Abstract
The goal of cardiovascular disease (CVD) research using linked bespoke studies and electronic health records (CALIBER) is to provide evidence to inform health care and public health policy for CVDs across different stages of translation, from discovery, through evaluation in trials to implementation, where linkages to electronic health records provide new scientific opportunities. The initial approach of the CALIBER programme is characterized as follows: (i) Linkages of multiple electronic heath record sources: examples include linkages between the longitudinal primary care data from the Clinical Practice Research Datalink, the national registry of acute coronary syndromes (Myocardial Ischaemia National Audit Project), hospitalization and procedure data from Hospital Episode Statistics and cause-specific mortality and social deprivation data from the Office of National Statistics. Current cohort analyses involve a million people in initially healthy populations and disease registries with ∼105 patients. (ii) Linkages of bespoke investigator-led cohort studies (e.g. UK Biobank) to registry data (e.g. Myocardial Ischaemia National Audit Project), providing new means of ascertaining, validating and phenotyping disease. (iii) A common data model in which routine electronic health record data are made research ready, and sharable, by defining and curating with meta-data >300 variables (categorical, continuous, event) on risk factors, CVDs and non-cardiovascular comorbidities. (iv) Transparency: all CALIBER studies have an analytic protocol registered in the public domain, and data are available (safe haven model) for use subject to approvals. For more information, e-mail s.denaxas@ucl.ac.uk
Keywords: electronic heath records, linkages, cardiovascular
Data resources basics
Using linked electronic health records for translational research
The goal of cardiovascular disease (CVD) research using linked bespoke studies and electronic health records (CALIBER) is to provide evidence across different stages of translation, from discovery, through evaluation to implementation where electronic health records provide new scientific opportunities. The expanding role of such record research with clinical and public health impact has been extensively reviewed.1–4 With healthy cohort sample sizes ×106 and disease registries 105, such records offer a larger scale and higher degree of phenotypic resolution5 than has been available in investigator led studies.
Opportunity
The UK is the only country in the world that has both detailed electronic primary care records, and CVD and procedure registries at a national scale, as well as more standard sources such as cause-specific hospitalization and mortality records and census data (see Table 1 and Table 2). Neither Sweden nor Denmark has comparably rich national primary care data in their respective linkage programmes. Scotland14,15 and Wales16,17 have established record-linkage programmes that have focused on diabetes and public health research, respectively. Some centres have had prominent research programmes in CVD with a focus on prognosis and quality of care and drug safety research in all phases of translational research in smaller (105) populations such as the Institute of Clinical and Evaluative Sciences in Canada18 and Yale, Duke, Intermountain Heart Centre and the Mayo Clinic in the USA.19 There have been few, if any, attempts to establish an Electronic Health Record (EHR)-based population-based cohort research platform in CVDs.
Table 1.
Availability of primary care data for research in different countries
| Country | National or regional | Primary and ambulatory care data available for research linkages |
|---|---|---|
| UK | National | CPRD, access through Academic Health Sciences Networks. See Table 2 |
| Sweden | National | Primary care is organized regionally; national initiatives in SwedeHeart6 and the National Registry of Secondary Prevention (SEPHIA) |
| Denmark | National | Register of Medicinal Product Statistics7,8 |
| Canada | Regional | Ontario, Institute for Clinical Evaluative Sciences9 |
| Ontario Health Insurance Plan Physician claims database | ||
| USA | National | Medicare (for people aged ≥65 years) |
| National | Million Veteran Programme | |
| Regional | Mayo Clinic10 | |
| Rochester Epidemiology Project, Olmsted County | ||
| Regional | Kaiser Permanente California Research Program on Genes, Environment, and Health11 | |
| Regional | Intermountain Healthcare12 | |
| South Korea | National | National health insurance claims database from the Health Insurance Review & Assessment Service13 |
Table 2.
Linked electronic health record sources in CALIBER: types of data, coding system used and data recording details
| Sources | Types of data | Coding system | When and by whom data is coded? |
|---|---|---|---|
| Primary care: CPRD and other sources |
|
Data recorded using the Read clinical terminology system, version 3 contains ∼99 000 codes |
|
| Social deprivation: ONS | Small area patient social deprivation data | Index of Multiple Deprivation (2007) and Townsend score | Derived from multiple national administrative data sets |
| Disease registry: MINAP |
|
In all, 120 fields most with multiple response categories, as defined by the MINAP steering group |
|
| Secondary care: HES |
|
|
Recorded by non-clinical trained coders based on the discharge summary weeks after discharge |
| Mortality: ONS |
|
The primary, underlying and up to 14 secondary causes of death are recorded using ICD-10 | Doctor (general practitioner or hospital) completes death certificate with cause of death. ICD codes added by trained non-clinical coders |
aEmergency, critical care and maternity data not included in CALIBER for now.
Who set CALIBER up and how was it funded?
In 2012, the UK Government launched four new centres of EHR research in London, Manchester, Dundee and Swansea. CALIBER is led from the London centre, Centre for Health Service and Academic Partnership in Translational Electronic health record Research, Director Hemingway (CHAPTER) incorporating the National Institute for Cardiovascular Outcomes Research (NICOR). CHAPTER is led from University College London (UCL) and Partners, which include UCL, the London School of Hygiene and Tropical Medicine and Queen Mary University of London. CALIBER investigators represent a collaboration between epidemiologists, clinicians, statisticians, health informaticians and computer scientists with initial funding from the Wellcome Trust and the National Institute for Health Research. The first tranche of raw-linked data was received in 2011. Third party linkage was coordinated by the Medicines and Healthcare products Regulatory Agency.
Challenges
Researchers wishing to harness large-scale national data sources or rich regional resources on imaging and bioresources, on CVDs in the UK, face major challenges:
Lack of CVD–EHR research programmes spanning the entire translational cycle.20
Major national EHR sources (e.g. in NICOR) remain unlinked among themselves or to investigator led studies.
Lack of understanding of the potential of different record sources. Although the research community is familiar with death and hospitalization records coded with the International Classification of Disease, other EHR data sources, e.g. primary care, have been much less used by cardiovascular researchers. Poor adoption of meta-data standards21 has led to little information being available on how raw data might be reliably converted into a useable form for researchers, and data inconsistency problems, both of which hinder replication of results and data sharing.
Lack of transparency initiatives, which might address well-recognized problems of selective non-publication, selective reporting and significance chasing biases.22
Data quality has been inadequately tied to the quality of the clinical care received.
CALIBER principles
CALIBER was established within CHAPTER, a centre seeking to address these challenges. We set out below our approach for:
Linkage of multiple EHR data sources. (See Table 2 and Figure 1 for key features of five sources of data currently linked in CALIBER.)
Linkage with bespoke investigator led cohort studies.
Establishing a common data model with reproducible data variables and meta-data (see Figure 2 and Figure 3).
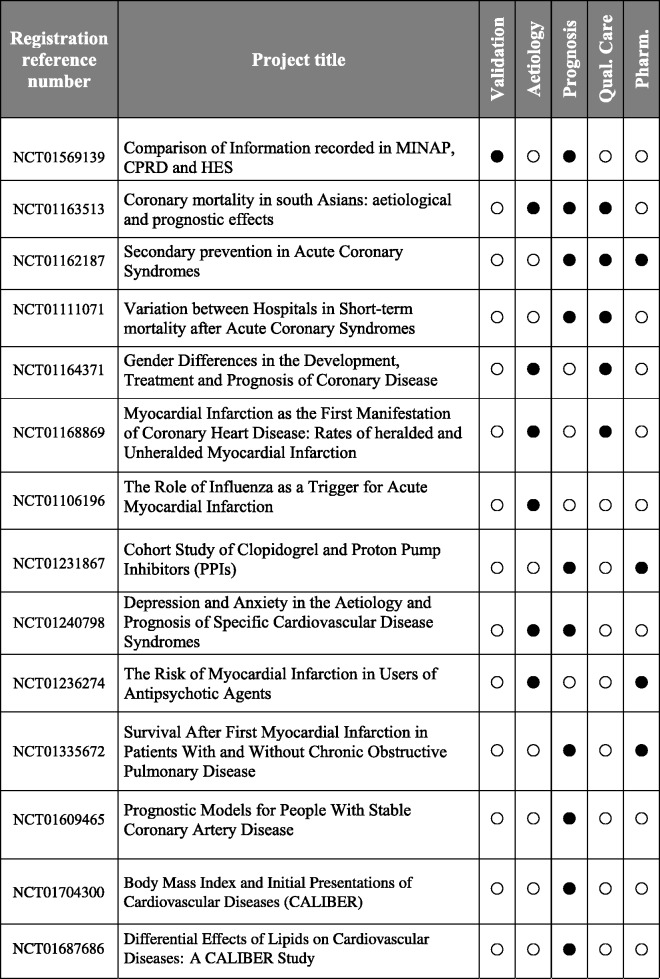
Transparency—CALIBER studies are registered in the public domain on clinicaltrials.gov, and their analytic protocol is made available for download (see Figure 4).
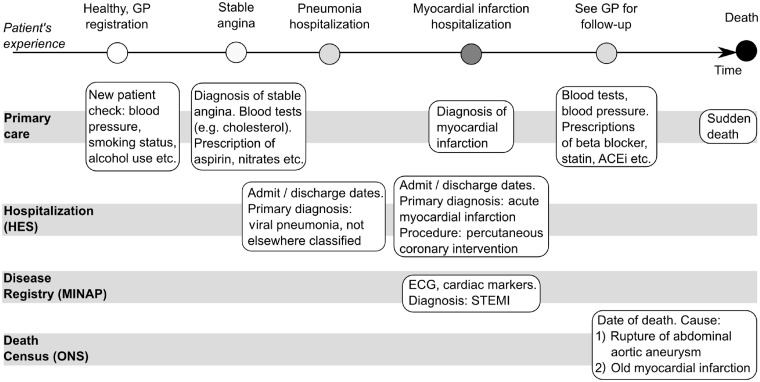
Figure 1.
Longitudinal nature of multiple linked data sources in CALIBER. ECG = Electrocardiography, STEMI = ST-segment elevation Myocardial Infarction, ACEI = Angiotensin-converting-enzyme Inhibitor
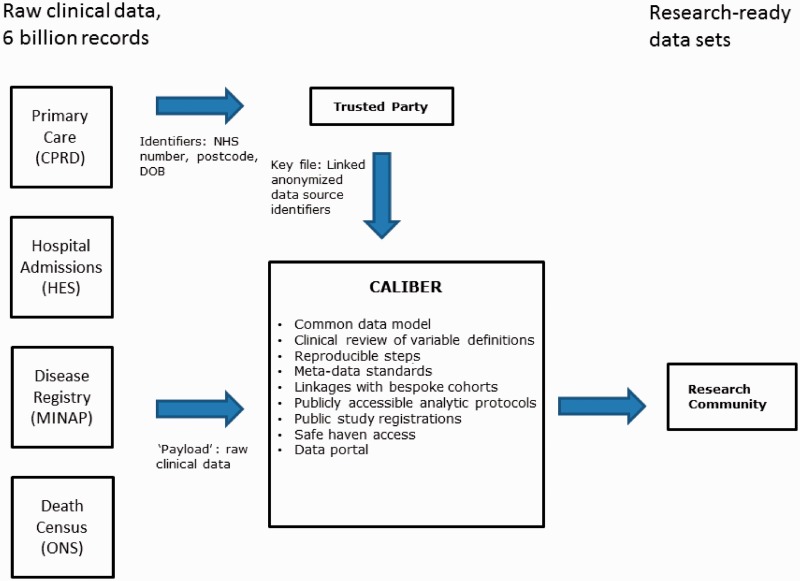
Figure 2.
The CALIBER framework of transforming raw electronic health record data into usable research-ready data sets
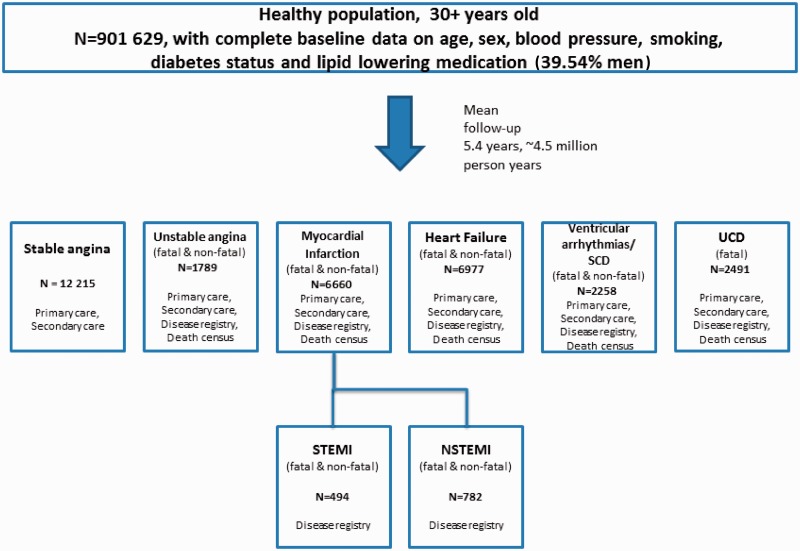
Figure 3.
Example of a CALIBER cohort showing initial presentation of specific cardiac endpoints (n = 32 390) with counts and sources. Appendix A illustrates the approach to defining cardiovascular diseases using multiple record sources in CALIBER
Figure 4.
Example of CALIBER research projects registered in the public domain
Data resource area and population coverage
Figure 1 illustrates the longitudinal, population-based nature of CALIBER data sets and how each source captures a different aspect of the patient journey. Current linkages in CALIBER involve the following: the Clinical Practice Research Datalink (CPRD),23 the Myocardial Ischaemia National Audit Project (MINAP),24 Hospital Episodes Statistics (HES)25 and the Office for National Statistics (ONS) mortality26 and social deprivation data (Table 2).
CPRD is a longitudinal primary care data set and records data on symptoms, diagnosis prescriptions, investigations, referrals and vaccinations. Roughly 97% of the population in the UK are registered with a general practitioner (GP).27 Diagnostic data are coded using Read codes,28 used in several countries in Europe and which map to Systematic Nomenclature of Medicine – Clinical Terms.29 The population of all CPRD practices has been shown to be broadly representative of the population of UK;23 the subset of practices consenting to data-linkage shares the demographic profile of the full CPRD data set. The CPRD has two key methods of ensuring quality of the data made available to the research community: the ‘acceptable research quality’ flag, a patient-level quality marker; and the Up To Standard date, a practice-level quality marker.
MINAP is a national registry of patients admitted to 230 hospitals in the UK with acute coronary syndrome (ACS) events. For each admission, MINAP records patient demographic characteristics, limited medical history, clinical features and investigations, drug treatment (pre-, during and post-admission) and final diagnosis, including differentiation into ST elevation MI, non-ST elevation MI and unstable angina.
HES is a national data set of all admissions to National Health Service (NHS) hospitals in the UK. Diagnoses are recorded using the International Statistical Classification of Diseases and Health-Related Problems, 10th revision (ICD-10)30 and procedures using the Office of Population Censuses and Surveys Classification of Interventions and Procedures (OPCS) Version 4.31
The ONS provides data on cause-specific mortality for all residents of the UK. Cause-specific mortality data are extracted from death certificates and recorded using ICD-10. Small-area measures of social deprivation are recorded using the Index of Multiple Deprivation 200732 and Townsend scores.33
Linkage
A 10-digit numeric identifier, known as the NHS number, uniquely identifies patients in the UK. Number generation is centrally managed and not derived from demographic information, enabling the identification of patients throughout the NHS in an unambiguous and unique way, making NHS numbers a reliable and robust identifier for record linkage and data sharing while preserving patient confidentiality. Roughly 45% of 592 CPRD practices in the UK consented to linkage of their registered patients. For these participating practices, all patients registered at the practice were included. Following the ‘separation principle’,16 data were linked on NHS number, date of birth, sex and post code by a Trusted Party (see Figure 2).
Measures
Converting raw data to research-ready data: curated common data model
Figure 2 illustrates how CALIBER curates data from multiple EHR sources, generating research-ready data from raw data. CALIBER contains >3 billion records of raw data originating from multiple sources. The recorded data are of variable quality, completeness and specificity and unusable for research without proper processing. We have created >300 variables on medical history, diagnosis, investigations, procedures and prescriptions. Data from all of the source data sets were used in the creation of these variables, and each definition has been reviewed and agreed by both clinical and non-clinical researchers in a transparent and reproducible manner. Coding lists containing Read codes, ICD-10 and OPCS-4 codes were compiled for each variable in a reproducible manner.34 Variables contain detailed response categories to provide individual researchers with the flexibility to adjust the sensitivity and specificity of their definitions according to their needs.
Variables are curated using established meta-data standards35 and are versioned using a distributed source control repository system (http://git-scm.com/), which facilitates accurate tracking of changes over time as it keeps all previous revisions of the variables and records the changes between versions. CALIBER researchers have access to the CALIBER online data portal, which includes individual coding lists and programming scripts used to extract both data and clinical coding lists. For each variable, the data portal entry contains the type, source data files, valid range (if applicable), response categories, revision, implementation details and any other relevant information such as references to published literature or descriptive notes. This transparent and reproducible manner of operation ensures that knowledge is fed back into the platform and subsequently becomes available for researchers to make further use of. For example, if a potential researcher is working on an exposure previously not defined within CALIBER, the new variables they define would be incorporated back into the data portal (following peer review) and made available to other future researchers for use. Metadata enable the automatic generation of the centralized data portal and documentation, keeping the variable definition process synchronized with the research data set creation and curation.
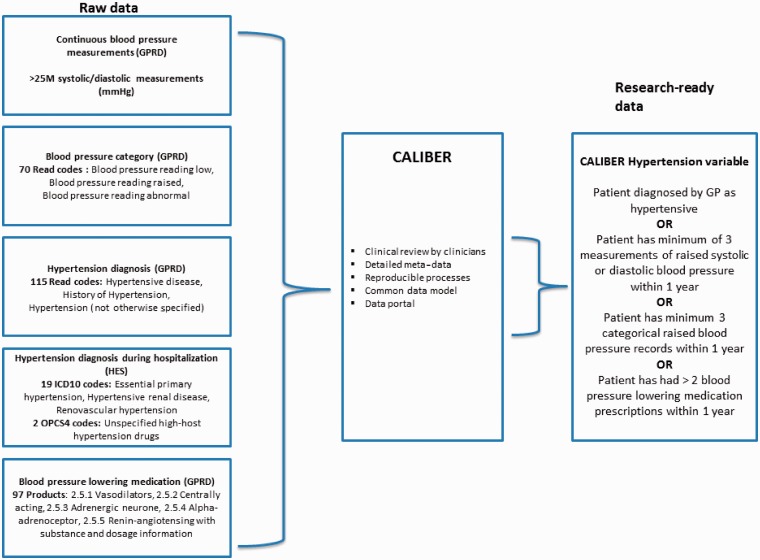
Figure 5 illustrates how CALIBER exploits multiple EHR sources to create a robust data variable (CALIBER hypertension). In total, >700 clinical terms were combined from three EHR sources to create this composite variable, which defines a patient's hypertensive status at a given time point. A patient is considered hypertensive if they have been diagnosed by a GP or within the past year have had at least three measurements of raised systolic or diastolic blood pressure or at least three records of raised blood pressure or at least two prescriptions of blood pressure lowering medication.
Figure 5.
Example of one CALIBER research variable, hypertension, created from multiple raw electronic health record sources. The variable uses a combination of (i) repeat continuous blood pressure measurements; (ii) categorical data on measured blood pressure (over 130 Read codes); (iii) hypertension diagnosis in primary care (over 180 Read codes); and (iv) prescription of blood pressure lowering medications
CALIBER data include all diagnosed co-existing conditions irrespective of hospital attendance and values of clinically collected circulating biomarkers such as haemoglobin and prescriptions issued, including medication type, timing and quantity. Detailed mental health data on diagnosis and hospitalization for numerous morbidities such as depression, anxiety, bipolar disorder, schizophrenia and psychosis have been included in the data portal (D Batty, personal communication).
Frequency of data collection on patients
Unlike traditional cohorts, in EHR cohorts follow-up is continuous and initiated by capture within one or more EHR source (Figure 1). In the example cohort (Figure 3), the median observation time from study entry date is 5.5 years, with an inter-quartile range (IQR) of 2.1–9.1. Roughly 75.5% of people have at least two blood pressure measurements, and the median number of measurements in people with at least one measurement is 9 (IQR 4–20). The median number of BMI measurements in people with at least one measurement is 3 (IQR 2–7), and similarly the median number of total cholesterol measurements are 3 (IQR 1–6). The mean annual consultation rate at baseline is 7.9.
Biobanks and bespoke cohort linkages
Further linkages have also been established between bespoke investigator led cohort studies and MINAP. Such linkages provide traditional cohort studies with higher-resolution ACS endpoints. Specifically, we have coordinated the linkage between MINAP and (i) the UK Collaborative Trial of Ovarian Cancer Screening, a randomized trial of 200 000 post-menopausal women aged 50–74 years;36 (ii) Whitehall II, a cohort of ∼10 000 civil servants aged 35–55 years;37 and (iii) UK Biobank, a cohort of 500 000 middle-aged adults with a range of physical measurements and biological samples.38 Cross-referencing of acute myocardial infarction in four sources is under way.
Data resource use
One important use is to define cohorts using the primary care as denominator population. An example of such a CALIBER cohort (entry between years 2001–10) is shown in Figure 3. The sample consists of 901 629 adults aged ≥30 years (39.54% men) registered with a CPRD practice, which participates in the data linkage and that have data of acceptable research quality, no prior atherosclerotic disease and complete baseline data on age, sex, blood pressure, smoking, diabetes status and lipid lowering medication information. CALIBER resources are contributing new knowledge at different stages in translational pathways, e.g.:
Risk factors for initial presentation of CVDs: CALIBER cohorts allow investigation of initial presentation of a wide range of CVDs, which has hitherto not been possible in smaller, less-well clinically phenotyped cohorts. For example smoking has heterogeneous associations with different CVD endpoints, with no association with initial presentation with ventricular arrhythmia or cardiac arrest, a modest association with chronic stable angina and heart failure and strong associations with ACSs.39
Prognosis: A new prognostic model for people with stable coronary artery disease (CAD)40 has been developed and validated in a population of 100 000 patients with stable angina or who have become stable after an ACS. We show that clinically collected information, including biomarkers, provides significant improvements in discrimination, risk re-classification and clinical cost-effectiveness beyond that achieved based on age, sex, CAD type and clinical history alone.
Quality of care and outcomes research: Two countries in the world have MI registries, which include data on consecutive patients in all hospitals. In comparisons of over half a million patients in Sweden and the UK, there were important treatment differences, and the 30-day mortality was lower in Sweden.41
Pharmacoepidemiology: Although primary care data have long been used for pharmacoepidemiology, new CALIBER linkages with hospital event data from MINAP and HES allowed CALIBER to contribute new policy-relevant evidence on the potential harms of clopidogrel discontinuation42 and the lack of robust evidence of interaction between clopidogrel use and proton pump inhibitors.43
Future developments
The data resources accessible via CALIBER will grow in CHAPTER in several respects including increasing the scale of population coverage to design and implement randomized trials within the primary care record and within disease and procedure registries (NICOR), to incorporate bioresources allowing genomic approaches to discovery and to use natural language processing of text recorded in primary care. Planned extensions include wider coverage of primary care data (current linked CPRD data cover 8% and aims to include 25% by end of 2012, maximizing the coverage by March 2016) and linkage with wider disease and procedure registries in NICOR. A key next step is to incorporate linkages with integrated hospital records and integrate clinical research with medical care on a large scale by unlocking the full potential of data collected in such settings. Existing platforms such as the National Institute for Health Research Cardiovascular Biomedical Research Unit, an integrated cardiovascular hospital records platform spanning cardiac data, general trust-level systems, radiology and genomics systems for 25 000 patients will be expanded.
Strengths and weaknesses
CALIBER has potential strengths in the degree of clinical, longitudinal phenotyping spanning ambulatory and hospitalized care in large population samples. This allows opportunities for a ‘higher resolution’ approach to cardiovascular epidemiology in three respects. First, most existing investigator led (bespoke) cardiovascular cohorts study broad aggregates [CVD, coronary heart disease (CHD)], or at most distinguish a narrow range (typically two or three) of different diseases of interest. As the causes, treatment and prognosis vary across a range of disease phenotypes, CALIBER data sets have the statistical size and clinical phenotyping allowing the opportunity to ‘resolve’ these broad aggregates into distinct disease phenotypes in cerebral, peripheral and coronary circulations. Figure 3 illustrates that cardiac diseases include the following: chronic stable angina, unstable angina, ST elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI), heart failure, ventricular arrhythmias and cardiac arrest, sudden cardiac death and unheralded coronary death. Use of four sources of data to define endpoints is illustrated in Appendix A. Second, unlike many investigator led cardiovascular cohorts, CALIBER data sets have the temporal resolution to distinguish whether a first event (e.g. heart attack or stroke) was the first manifestation of CVD or whether it was preceded by any of a range of non-fatal (and often diagnosed in primary care) manifestations of CVD. Third, most previous studies have been too small to understand the interplay between different causal domains. For example, even addressing the simple question of gender differences in the smoking effect across different specific cardiovascular endpoints necessitates large samples. CALIBER cohorts have a sufficient number of events and patients on which accurate estimates of risk can be based.
The main weaknesses of any EHR research platform relate to data quality.
Data quality in single sources
Data quality in single sources requires evaluation on a case by case basis with a range of methods: cross-referencing against chart review, against data collected under research conditions and cross-sectional and prospective tests of validity.
Missing data
Although some measurements, such as BMI, are reasonably complete (82.6% of people with at least one BMI measurement), others, such as total cholesterol, are less complete (44.9% with at least one total cholesterol measurement). Developments in imputation of missing data within such primary care longitudinal cohorts are promising and might offer a partial solution to the problem.44 Variation in coding used across practices and over time, as well the use of broader diagnostic categories used (e.g. CHD Not Otherwise Specified),45 may prove more difficult to resolve.
Conflicting data
Fatal MI may be recorded in up to four different sources, which differ in their diagnostic granularity and timing accuracy. Multiple records at similar time points may reflect the same event or subsequent events—e.g. an MI is recorded in CPRD, and, after 30 days in MINAP, could point to a single or recurrent event (E Herrett, personal communication).
Linkage quality
There is empirical evidence46 that errors in data linkage can lead to significantly different conclusions about the associations of risk factors with outcomes. The linkage method used for linking CALIBER constituent data sets has been used before, and it has been shown to yield reasonably high quality matches.
Data resource access
Raw data are available for use by researchers subject to approval of the protocol by, and payment to, the bodies governing access to the constituent data sets. For CPRD, this involves scientific approval of the protocol by the Independent Scientific Advisory Committee and a signed licence outlining scope and data confidentiality of use of CPRD data. For MINAP, applications are made to the MINAP Academics Group, and to the NHS Information Centre47 for HES and ONS. Following such approvals, an application to the CALIBER Scientific Advisory Committee should be submitted.
CALIBER researchers register their study and publish their protocol in the public domain before receiving data.48 Study registration and protocol publication are of complementary nature; registration records present short, standardized elements of the full protocol in an accessible manner.
CALIBER welcomes collaborative research. Access to data for external researchers (those who are not affiliated with CALIBER investigators) is provided within the CALIBER ‘safe haven’ environment, which currently requires researchers to be physically based in either UCL (Clinical Epidemiology Group) or the London School of Hygiene and Tropical Medicine (Smeeth). Given the diverse clinical origins, complexity and size of the data sets, training with the CALIBER team on data sources, coding, quality and management is available.
Appendix A.
Overview of codes used to define a range of common CVDs. Details of how these codes are combined are given in the data portal. We provide a list of indicative diagnostic codes used across the data sources and not an exhaustive list
| Endpoint | Primary care | Disease Registry | Hospital procedures | Hospital diagnosesa | Causes of deathb |
|---|---|---|---|---|---|
| CPRD | MINAP | HES | HES | ONS | |
| Read codes | Registry specific | OPCS-4 | ICD-10 | ICD-10 | |
| Acute myocardial infarction |
|
MI with or without ST elevation based on initial ECG findings, raised troponins and clinical diagnosis | Not used (there is no code that is specific to primary coronary intervention) |
|
|
| Unstable angina | G311.13/G311100 Unstable angina, G233200 Angina at rest, G311400 Worsening angina + 13 other codes | Discharge diagnosis of unstable angina, no raised ST elevationNo raised troponin levels | nu |
|
nu |
| Stable angina |
|
nu |
|
Stable angina pectoris I20 excluding unstable angina I20.0 | nu |
| Coronary heart disease not otherwise specified | G3…00 Ischaemic Heart Disease + 90 other codes including CHD NOS, chronic ischaemic heart disease, silent myocardial infarction | nu | nu | CHD NOS, chronic ischaemic heart disease, silent myocardial infarction I25 excluding I25.2, old myocardial infarction | nu |
| Heart failure | G58.00 Heart Failure + 92 other Read codes for heart failure diagnosis | nu | nu |
|
|
| Ventricular arrhythmias, cardiac arrest and sudden cardiac death |
|
nu | Implanted cardiac defibrillation device X50, Implantation, revision and renewal of cardiac defibrillator K59 |
|
|
| Unheralded coronary death | Any CVD excluded | Any CVD excluded | Any CVD excluded | Any CVD excluded | I20 Angina Pectoris, I21 Acute myocardial infarction, I22 Subsequent myocardial infarction, I23 Certain current complications following acute myocardial infarction, I24 Other acute ischaemic heart diseases and I25 Chronic ischaemic heart disease not preceded by any other CVD presentation |
| Ischaemic stroke | G64.11 CVA – cerebral artery occlusion, G64.13 Stroke due to cerebral arterial occlusion, G6W.00 Cereb infarct due unspecified occlusion/stenosis of precerebral arteries, G6X.00 cerebral infarction due to unspecified occlusion or stenosis of cerebral arteries plus 8 other codes | nu | nu | I63 cerebral infarction | I63 cerebral infarction |
| Haemorrhagic stroke | 93 codes for subarachnoid haemorrhage, intracerebral haemorrhage, and intracranial haemorrhage not otherwise specified | nu | I60 Subarachnoid haemorrhage, I61.0 Intracerebral haemorrhage in hemisphere, subcortical, I61.1 Intracerebral haemorrhage in hemisphere, cortical, I61.2 Intracerebral haemorrhage in hemisphere unspecified, I61.3 Intracerebral haemorrhage in brain stem, I61.4 Intracerebral hemorrhage in cerebellum, I61.5 Intracerebral haemorrhage intraventricular, I61.6 Intracerebral haemorrhage, multiple localized, I61.8 Other intracerebral haemorrhage, I61.9 Intracerebral haemorrhage | nu | |
| Peripheral arterial disease |
|
nu |
|
|
|
| Abdominal aortic aneurysm (AAA) |
|
nu |
|
|
|
aPrimary cause of admission.
bUnderlying cause of death.
Nu, not used in definition.
The CALIBER data portal is available for consultation online at http://www.caliberresearch.org/. Existing and potential collaborators are encouraged to provide feedback on potential enhancements to the portal to facilitate the wider use of these data resources.
For more information, visit http://www.caliberresearch.org/ or contact Dr Spiros Denaxas at s.denaxas@ucl.ac.uk.
Acknowledgements
Tjeerd van Staa coordinated linkages involving the Clinical Practice Research Datalink; David Cunningham, National Institute for Cardiovascular Outcomes Research, coordinated linkages involving MINAP; Marina Daskalopoulou Clinical Epidemiology Group, University College London.
Funding
CALIBER is funded by a Wellcome Trust project grant (086091/Z/08/Z), a National Institute of Health Research (NIHR) programme grant (RP-PG-0407-10314) and a consortium of 10 UK government and charity funders, led by the Medical Research Council (MRC), which funds the Centre for Health service and Academic Partnership in Translational Electronic health records Research (CHAPTER). The funders are: Cancer Research UK (CRUK), Chief Scientist Office, Scottish Government Health Directorates (CSO), Engineering and Physical Sciences Research Council (EPSRC), Economic and Social Research Council (ESRC), National Institute for Health Research (NIHR), National Institute for Social Care and Health Research (NISCHR) and The Wellcome Trust.
A.D.H., A.T., and H.H. are partners in the Medical Research Council PROGnosis RESearch Strategy (PROGRESS) Partnership (www.progress-partnership.org), supported by the Medical Research Council [grant number G0902393/1] which supports research using CALIBER. M.K. is supported by an Economic and Social Research Council (ESRC) professorial fellowship. L.S. is supported by a Senior Clinical Fellowship from the Wellcome Trust. A.S. is supported by a Wellcome Trust Clinical Research Training Fellowship [0938/30/Z/10/Z] E.H. is supported by an MRC studentship, and J.G. was funded by a NIHR Doctoral Fellowship [DRF-2009-02-50].
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR PHR Programme or the Department of Health.
Conflict of interest: None declared.
Key messages.
CALIBER data resources provide a common framework for addressing questions of:
Risk factors for initial presentation of a wide range of pathologically diverse CVDs: We have shown that smoking has heterogeneous associations with different CVD endpoints.
Prognosis: We have developed a new prognostic model for people with stable CAD, which makes use of clinically collected information, including biomarkers.
Quality of care and outcomes research: We observed significant treatment differences and lower 30-day mortality in Sweden when comparing over half a million MI patients with the UK ACS registry.
Pharmacoepidemiology: We have illustrated the potential harms of clopidogrel discontinuation and the lack of robust evidence of interaction between clopidogrel use and proton pump inhibitors.
References
- 1.UK Clinical Research Collaboration. Report of Research Simulations. London: UKCRC; 2007. (UKCRC) Advisory Group to Connecting for Health. [Google Scholar]
- 2.Medical Research Council (MRC) UK E-Health Records Research Capacity and Capability. London: MRC; 2011. [Google Scholar]
- 3.Department of Health. The Power of Information: Putting All of Us in Control of the Health and Care Information We Need. London: Department of Health; 2012. [Google Scholar]
- 4.Department of Business Innovation & Skills. Strategy for UK Life Sciences. London: Department of Business, Innovation and Skills; 2011. [Google Scholar]
- 5.Timmis AD, Feder G, Hemingway H. Prognosis of stable angina pectoris: why we need larger population studies with higher endpoint resolution. Heart. 2007;93:786–91. doi: 10.1136/hrt.2006.103119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jernberg T, Attebring MF, Hambraeus K, et al. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART) Heart. 2010;96:1617–21. doi: 10.1136/hrt.2010.198804. [DOI] [PubMed] [Google Scholar]
- 7.Lindhardsen J, Ahlehoff O, Gislason GH, et al. Risk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort study. BMJ. 2012;344:e1257. doi: 10.1136/bmj.e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sørensen R, Hansen ML, Abildstrom SZ, et al. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet. 2009;374:1967–74. doi: 10.1016/S0140-6736(09)61751-7. [DOI] [PubMed] [Google Scholar]
- 9.Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378:991–96. doi: 10.1016/S0140-6736(11)60990-2. [DOI] [PubMed] [Google Scholar]
- 10.St Sauver JL, Jacobson DJ, McGree ME, et al. Associations between longitudinal changes in serum estrogen, testosterone, and bioavailable testosterone and changes in benign urologic outcomes. Am J Epidemiol. 2011;173:787–96. doi: 10.1093/aje/kwq438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Research Program on Genes, Environment, and Health. http://www.dor.kaiser.org/external/DORExternal/rpgeh/index.aspx (14 November 2012, date last accessed) [Google Scholar]
- 12.Intermountain Healthcare Cardiovascular Research. http://intermountainhealthcare.org/services/heart/research/pages/home.aspx (14 November 2012, date last accessed) [Google Scholar]
- 13.Jang MJ, Bang SM, Oh D. Incidence of pregnancy-associated venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haem. 2011;9:2519–21. doi: 10.1111/j.1538-7836.2011.04518.x. [DOI] [PubMed] [Google Scholar]
- 14.Colhoun HM. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009;52:1755–65. doi: 10.1007/s00125-009-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scottish Health Informatics Programme. A Blueprint for Health Records Research in Scotland. Dundee: Scottish Health Informatics Programme, 2011.
- 16.Ford DV, Jones KH, Verplancke JP, et al. The SAIL Databank: building a national architecture for e-health research and evaluation. BMC Health Serv Res. 2009;9:157. doi: 10.1186/1472-6963-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyons RA, Jones KH, John G, et al. The SAIL databank: linking multiple health and social care datasets. BMC Med Inf Dec Mak. 2009;9:3. doi: 10.1186/1472-6947-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Institute for Clinical Evaluative Sciences (ICES), http://www.ices.on.ca/ (14 November 2012, date last accessed)
- 19.McCarty CA, Chisholm RL, Chute CG, et al. The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Gen. 2011;4:13. doi: 10.1186/1755-8794-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westfall JM, Mold J, Fagnan L. Practice-based research–“Blue Highways” on the NIH roadmap. JAMA. 2007;297:403–06. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 21. International Organization for Standardization (ISO). Health Informatics - Good Principles and Practices for a Clinical Data Warehouse (ISO/TR 22221:2006). Geneva: ISO, 2006.
- 22.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walley T, Mantgani A. The UK General Practice Research Database. Lancet. 1997;350:1097–99. doi: 10.1016/S0140-6736(97)04248-7. [DOI] [PubMed] [Google Scholar]
- 24.Herrett E, Smeeth L, Walker L, Weston C. The Myocardial Ischaemia National Audit Project (MINAP) Heart. 2010;96:1264–67. doi: 10.1136/hrt.2009.192328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centre TH and SCI. Hospital Episodes Statistics (HES) 2011. Available from: http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937 (14 November 2012, date last accessed) [Google Scholar]
- 26.Office for National Statistics. Mortality Statistics: Metadata 2010 Statistics. London: 2011. (14 November 2012, date last accessed) [Google Scholar]
- 27.Simon C. Overview of the GP contract. InnovAiT. 2008;1:134–39. [Google Scholar]
- 28.Chisholm J. The Read clinical classification. BMJ. 1990;300:1092. doi: 10.1136/bmj.300.6732.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. International Health Terminology Standards Development Organization. Systematized Nomenclature of Medicine—Clinical Terms (SNOMED-CT) [Internet]. http://www.ihtsdo.org/snomed-ct/ (14 November 2012, date last accessed)
- 30. ICD. International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM). 2011 release. Available from: http://www.cdc.gov/nchs/icd/icd10cm.htm (14 November 2012, date last accessed)
- 31. OPCS-4 Classification — NHS Connecting for Health. Available from: http://www.connectingforhealth.nhs.uk/systemsandservices/data/clinicalcoding/codingstandards/opcs4/index_html (27 November 2012, date last accessed)
- 32.Noble M, Mclennan D, Wilkinson K, Whitworth A. The English Indices of Deprivation 2007. London: Communities; 2007. [Google Scholar]
- 33.Townsend P. Health and Deprivation: Inequality and the North. London: Routledge; 1988. [Google Scholar]
- 34.Dave S, Petersen I. Creating medical and drug code lists to identify cases in primary care databases. Pharm Drug Saf. 18:704–07. doi: 10.1002/pds.1770. [DOI] [PubMed] [Google Scholar]
- 35.Data Documentation Initiative (DDI) website. http://www.ddialliance.org/Specification/ (14 November 2012, date last accessed) [Google Scholar]
- 36. UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), 2012. http://www.ukctocs.org.uk (14 November 2012, date last accessed)
- 37.Marmot M, Brunner E. Cohort Profile: the Whitehall II study. Int J Epidemiol. 2005;34:251–56. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 38.Manolio TA, Weis BK, Cowie CC, et al. New models for large prospective studies: is there a better way? Am J Epidemiol. 2012;175:859–66. doi: 10.1093/aje/kwr453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.George J, Herrett E, Denaxas S, et al. Differential Effects of Smoking on Specific Cardiovascular Presentations in Men and Women: Prospective Cohort Study in 900,000 Patients Using CALIBER Linked Electronic Health Records. Los Angeles: American Heart Association Scientific Sessions, 2012. [Google Scholar]
- 40.Rapsomaniki E, Shah AD, Denaxas S, et al. Prognostic Models for People with Stable Coronary Artery Disease Based on 115,500 Patients from the CALIBER Study. Munich: European Society of Cardiology (ESC), 2012. [Google Scholar]
- 41.Chung SC, Gedeborg R, Nicholas O, et al. Comparative Effectiveness of Acute Myocardial Infarction Care Delivered in Sweden and the United Kingdom Using National Outcome Registries. Los Angeles: American Heart Association Scientific Sessions, 2012. [Google Scholar]
- 42.Boggon R, van Staa TP, Timmis A, et al. Clopidogrel discontinuation after acute coronary syndromes: frequency, predictors and associations with death and myocardial infarction–a hospital registry-primary care linked cohort (MINAP-GPRD) Eur Heart J. 2011;32:2376–86. doi: 10.1093/eurheartj/ehr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Douglas IJ, Evans SJW, Hingorani AD, et al. Clopidogrel and interaction with proton pump inhibitors: comparison between cohort and within person study designs. BMJ. 2012;345:e4388. doi: 10.1136/bmj.e4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marston L, Carpenter JR, Walters KR, Morris RW, Nazareth I, Petersen I. Issues in multiple imputation of missing data for large general practice clinical databases. Pharmacoepidemiol Drug Saf. 2010;19:618–26. doi: 10.1002/pds.1934. [DOI] [PubMed] [Google Scholar]
- 45.Bhattarai N, Charlton J, Rudisill C, Gulliford MC. Coding, recording and incidence of different forms of coronary heart disease in primary care. PLoS ONE. 2012;7:e29776. doi: 10.1371/journal.pone.0029776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lariscy JT. Differential record linkage by Hispanic ethnicity and age in linked mortality studies: implications for the epidemiologic paradox. J Aging and Health. 2011;23:1263–84. doi: 10.1177/0898264311421369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.NHS Information Centre website. http://www.ic.nhs.uk/ (14 November 2012, date last accessed) [Google Scholar]
- 48.CALIBER. Cardiovascular Disease Research using Linked Bespoke Studies and Electronic Records Data Portal. 2010. http://caliberresearch.org/ (14 November 2012, date last accessed) [Google Scholar]