar]
- 12.Littlefield M, Fulton R. Population estimates; backseries methodology for 1992-2000. Popul Trends. 2005;122:18–26. [PubMed] [Google Scholar]
- 13.Adams J, White M. Removing the health domain from the index of multiple deprivation 2004—effect on measured inequalities in census measure of health. J Public Health. 2006;28:379–83. doi: 10.1093/pubmed/fdl061. [DOI] [PubMed] [Google Scholar]
- 14.Briggs D, Abellan JJ, Fecht D. Environmental inequity in England: small area associations between socio-economic status and environmental pollution. Soc Sci Med. 2008;67:1612–29. doi: 10.1016/j.socscimed.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 15.McLennan D, Barnes H, Noble M, Davies J, Garratt E. The English Indices of Deprivation, 2010: Technical Report. London: HMSO; 2011. [Google Scholar]
- 16.Nobel M, McLennan D, Wilkinson K, Whitworth A, Barnes H. The English Indices of Deprivation, 2007. London: HMSO; 2008. [Google Scholar]
- 17.Besag J, York J, Mollie A. Bayesian image restoration, with two applications in spatial statistics. Ann Inst Stat Math. 1991;43:1–20. [Google Scholar]
- 18.Wakefield JC, Best NG, Waller L. Bayesian approaches to disease mapping. In: Elliott P, Wakefield JC, Best NG, Briggs DJ, editors. Spatial Epidemiology: Methods and Applications. Oxford: Oxford University Press; 2000. pp. 104–27. [Google Scholar]
- 19.Office of National Statistics. Urban/Rural Definition (England and Wales) http://www.ons.gov.uk/ons/guide-method/geography/products/area-classifications/rural-urban-definition-and-la/rural-urban-definition-england-and-wales-/index.html (11 July 2012, date last accessed)
- 20.Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol. 2002;64:583–639. [Google Scholar]
- 21.Richardson S, Thomson A, Best N, Elliott P. Interpreting posterior relative risk estimates in disease-mapping studies. Environ Health Perspect. 2004;112:1016–25. doi: 10.1289/ehp.6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch J, Davey Smith G, Harper S, Bainbridge K. Explaining the social gradient in coronary heart disease: comparing relative and absolute risk approaches. J Epidemiol Community Health. 2006;60:436–41. doi: 10.1136/jech.2005.041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolte E, McKee M. Measuring the health of nations: analysis of mortality amenable to health care. BMJ. 2003;327:1129. doi: 10.1136/bmj.327.7424.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marmot M, Allen J, Goldblatt P, et al. Fair Society, Healthy Lives: Strategic Review of Health Inequalities in England Post 2010. London: 2010. [Google Scholar]
- 25.Boyle P, Norman P, Rees P. Changing places. Do changes in the relative deprivation of areas influence limiting long-term illness and mortality among non-migrant people living in non-deprived households? Soc Sci Med. 2004;58:2459–71. doi: 10.1016/j.socscimed.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell G, Norman P. Longitudinal environmental justice analysis: co-evolution of environmental quality and deprivation in England, 1960–2007. Geoforum. 2012;43:44–57. [Google Scholar]
- 27.Griffiths C, Brock A, Rooney C. The impact of introducing ICD-10 on trends in mortality from circulatory diseases in England and Wales. Health Stat Q. 2004;22:14–20. [PubMed] [Google Scholar]
- 28.Arnold R. Small area health statistics unit procedures for estimating populations in small areas. In: Arnold R, Elliott P, Wakefield J, Quinn M, editors. Population Counts in Small Areas: Implications for Studies of Environment and Health. London: HMSO, Office of National Statistics, Studies on Medical Populations and Subjects; 1999. pp. 10–24. [Google Scholar]
- 29.Arnold R, Diamond I, Wakefield JC. The use of population data in spatial epidemiology. In: Elliott P, Wakefield JC, Best NG, Briggs DJ, editors. Bayesian Approaches to Disease Mapping. Oxford: Oxford University Press; 2000. pp. 30–50. [Google Scholar]
- 30.Rees P, Brown D, Norman P, Dorling D. Are socioeconomic inequalities in mortality decreasing or increasing within some British regions? An observational study, 1990-1998. J Public Health. 2003;25:208–14. doi: 10.1093/pubmed/fdg055. [DOI] [PubMed] [Google Scholar]
- 31.Wakefield J, Wallace C. Implications of estimated counts for small area studies of environment and health. In: Arnold R, Elliott P, Wakefield C, Quinn M, editors. Population Counts in Small Areas: Implications for Studies of Environment and Health. London: HMSO, Office of National Statistics, Studies on Medical Populations and Subjects; 1999. pp. 63–74. [Google Scholar]
- 32.Boyle PJ, Norman P, Popham F. Social mobility: evidence that it can widen health inequalities. Soc Sci Med. 2009;68:1835–42. doi: 10.1016/j.socscimed.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 33.Norman P, Boyle P, Rees P. Selective migration, health and deprivation: a longitudinal analysis. Soc Sci Med. 2005;60:2755–71. doi: 10.1016/j.socscimed.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Connolly S, O’Reilly D, Rosato M. Increasing inequalities in health: is it an artifact caused by the selective movement of people? Soc Sci Med. 2007;64:2008–15. doi: 10.1016/j.socscimed.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Wannamethee SG, Shaper AG, Whincup PH, Walker M. Migration within Great Britain and cardiovascular disease: early life and adult environmental factors. Int J Epidemiol. 2002;31:1054–60. doi: 10.1093/ije/31.5.1054. [DOI] [PubMed] [Google Scholar]
- 36.Elford J, Phillips AN, Thomson AG, Shaper AG. Migration and geographic variations in ischaemic heart disease in Great Britain. Lancet. 1989;1:343–46. doi: 10.1016/s0140-6736(89)91722-4. [DOI] [PubMed] [Google Scholar]
- 37.Strachan D, Leon D, Dodgeon B. Mortality from cardiovascular disease among interregional migrants in England and Wales. BMJ. 1995;310:423–27. doi: 10.1136/bmj.310.6977.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ezzati M, Friedman AB, Kulkarni SC, Murray CJ. The reversal of fortunes: trends in county mortality and cross-county mortality disparities in the United States. PLoS Med. 2008;5:557–68. doi: 10.1371/journal.pmed.0050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scarborough P, Bhatnagar P, Kaur A, Smolina K, Wickramasinghe K, Rayner M. Ethnic Differences in Cardiovascular Disease. 2010 edn. Oxford: British Heart Foundation Statistics Database; 2010. [Google Scholar]
- 40.Davey Smith G, Shaw M, Dorling D. Shrinking areas and mortality. Lancet. 1998;352:1439–40. doi: 10.1016/S0140-6736(05)61261-5. [DOI] [PubMed] [Google Scholar]
- 41.Bajekal M, Scholes S, O’Flaherty M, Raine R, Norman P, Capewell S. Trends in coronary heart disease mortality in England by socio-economic circumstances, 1982-2006. J Epidemiol Community Health. 2010;64:A2. [Google Scholar]
- 42.Thomas B, Dorling D, Davey Smith G. Inequalities in premature mortality in Britain: observational study from 1921 to 2007. BMJ. 2010;341:c3639. doi: 10.1136/bmj.c3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leyland A. Increasing inequalities in premature mortality in Great Britain. J Epidemiol Community Health. 2004;58:296–302. doi: 10.1136/jech.2003.007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hacking JM, Muller S, Buchan IE. Trends in mortality from 1965 to 2008 across the English north-south divide: comparative observational study. BMJ. 2011;342:d508. doi: 10.1136/bmj.d508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuulasmaa K, Tunstall-Pedoe H, Dobson A, et al. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. Lancet. 2000;355:675–87. doi: 10.1016/s0140-6736(99)11180-2. [DOI] [PubMed] [Google Scholar]
- 46.Unal B, Critchley JA, Capewell S. Modelling the decline in coronary heart disease deaths in England and Wales, 1981-2000: comparing contributions from primary prevention and secondary prevention. BMJ. 2005;331:614. doi: 10.1136/bmj.38561.633345.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adler NE, Snibbe AC. The role of psychosocial processes in explaining the gradient between socioeconomic status and health. Curr Dir Psychol Sci. 2003;12:119–23. [Google Scholar]
- 48.Bosma H, Marmot MG, Hemingway H, Nicholson AC, Brunner E, Stansfeld SA. Low job control and risk of coronary heart disease in Whitehall II (prospective cohort) study. BMJ. 1997;314:558–65. doi: 10.1136/bmj.314.7080.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris R, Whincup P, Lampe F, Walker M, Wannamethee SG, Shaper A. Geographic variation in incidence of coronary heart disease in Britain: the contribution of established risk factors. Heart. 2001;86:277–83. doi: 10.1136/heart.86.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kivimäki M, Shipley MJ, Ferrie JE, et al. Best-practice interventions to reduce socioeconomic inequalities of coronary heart disease mortality in UK: a prospective occupational cohort study. Lancet. 2008;372:1648–54. doi: 10.1016/S0140-6736(08)61688-8. [DOI] [PubMed] [Google Scholar]
- 51.House of Commons Health Committee Report. Health Inequalities. London: Third Report of Session 2008-2009; 2009. [Google Scholar]
- 52.Pollock AM, Price D, Roderick P, et al. How the health and social care bill 2011 would end entitlement to comprehensive health care in England. Lancet. 2012;6736:2011–13. doi: 10.1016/S0140-6736(12)60119-6. [DOI] [PubMed] [Google Scholar]
- 53.McKee M, Pollock AM, Clarke A, et al. In defence of the NHS: why writing to the House of Lords was necessary. BMJ. 2011;343:d6535. doi: 10.1136/bmj.d6535. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds L, McKee M. GP commissioning and the NHS reforms: what lies behind the hard sell? J R Soc Med. 2012;105:7–10. doi: 10.1258/jrsm.2011.110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dixon J, Smith P, Gravelle H, et al. A person based formula for allocating commissioning funds to general practices in England: development of a statistical model. BMJ. 2011;343:d6608. doi: 10.1136/bmj.d6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.