Abstract
Background The association between smoking and the risk of skin cancer has not been well established.
Methods In two large cohorts in the USA, we prospectively examined the risks of melanoma, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) among participants grouped according to smoking variables.
Results Among men, compared with never smokers, ever smokers had a significantly lower risk of melanoma [relative risk (RR) = 0.72; 95% confidence interval (CI): 0.58–0.86]; those who smoked for ≥30 years had an RR of 0.65 (95% CI: 0.48–0.89) (Ptrend = 0.003); those who smoked ≥15 cigarettes per day had an RR of 0.32 (95% CI: 0.13–0.78) (Ptrend = 0.006) and those who smoked for > 45 pack years had an RR of 0.66 (95% CI: 0.45–0.97) (Ptrend = 0.03). Ever smokers also had a slightly lower risk of BCC (RR = 0.94; 95% CI: 0.90–0.98). There was no significant association for SCC (RR = 0.99; 95% CI: 0.89–1.12). In women, no significant association was found for melanoma (RR = 0.96; 95% CI: 0.83–1.10). Compared with never smokers, ever smokers had a slightly higher risk of BCC (RR = 1.06; 95% CI: 1.03–1.08) and a higher risk of SCC (RR = 1.19; 95% CI: 1.08–1.31). A significant inverse association between smoking and melanoma was limited to the head and neck (RR = 0.65; 95% CI: 0.42–0.89).
Conclusions Smoking was inversely associated with melanoma risk, especially on the head and neck. Further studies are warranted to investigate the underlying mechanism(s).
Keywords: Smoking, skin cancer, cohort study, meta-analysis
Introduction
Skin cancers, mainly melanoma, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), are the most frequently diagnosed malignant tumors among White people in the USA. One in five Americans develops skin cancer in his or her lifetime.1 An annual increase of 3–7% has been noted in incidence of these three cancers in recent years.2,3 Though malignant melanoma is a lethal form of skin cancer, BCC and SCC also present a substantial and costly health problem because of their high prevalence and morbidity.
Although there is some variation among melanoma, BCC and SCC, an individual’s risk of developing skin cancers depends on both constitutional and environmental factors. It is generally accepted that constitutional factors, such as skin phototype, eye and hair colour and tanning ability, are risk factors for skin cancers, whereas ultraviolet (UV) radiation is an established environmental risk factor.4 Tobacco smoke contains a number of carcinogenic compounds and has been shown to have an aetiological effect in at least 18 types of cancer,5 but the evidence for skin cancers is contradictory. On the one hand, smoking has been demonstrated to decrease cutaneous blood flow and suppress immune responses, which may increase skin cancer risk;6 on the other hand, there is the possibility that smoking may protect the skin from the inflammatory reaction induced by UV radiation, thus reducing skin cancer risk.7
Previous epidemiological studies investigating the association between cigarette smoking and skin cancer have focused exclusively on one type of cancer, limiting the direct comparison of the three cancers within the same at-risk population. Most reports were from case–control studies and were limited by small sample sizes. Few studies have presented associations stratified by sex and body sites, and most studies did not incorporate measures of frequency or duration of smoking (such as total years smoked, cigarettes per day and pack years), nor did they exhaustively adjust for potential confounders.8–14 The objective of the current study was to investigate the associations between cigarette smoking and melanoma, BCC and SCC in both men and women and to assess whether there is a dose–response relationship between cigarette smoking and skin cancer in the Health Professionals Follow-up Study (HPFS) and the Nurses’ Health Study (NHS). In addition, we summarized previous results on the association between smoking and skin cancer by conducting a meta-analysis.
Methods
Study population
The NHS was established in 1976, when 121 700 registered nurses aged 30–55 years in 11 US states responded to a baseline questionnaire regarding risk factors for cancer and cardiovascular diseases. Participants completed self-administered mailed follow-up questionnaires biennially with updated information on their lifestyle, diet and medical history. The HPFS began in 1986, when 51 529 US male health professionals, including dentists, veterinarians, pharmacists and optometrists, aged 40–75 years completed a baseline questionnaire on lifestyle, diet and newly diagnosed diseases. The information was updated biennially with follow-up questionnaires. These studies were approved by the Human Research Committee at the Brigham and Women’s Hospital (Boston, MA), with written informed consent from all participants.
Assessment of smoking and skin cancer risk factors
Information on smoking was obtained at baseline (in 1976 for the NHS and 1986 for the HPFS). For past smokers, we asked the ages of initiation and quitting and the number of cigarettes per day while smoking. We assumed that a past smoker had smoked the same number of cigarettes throughout the reported years of smoking. Current smokers reported intensity of smoking (cigarettes per day). Current smoking status and intensity of smoking were updated biennially in subsequent questionnaires for all cohort members. For each questionnaire, participants who reported smoking were defined as current smokers during that 2-year period. We estimated pack years (the equivalent of smoking 20 cigarettes a day for 1 year) by multiplying the number of packs smoked per day by the number of years of smoking. The time since quitting was calculated for those who quit smoking during follow-up. Categories were derived for duration of smoking, intensity of smoking, pack years of smoking and years since quitting smoking.
Data on skin cancer risk factors were obtained from cohort questionnaires in both cohorts in the 1980s. These risk factors included adolescent sunburn reactions, family history of melanoma, number of severe sunburns, mole count on the left arm, natural hair colour and UV index at birth, age 15 and age 30 years. In 2008, we asked how many hours per week were spent outdoors in direct sunlight in the middle of the day in summer months, including work and recreation at different age intervals (high school/college-age, 25–35, 36–59 and 60–65 years), in both cohorts.
Identification of skin cancer cases
Skin cancer identification was performed routinely in both cohorts (from 1984 in the NHS and from 1986 in the HPFS). Participants reported new diagnoses biennially. With their permission, participants’ medical records were obtained and reviewed by physicians to confirm their self-reported diagnosis. Only pathologically confirmed invasive cases of melanoma and SCC were included in this study. Medical records were not obtained for self-reported cases of BCC, and the validity of BCC self-reports was > 90%.15,16
Statistical analysis
A Cox proportional hazard model was used to assess the association between smoking and the incidence of skin cancers. We constructed interaction terms between cigarette smoking and calendar year and performed likelihood ratio tests. The statistical non-significance of these interaction terms indicated that the proportional hazard assumption was met.
Participants contributed person time from the date of return of the baseline questionnaire (1984 in the NHS and 1986 in the HPFS) until date of self-report of the first BCC, date of diagnosis of confirmed melanoma or SCC, date of death or the end of follow-up (31 May 2008), whichever came first. Those who were lost to follow-up we censored after the return date of the last questionnaire. Smoking was analysed as smoking status (never, past and current), intensity of smoking (never, 1–14 and ≥15 cigarettes/day), duration of smoking (never, < 20, 20–29 and ≥30 years), years since quitting smoking (never, <10 and ≥10 years) and pack years of smoking (never, <10, 10–24, 25–44 and ≥45 pack years). All categorical variables of smoking were used as indicator variables, with the lowest level as the reference. Trend tests for categorical measures of smoking were performed using mid-points of categories for variables with numerical values, e.g. years and pack years. Confounding factors were adjusted in the multivariable analysis. These factors included body mass index; physical activity (quintiles); history of smoking-related diseases, such as cardiovascular diseases, type 2 diabetes, hypertension, hypercholesterolaemia and non-skin cancer (any of which could influence an individual’s outdoor activities); childhood reaction to sun; moles; hair colour; family history of melanoma; severe sunburns; sun exposures at different age intervals and UV index at birth, age 15 and age 30 years.
We examined the effect modification by major risk factors on the association between smoking and skin cancer. To summarize multiple variables, we constructed a multivariable confounder score17 as a susceptibility score for melanoma. Briefly, we applied the Cox regression coefficients from a multivariable model, including age, hair colour, severe sunburns, moles and family history of melanoma, to each individual’s values for the latter four of these variables and summed the values to compute a susceptibility risk score. We used this score to identify participants with low and high susceptibility based on the mean score. Participants with high scores had an increased risk of melanoma [among men, relative risk (RR) = 2.42; 95% confidence interval (CI): 1.96–2.99 and among women, RR = 2.31; 95% CI: 1.97–2.70] compared with those with low scores. We tested a single multiplicative interaction term by the likelihood ratio test comparing the model with the single interaction term with the model containing just the main effects of the susceptibility score and smoking along with the same covariates. We also analysed melanoma at four different specific body sites: the head and neck, trunk, upper extremity and lower extremity. Statistical analyses were conducted using SAS software (version 9, SAS Institute, Cary, NC, USA). All statistical tests were two sided.
To summarize previous results on the association between smoking and skin cancer, we conducted a PubMed search using keywords ‘smoking’, ‘skin cancer’ and ‘melanoma’. Meta-analyses were performed for the association between smoking and skin cancer risks, and all the cohort studies and case–control studies were included. Including our study, four studies were used in the male cohort analysis for smoking and melanoma,7,18,19 and three studies were used in the female cohort analysis.18,19 The study in the Norwegian cohort was not included because the authors cannot provide separate results for male and female subjects,20 but the exclusion of this study had little influence on the pooled results. Seven studies were included in the case–control analysis for smoking and melanoma.6,14,21–25 Five were included in the analysis for smoking and BCC,6,10,11,26 and six were included in the analysis for smoking and SCC.6,27–31 The aforementioned studies did not provide data stratified by sex, so we performed a meta-analysis for men and women combined. All meta-analyses were performed using the STATA software (version 11.0; Stata Corp, College Station, TX, USA).
Results
A total of 145 709 eligible participants were included in the analyses (44 799 male health professionals and 100 910 female nurses). Baseline characteristics of participants were similar among never smokers, past smokers and current smokers in both cohorts (Table 1).
Table 1.
Age-adjusted variables by smoking status for HPFS and NHS
| HPFS |
NHS |
|||||
|---|---|---|---|---|---|---|
| Variables | Never smoker (n = 20 683) | Past smoker (n = 19 658) | Current smoker (n = 4458) | Never smoker (n = 45 511) | Past smoker (n = 30 765) | Current smoker (n = 24 634) |
| Age (years), 1986a | 52.8 (9.9) | 55.9 (9.7) | 54.0 (9.3) | 52.2 (7.4) | 52.5 (7.1) | 52.0 (7.0) |
| Body mass index (kg/m2), 1986 | 25.4 (3.3) | 25.8 (3.4) | 25.4 (3.4) | 25.5 (4.9) | 25.5 (4.9) | 24.4 (4.4) |
| Activity (met-hour/week), 1986b | 21.5 (29.5) | 20.9 (29.3) | 15.1 (21.5) | 13.9 (19.6) | 15.4 (21.8) | 11.7 (18.6) |
| Family history of melanoma (%) | 3 | 3 | 2 | 3 | 3 | 2 |
| Number of sun burns: 6 + (%) | 34 | 37 | 35 | 50 | 54 | 48 |
| Number of moles on the left arm: 6 + (%) | 5 | 5 | 5 | 5 | 5 | 4 |
| Tanning ability (%) | 30 | 29 | 34 | 21 | 25 | 27 |
| Red or blonde hair (%) | 13 | 14 | 16 | 15 | 16 | 16 |
| College/high school sun exposure: 11 + h (%) | 51 | 48 | 51 | 13 | 14 | 16 |
| Age = 25–35 years, sun exposure: 11 + h (%) | 32 | 32 | 32 | 13 | 13 | 14 |
| Age = 36–59 years, sun exposure: 11 + h (%) | 27 | 28 | 30 | 9 | 10 | 10 |
| Age = 60 + years, sun exposure: 11 + h (%) | 25 | 28 | 28 | 8 | 8 | 8 |
| At birth, UV index: ≥7 (%) | 26 | 26 | 27 | 12 | 9 | 9 |
| Age = 15 years, UV index: ≥7 (%) | 27 | 27 | 29 | 12 | 9 | 9 |
| Age = 30 years, UV index: ≥7 (%) | 31 | 31 | 33 | 16 | 14 | 13 |
Values are means (standard deviation) or percentages and are standardized to the age distribution of the study population.
aValue is not age adjusted.
bMetabolic equivalents from recreational and leisure time activities.
During the follow-up period, 1067 participants developed melanoma in the two cohorts. The association between smoking variables and melanoma risk is shown in Table 2. Among men, compared with never smokers, past smokers had an RR of 0.78 (95% CI: 0.63–0.95), and current smokers had an RR of 0.53 (95% CI: 0.32–0.89). However, such inverse associations were not found in women. In men, there was a monotonic relationship between longer duration of smoking and lower melanoma risk (Ptrend = 0.003). Significant inverse associations with melanoma risk were also detected for daily smoking quantity in current smokers (Ptrend = 0.006) and pack years of smoking (Ptrend = 0.03). Compared with never smokers, those who smoked for >30 years had an RR of 0.65 (95% CI: 0.48–0.89), current smokers who smoked >15 cigarettes per day had an RR of 0.32 (95% CI: 0.13–0.78) and those who smoked for > 45 pack years had an RR of 0.66 (95% CI: 0.45–0.97) (Table 2).
Table 2.
The association between smoking and melanoma in HPFS and NHS
| Men (HPFS) |
Women (NHS) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Smoking variables | Cases | Person years | Age-adjusted RR | Multivariable-adjusted RR | Cases | Person years | Age-adjusted RR | MV-adjusted RR |
| Smoking status | ||||||||
| Never | 209 | 304 860 | 1.00 | 1.00 | 305 | 1 308 273 | 1.00 | 1.00 |
| Past smoker | 182 | 311 523 | 0.78 (0.64–0.96) | 0.78 (0.63–0.95) | 246 | 1 017 325 | 1.00 (0.84–1.18) | 0.96 (0.81–1.14) |
| Current smoker | 16 | 46 866 | 0.53 (0.32–0.88) | 0.53 (0.32–0.89) | 109 | 5 51 922 | 0.93 (0.74–1.16) | 0.97 (0.77–1.21) |
| Ever smoker | 198 | 358 389 | 0.72 (0.58–0.86) | 0.72 (0.58–0.86) | 355 | 1 569 247 | 0.97 (0.84–1.10) | 0.96 (0.83–1.10) |
| Duration of smoking | ||||||||
| Never | 209 | 304 860 | 1.00 | 1.00 | 305 | 1 308 273 | 1.00 | 1.00 |
| <20 years | 99 | 168 191 | 0.84 (0.66–1.06) | 0.84 (0.66–1.07) | 152 | 636 589 | 1.06 (0.87–1.29) | 1.01 (0.83–1.23) |
| 20–29 years | 39 | 72 825 | 0.75 (0.54–1.06) | 0.73 (0.51–1.03) | 75 | 395 583 | 0.85 (0.66–1.10) | 0.86 (0.67–1.12) |
| 30+ years | 54 | 100 762 | 0.68 (0.50–0.92) | 0.65 (0.48–0.89) | 128 | 536 775 | 0.96 (0.78–1.19) | 0.97 (0.78–1.20) |
| P for trend | 0.006 | 0.003 | 0.41 | 0.50 | ||||
| Daily smoking in current smokers | ||||||||
| Never | 209 | 304 860 | 1.00 | 1.00 | 305 | 1 308 273 | 1.00 | 1.00 |
| 1–14 daily | 7 | 14 979 | 0.71 (0.34–1.52) | 0.77 (0.36–1.63) | 38 | 177 101 | 0.97 (0.70–1.37) | 1.00 (0.71–1.41) |
| 15+ daily | 5 | 24 259 | 0.33 (0.13–0.80) | 0.32 (0.13–0.78) | 69 | 368 135 | 0.90 (0.69–1.17) | 0.94 (0.72–1.23) |
| P for trend | 0.007 | 0.006 | 0.31 | 0.40 | ||||
| Pack years for smokers | ||||||||
| Never | 209 | 304 860 | 1.00 | 1.00 | 305 | 1 308 273 | 1.00 | 1.00 |
| <10 years | 32 | 66 264 | 0.71 (0.49–1.04) | 0.72 (0.49–1.05) | 114 | 494 549 | 1.01 (0.81–1.25) | 0.96 (0.77–1.19) |
| 10–24 years | 72 | 126 511 | 0.80 (0.61–1.05) | 0.81 (0.61–1.05) | 108 | 463 181 | 1.02 (0.82–1.28) | 1.03 (0.82–1.29) |
| 25–44 years | 55 | 92 689 | 0.79 (0.59–1.07) | 0.79 (0.58–1.07) | 85 | 395 076 | 0.93 (0.73–1.18) | 0.93 (0.73–1.19) |
| >45 years | 31 | 52 910 | 0.72 (0.49–1.05) | 0.66 (0.45–0.97) | 48 | 216 440 | 0.89 (0.65–1.21) | 0.89 (0.66–1.22) |
| P for trend | 0.08 | 0.03 | 0.59 | 0.68 | ||||
| Years since quitting in past smokers | ||||||||
| Never | 209 | 304 860 | 1.00 | 1.00 | 305 | 1 308 273 | 1.00 | 1.00 |
| 10+ years | 153 | 249 050 | 0.80 (0.65–0.99) | 0.79 (0.64–0.98) | 190 | 726 339 | 1.05 (0.88–1.26) | 1.00 (0.83–1.20) |
| <10 years | 28 | 60 311 | 0.71 (0.48–1.06) | 0.72 (0.48–1.07) | 56 | 290 987 | 0.85 (0.64–1.14) | 0.85 (0.63–1.13) |
MV-adjusted RR: multivariable adjusted for body mass index; physical activity (quintiles); history of cardiovascular diseases, such as type 2 diabetes, hypertension, hypercholesterolaemia and non-skin cancer; childhood reaction to sun; severe sunburns; moles; hair colour; family history of melanoma; sun exposures at different age intervals and UV index at birth, age 15 and age 30 years.
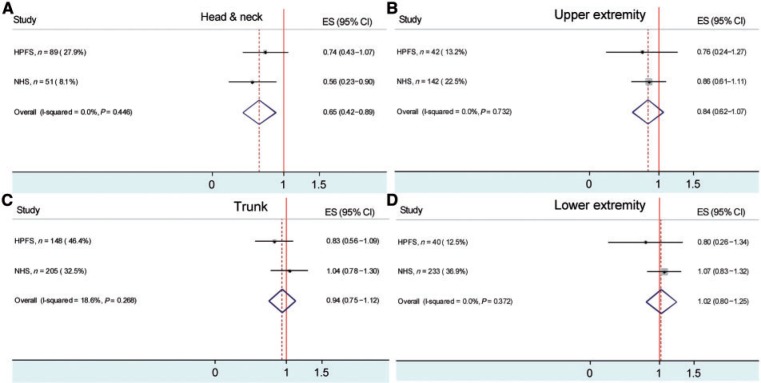
In the body site analysis, a significant association was found only between smoking and melanoma of the head and neck. When male and female participants were combined, compared with never smokers, ever smokers had a lower risk of melanoma (RR = 0.65; 95% CI: 0.42–0.89) (Figure 1A). There was a suggestive inverse association for melanoma in the upper extremity (RR = 0.84; 95% CI: 0.62–1.07) (Figure 1B). No association was found for melanoma on the trunk (RR = 0.94; 95% CI: 0.75–1.12) (Figure 1C) or the lower extremity (RR = 1.02; 95% CI: 0.80–1.25) (Figure 1D). We found no significant effect of the interactions between the susceptibility score and smoking on melanoma risk (data not shown).
Figure 1.
The association between smoking (ever smokers vs never smokers) and melanoma in different body sites (A, Head and neck; B, Upper extremity; C, Trunk; D, Lower extremity) in HPFS and NHS
A total of 28 799 participants developed BCC in the two cohorts. The association between smoking variables and BCC risk is shown in Table 3. In men, compared with never smokers, ever smokers had a lower risk of BCC (RR = 0.93; 95% CI: 0.89–0.97). Dose–response relationships were found for duration of smoking (Ptrend < 0.0001), daily smoking (Ptrend = 0.0003) and pack years of smoking (Ptrend < 0.0001). In women, compared with never smokers, ever smokers had a slightly elevated risk of BCC (RR = 1.06; 95% CI: 1.03–1.08), but dose–response relationships were not significant.
Table 3.
The association between smoking and BCC in HPFS and NHS
| Men (HPFS) |
Women (NHS) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Smoking variables | Cases | Person years | Age-adjusted RR | Multivariable-adjusted RR | Cases | Person years | Age-adjusted RR | MV-adjusted RR | |
| Smoking status | |||||||||
| Never | 3712 | 300 185 | 1.00 | 1.00 | 9015 | 1 303 161 | 1.00 | 1.00 | |
| Past smoker | 4047 | 305 110 | 0.97 (0.93–1.02) | 0.97 (0.93–1.02) | 8871 | 1 012 350 | 1.15 (1.11–1.18) | 1.08 (1.05–1.11) | |
| Current smoker | 430 | 46 051 | 0.80 (0.73–0.89) | 0.85 (0.77–0.94) | 2724 | 550 275 | 0.99 (0.95–1.03) | 1.00 (0.95–1.04) | |
| Ever smoker | 4477 | 351 161 | 0.93 (0.89–0.97) | 0.94 (0.90–0.98) | 11 595 | 1 562 625 | 1.08 (1.05–1.11) | 1.06 (1.03–1.08) | |
| Duration of smoking | |||||||||
| Never | 3712 | 300 185 | 1.00 | 1.00 | 9015 | 1 303 161 | 1.00 | 1.00 | |
| <20 years | 2153 | 165 274 | 1.00 (0.95–1.06) | 1.01 (0.96–1.06) | 4494 | 634 042 | 1.21 (1.16–1.25) | 1.12 (1.08–1.16) | |
| 20–29 years | 862 | 71 454 | 0.96 (0.89–1.03) | 0.96 (0.89–1.03) | 2477 | 394 470 | 1.13 (1.08–1.18) | 1.09 (1.05–1.14) | |
| 30+ years | 1241 | 98 182 | 0.87 (0.81–0.93) | 0.87 (0.82–0.93) | 4624 | 534 113 | 1.01 (0.98–1.05) | 0.99 (0.96–1.03) | |
| P for trend | <0.0001 | <0.0001 | 0.20 | 0.86 | |||||
| Daily smoking in current smokers | |||||||||
| Never | 3712 | 300 185 | 1.00 | 1.00 | 9015 | 1 303 161 | 1.00 | 1.00 | |
| 1–14 daily | 156 | 14 700 | 0.87 (0.74–1.02) | 0.91 (0.78–1.07) | 934 | 179 392 | 0.92 (0.86–0.99) | 0.92 (0.86–0.99) | |
| 15+ daily | 202 | 23 819 | 0.74 (0.64–0.85) | 0.77 (0.67–0.89) | 1733 | 369 976 | 1.02 (0.97–1.07) | 1.03 (0.97–1.08) | |
| P for trend | <0.0001 | 0.0003 | 0.67 | 0.75 | |||||
| Pack years for smokers | |||||||||
| Never | 3712 | 300 185 | 1.00 | 1.00 | 9015 | 1 303 161 | 1.00 | 1.00 | |
| <10 years | 816 | 65 205 | 1.01 (0.94–1.09) | 1.00 (0.93–1.08) | 3701 | 492 422 | 1.19 (1.15–1.24) | 1.12 (1.07–1.16) | |
| 10–24 years | 1583 | 124 346 | 0.98 (0.93–1.04) | 1.00 (0.94–1.06) | 3188 | 461 339 | 1.12 (1.08–1.17) | 1.08 (1.04–1.13) | |
| 25–44 years | 1172 | 90 712 | 0.94 (0.88–1.01) | 0.95 (0.89–1.02) | 2879 | 393 469 | 1.07 (1.03–1.12) | 1.04 (1.00–1.09) | |
| >45 years | 644 | 51 313 | 0.84 (0.77–0.92) | 0.82 (0.76–0.90) | 1827 | 215 394 | 0.98 (0.94–1.04) | 0.95 (0.90–1.00) | |
| P for trend | <0.0001 | <0.0001 | 0.30 | 0.03 | |||||
| Years since quitting in past smokers | |||||||||
| Never | 3172 | 300 185 | 1.00 | 1.00 | 9015 | 1 303 161 | 1.00 | 1.00 | |
| 10+ years | 3363 | 243 796 | 0.98 (0.93–1.02) | 0.97 (0.92–1.02) | 7040 | 722 424 | 1.20 (1.16–1.24) | 1.12 (1.09–1.16) | |
| <10 years | 648 | 59 200 | 0.95 (0.87–1.03) | 0.98 (0.90–1.07) | 1831 | 289 926 | 0.99 (0.94–1.04) | 0.95 (0.91–1.00) | |
MV-adjusted RR: multivariable-adjusted for body mass index; physical activity (quintiles); history of cardiovascular diseases, such as type 2 diabetes, hypertension, hypercholesterolaemia and non-skin cancer; childhood reaction to sun; severe sunburns; moles; hair colour; family history of melanoma; sun exposures at different age intervals and UV index at birth, age 15 and age 30 years.
A total of 2332 participants developed SCC in the two cohorts. The association between smoking variables and SCC risk is shown in Table 4. In men, current smokers had a significantly increased risk of SCC (RR = 1.31; 95% CI: 1.01–1.70) compared with never smokers, and dose–response relationships were not significant. In women, current smokers had a significantly increased risk of SCC (RR = 1.38; 95% CI: 1.16–1.64) compared with never smokers. Compared with never smokers, individuals who smoked for ≥30 years had an increased risk of SCC (RR = 1.24; 95% CI: 1.08–1.42) (Ptrend = 0.005), and current smokers who smoked ≥15 cigarettes per day also had an increased risk (RR = 1.54; 95% CI: 1.25–1.89) (Ptrend = 0.0003). The dose–response relationship for pack years of smoking was also significant (Ptrend = 0.02).
Table 4.
The association between smoking and SCC in HPFS and NHS
| Men (HPFS) |
Women (NHS) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Smoking variables | Cases | Person years | Age-adjusted RR | Multivariable-adjusted RR | Cases | Person years | Age-adjusted RR | MV-adjusted RR |
| Smoking status | ||||||||
| Never | 440 | 303 318 | 1.00 | 1.00 | 561 | 1 308 051 | 1.00 | 1.00 |
| Past smoker | 484 | 308 595 | 0.92 (0.81–1.05) | 0.93 (0.82–1.06) | 596 | 1 017 034 | 1.16 (1.03–1.30) | 1.11 (0.98–1.24) |
| Current smoker | 67 | 46 401 | 1.15 (0.89–1.49) | 1.31 (1.01–1.70) | 182 | 551 844 | 1.34 (1.13–1.58) | 1.38 (1.16– 1.64) |
| Ever smoker | 551 | 354 996 | 0.95 (0.84–1.06) | 0.99 (0.89–1.12) | 778 | 1 568 878 | 1.21 (1.09–1.32) | 1.19 (1.08–1.31) |
| Duration of smoking | ||||||||
| Never | 440 | 303 318 | 1.00 | 1.00 | 561 | 1 308 051 | 1.00 | 1.00 |
| <20 years | 279 | 167 100 | 1.06 (0.91–1.23) | 1.10 (0.94–1.28) | 285 | 636 498 | 1.22 (1.06–1.41) | 1.12 (0.97–1.30) |
| 20–29 years | 82 | 72 230 | 0.75 (0.60–0.96) | 0.74 (0.58–0.94) | 137 | 395 828 | 1.07 (0.89–.29) | 1.05 (0.87–1.27) |
| 30+ years | 167 | 99 217 | 0.91 (0.76–1.09) | 0.92 (0.77–1.11) | 356 | 536 552 | 1.23 (1.07–1.40) | 1.24 (1.08–1.42) |
| P for trend | 0.06 | 0.08 | 0.008 | 0.005 | ||||
| Daily smoking in current smokers | ||||||||
| Never | 440 | 303 318 | 1.00 | 1.00 | 561 | 1 308 051 | 1.00 | 1.00 |
| 1–14 daily | 31 | 14 823 | 1.52 (1.06–2.20) | 1.68 (1.16–2.43) | 69 | 177 072 | 1.24 (0.96–1.59) | 1.21 (0.94–1.56) |
| 15+ daily | 28 | 23 991 | 0.97 (0.66–1.43) | 1.03 (0.70–1.52) | 112 | 368 086 | 1.45 (1.18–1.78) | 1.54 (1.25–1.89) |
| P for trend | 0.46 | 0.42 | 0.0004 | 0.0003 | ||||
| Pack years for smokers | ||||||||
| Never | 440 | 303 318 | 1.00 | 1.00 | 561 | 1 308 051 | 1.00 | 1.00 |
| <10 years | 93 | 65 892 | 0.97 (0.78–1.21) | 0.99 (0.79–1.25) | 237 | 494 457 | 1.22 (1.04–1.42) | 1.12 (0.96–1.31) |
| 10–24 years | 208 | 125 684 | 1.05 (0.89–1.24) | 1.09 (0.92–1.28) | 200 | 463 112 | 1.15 (0.98–1.35) | 1.10 (0.93–1.29) |
| 25–44 years | 131 | 91 742 | 0.84 (0.69–1.02) | 0.84 (0.69–1.02) | 199 | 394 961 | 1.24 (1.05–1.45) | 1.24 (1.06–1.46) |
| >45 years | 94 | 51 848 | 0.95 (0.76–1.19) | 0.94 (0.75–1.18) | 142 | 216 347 | 1.17 (0.97–1.41) | 1.19 (0.99–1.44) |
| P for trend | 0.34 | 0.28 | 0.07 | 0.02 | ||||
| Years since quitting in past smokers | ||||||||
| Never | 440 | 303 318 | 1.00 | 1.00 | 561 | 1 308 051 | 1.00 | 1.00 |
| 10+ years | 416 | 246 695 | 0.93 (0.81–1.07) | 0.93 (0.81–1.07) | 486 | 726 092 | 1.19 (1.05–1.34) | 1.12 (0.99–1.27) |
| <10 years | 64 | 59 760 | 0.85 (0.66–1.11) | 0.90 (0.69–1.18) | 110 | 290 942 | 1.04 (0.85–1.28) | 1.04 (0.85–1.28) |
MV-adjusted RR: multivariable adjusted for body mass index; physical activity (quintiles); history of cardiovascular diseases, such as type 2 diabetes, hypertension, hypercholesterolaemia and non-skin cancer; childhood reaction to sun; severe sunburns; moles; hair colour; family history of melanoma; sun exposures at different age intervals; UV index at birth, age 15 and age 30 years.
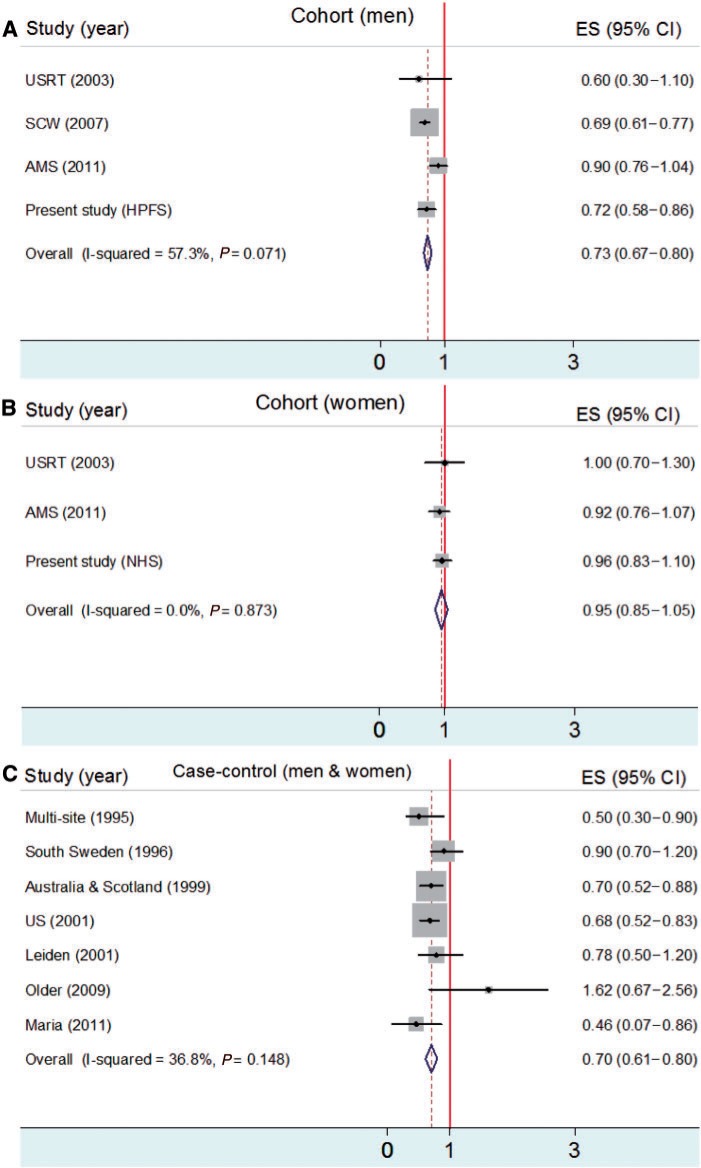
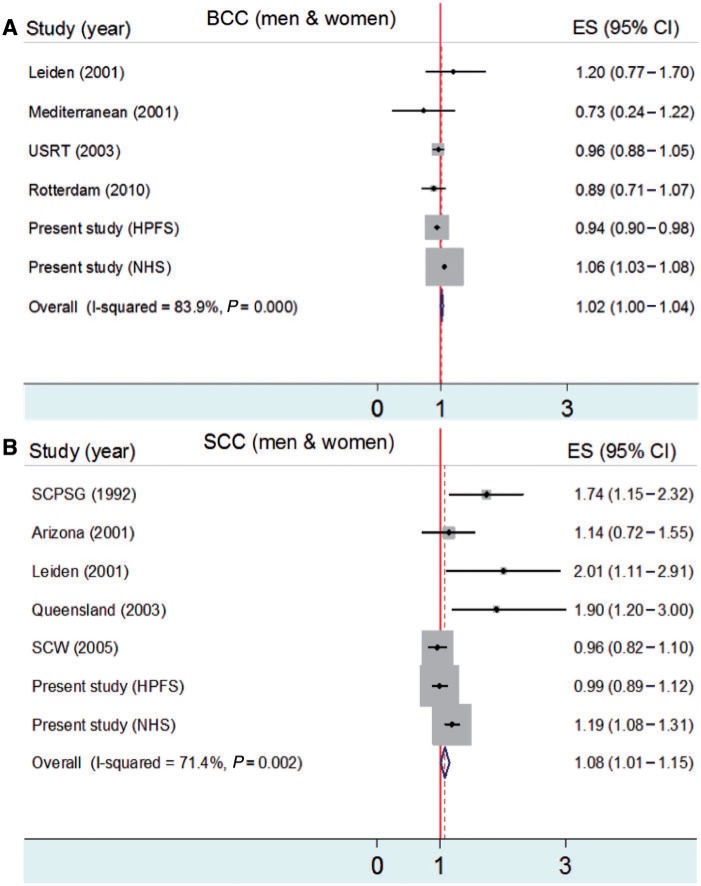
We performed meta-analyses to evaluate the associations between smoking and risks of melanoma, BCC and SCC. The association between smoking and melanoma risk is shown in Figure 2. In male cohort studies, ever smokers had a lower risk of melanoma than never smokers (RR = 0.73; 95% CI: 0.67–0.80) (Figure 2A). In female cohort studies, smoking was not significantly associated with melanoma risk (RR = 0.95; 95% CI: 0.85–1.05) (Figure 2B). In case–control studies, ever smokers had a lower risk of melanoma than never smokers [odds ratio (OR) = 0.70; 95% CI: 0.61–0.80] (Figure 2C). The associations between smoking and risks of BCC and SCC are shown in Figure 3. Ever smokers had slightly increased risks of BCC (OR = 1.02; 95% CI: 1.00–1.04) (Figure 3A) and SCC (OR = 1.08; 95% CI: 1.01–1.15) (Figure 3B) compared with never smokers.
Figure 2.
Meta-analysis for smoking (ever smokers vs never smokers) and melanoma risk in gender-specific cohort studies (A and B) compared with controls (C; men and women)
Figure 3.
Meta-analysis for smoking (ever smokers vs never smokers) and BCC (A) and SCC (B) risks
Discussion
In this study, an inverse association was found between smoking and the risk of melanoma, especially for melanoma of the head and neck. We detected trends between lower melanoma risk and longer duration of smoking, intensity of smoking in current smokers and pack years of smoking.
Previous studies with various designs suggested the possibility that smoking is inversely associated with melanoma risk, although most results from case–control studies were not statistically significant.6,14 Our results were consistent with the large cohort study of Swedish male construction workers, which reported a significant RR of 0.75 (95% CI: 0.63–0.91) for past smokers and 0.66 (95% CI: 0.58–0.77) for current smokers, compared with never smokers (dose–response relation for duration of smoking, Ptrend < 0.001).7 In another occupational cohort, Freedman et al.18 also found a non-significant risk reduction for melanoma among men who had ever smoked (RR = 0.6; 95% CI: 0.3–1.1), but not among women (RR = 1.0; 95% CI: 0.7–1.3).18 With regards to the association between smoking and BCC and SCC risks, our results were largely consistent with results from previous studies. Results from meta-analysis on the associations between smoking and risk of melanoma, BCC and SCC were similar to the results from our cohort study.
There are both causal and non-causal explanations for the relationship between smoking and melanoma risk. Results from our analysis of body sites tend to support the hypothesis that smoking protects melanocytes from the inflammatory reaction induced by long-term UV radiation7,32 because the protective effect was stronger at body sites often exposed to the sun. This effect of smoking may be partially caused by the long-term effect of the nicotine that accumulates in human tissues containing melanin.33 It has been reported that nicotine administration via a transdermal delivery system suppresses the cutaneous inflammatory response to sodium lauryl sulphate and UV-B.34,35 The anti-inflammatory effect of nicotine was also suggested to help prevent skin conditions that occur less often in smokers than non-smokers.36 Another possible mechanism is that smoking down-regulates gene expression of the Notch pathway, which has been reported to enhance the growth of melanoma cells, in contrast to BCC and SCC, whose growth is inhibited by Notch signalling.37–40 This is consistent with our findings of an inverse association between smoking and melanoma risk and the positive association between smoking and BCC and SCC risks. Moreover, tobacco smoke was found to impair the production of collagen and increase the production of tropoelastin and matrix metalloproteinases, which degrade matrix proteins and also cause an abnormal production of elastosis material.41 Solar elastosis has been found to be protective against melanoma.42,43
Non-causal explanations include inadequate adjustment for confounding, such as sunlight exposure, socio-economic status or other unknown factors. However, more complete adjustment for potential confounding factors actually strengthened the inverse association between cigarette smoking and malignant melanoma rather than diminishing it.14 The magnitude of RR changed little after adjustment for confounding in our study. Because of the prospective design, our results were unlikely to be substantially influenced by recall or selection bias. Participants in our study were all health-care professionals; the accuracy of the reports on diseases and factors is likely to be high. The variation of socio-economic status among our participants tends to be small, and its confounding effect should be limited. In addition, the homogeneity of the occupation cohort may decrease the misclassification of work-related sun exposure. Detection bias because of smoking status, if any, would be of similar direction and magnitude for the three types of skin cancers, and could not explain what we observed in this study.
Despite the well-known adverse effects of cigarette smoking on health, it is indeed beneficial to certain conditions. Most notable is its protective effect against Parkinson’s disease (PD),44,45 which has been found to be more significant in men than in women. The protective effect is attributable to nicotine’s ability to reduce the brain damage that triggers PD,46 e.g. improving compromised semantic processing possibly via enhanced expectancy or inhibitory mechanisms. Dopamine depletion in the substantia nigra, an area of the brain heavily populated by melanocytes, is associated with PD, whereas melanoma arises from melanocytes in the epidermis of the skin. Consistent with co-occurrence between melanoma and PD, reports from various studies suggested a 2- to 6-fold higher RR of melanoma in patients with PD than would be expected.47–49 There have been many speculations of an aetiological relationship between melanoma and PD, including changes in melanin synthesis, changes in PD-related genes or some low-penetrance genes and impaired autophagy in both diseases.50,51 Interestingly, rotating night shifts for a long period are associated with decreased risk of both PD and melanoma52,53 but increased risk of cancer at other sites. Similar patterns of association have also been found for short telomere length in peripheral blood lymphocytes.54,55 Based on these findings, it appears plausible that PD and melanoma share some common genetic or environmental risk factors.
The combined NHS and HPFS cohorts have enabled us to evaluate melanoma, BCC and SCC in both genders. Smoking is protective against melanoma in men but not in women. There are several possible explanations for this. Men and women generally differ in the type of cigarettes they smoke and their style of inhaling. They also differ in the body distribution and histopathology of melanoma. Because of up-regulation of Cytochrome P450 proteins (CYP) by oestrogen, women tend to have more active CYP enzyme activity, metabolizing more nicotine56 and reducing the anti-inflammatory effect of nicotine for melanoma. In our study, when melanoma cases were stratified according to body sites, no material differences in risk estimates were found between men and women at each body site. A significant inverse association between smoking and melanoma was found only on the head and neck, sites chronically exposed to the sun (RR = 0.65; 95% CI: 0.42–0.89), but not at other body sites. This finding may generally explain the gender difference because men were more likely to have melanoma on the head and neck than women (27.9% and 8.1%, respectively). This analysis was exploratory and needs further replication. In addition, it should be noted that in our study, smoking increased the risk of BCC and SCC in women, reflecting the difference in aetiology between melanoma and non-melanoma skin cancers. Melanoma originates from pigment-producing melanocytes in the basal layer of the epidermis. Non-melanoma skin cancers arise via the transformation of keratinocytes from different layers of the skin (SCC from the top layer and BCC from the basal layer).39 Clear differences in pathogenesis exist between melanoma and non-melanoma skin cancer, and smoking may have different effects in the development of different types of skin cancer.
Among men, the incidence of melanoma is lower by 13.4/100 000, the incidence of BCC is higher by 38.3/100 000 and the incidence of SCC is higher by 10.1/100 000 in ever smokers than in never smokers. Among women, the incidence of melanoma is lower by 0.7/100 000, the incidence of BCC is higher by 50.2/100 000 and the incidence of SCC is higher by 6.5/100 000 in ever smokers than in never smokers. In conclusion, the results from our cohort analysis, in agreement with the others, strongly suggest an inverse association between smoking and the risk of melanoma in men. Experimental studies are warranted to investigate the potential biological mechanisms and identify the putative protective compound(s) for chemoprevention of melanoma, a lethal cancer.
Funding
This work was supported by Departmental Funding and NIH CA87969 and CA055075.
Acknowledgements
The authors are indebted to the participants in the NHS and HPFS for their dedication to this study. They thank the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington and Wyoming.
Conflict of Interest: None declared.
Key Messages.
In the current literature, prospective studies suggest an inverse association between smoking and the risk of melanoma.
In this present prospective study combining two independent cohorts, we found that compared with never smokers, ever smokers had a significantly decreased risk of melanoma in men, but not in women.
The inverse association was only limited to the head and neck, which may explain the gender difference, because men were more likely to have melanoma on head and neck compared to women.
References
- 1.Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541–43. doi: 10.1001/jama.294.12.1541. [DOI] [PubMed] [Google Scholar]
- 2.Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375:673–85. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 3.Bloethner S, Scherer D, Drechsel M, Hemminki K, Kumar R. Malignant melanoma—a genetic overview. Actas Dermosifiliogr. 2009;100(Suppl 1):38–51. [PubMed] [Google Scholar]
- 4.Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978–86. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- 5.Secretan B, Straif K, Baan R, et al. A review of human carcinogens—part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–34. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 6.De Hertog SA, Wensveen CA, Bastiaens MT, et al. Relation between smoking and skin cancer. J Clin Oncol. 2001;19:231–38. doi: 10.1200/JCO.2001.19.1.231. [DOI] [PubMed] [Google Scholar]
- 7.Odenbro A, Gillgren P, Bellocco R, Boffetta P, Hakansson N, Adami J. The risk for cutaneous malignant melanoma, melanoma in situ and intraocular malignant melanoma in relation to tobacco use and body mass index. Br J Dermatol. 2007;156:99–105. doi: 10.1111/j.1365-2133.2006.07537.x. [DOI] [PubMed] [Google Scholar]
- 8.Aubry F, MacGibbon B. Risk factors of squamous cell carcinoma of the skin. A case-control study in the Montreal region. Cancer. 1985;55:907–11. doi: 10.1002/1097-0142(19850215)55:4<907::aid-cncr2820550433>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Boyd AS, Shyr Y, King LE., Jr Basal cell carcinoma in young women: an evaluation of the association of tanning bed use and smoking. J Am Acad Dermatol. 2002;46:706–09. doi: 10.1067/mjd.2002.120467. [DOI] [PubMed] [Google Scholar]
- 10.Corona R, Dogliotti E, D’Errico M, et al. Risk factors for basal cell carcinoma in a Mediterranean population: role of recreational sun exposure early in life. Arch Dermatol. 2001;137:1162–68. doi: 10.1001/archderm.137.9.1162. [DOI] [PubMed] [Google Scholar]
- 11.Freedman DM, Sigurdson A, Doody MM, Mabuchi K, Linet MS. Risk of basal cell carcinoma in relation to alcohol intake and smoking. Cancer Epidemiol Biomarkers Prev. 2003;12:1540–43. [PubMed] [Google Scholar]
- 12.Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med. 1997;90:371–74. doi: 10.1177/014107689709000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milan T, Verkasalo PK, Kaprio J, Koskenvuo M. Lifestyle differences in twin pairs discordant for basal cell carcinoma of the skin. Br J Dermatol. 2003;149:115–23. doi: 10.1046/j.1365-2133.2003.05352.x. [DOI] [PubMed] [Google Scholar]
- 14.Kessides MC, Wheless L, Hoffman-Bolton J, Clipp S, Alani RM, Alberg AJ. Cigarette smoking and malignant melanoma: a case-control study. J Am Acad Dermatol. 2011;64:84–90. doi: 10.1016/j.jaad.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 16.Hunter DJ, Colditz GA, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Risk factors for basal cell carcinoma in a prospective cohort of women. Ann Epidemiol. 1990;1:13–23. doi: 10.1016/1047-2797(90)90015-k. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen OS. Stratification by a multivariate confounder score. Am J Epidemiol. 1976;104:609–20. doi: 10.1093/oxfordjournals.aje.a112339. [DOI] [PubMed] [Google Scholar]
- 18.Freedman DM, Sigurdson A, Doody MM, Rao RS, Linet MS. Risk of melanoma in relation to smoking, alcohol intake, and other factors in a large occupational cohort. Cancer Causes Control. 2003;14:847–57. doi: 10.1023/b:caco.0000003839.56954.73. [DOI] [PubMed] [Google Scholar]
- 19.Delancey JO, Hannan LM, Gapstur SM, Thun MJ. Cigarette smoking and the risk of incident and fatal melanoma in a large prospective cohort study. Cancer Causes Control. 2011;22:937–42. doi: 10.1007/s10552-011-9766-z. [DOI] [PubMed] [Google Scholar]
- 20.Veierod MB, Thelle DS, Laake P. Diet and risk of cutaneous malignant melanoma: a prospective study of 50,757 Norwegian men and women. Int J Cancer. 1997;71:600–04. doi: 10.1002/(sici)1097-0215(19970516)71:4<600::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 21.Siemiatycki J, Krewski D, Franco E, Kaiserman M. Associations between cigarette smoking and each of 21 types of cancer: a multi-site case-control study. Int J Epidemiol. 1995;24:504–14. doi: 10.1093/ije/24.3.504. [DOI] [PubMed] [Google Scholar]
- 22.Green A, McCredie M, MacKie R, et al. A case-control study of melanomas of the soles and palms (Australia and Scotland) Cancer Causes Control. 1999;10:21–25. doi: 10.1023/a:1008872014889. [DOI] [PubMed] [Google Scholar]
- 23.Westerdahl J, Olsson H, Masback A, Ingvar C, Jonsson N. Risk of malignant melanoma in relation to drug intake, alcohol, smoking and hormonal factors. Br J Cancer. 1996;73:1126–31. doi: 10.1038/bjc.1996.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shors AR, Solomon C, McTiernan A, White E. Melanoma risk in relation to height, weight, and exercise (United States) Cancer Causes Control. 2001;12:599–606. doi: 10.1023/a:1011211615524. [DOI] [PubMed] [Google Scholar]
- 25.Nagore E, Hueso L, Botella-Estrada R, et al. Smoking, sun exposure, number of nevi and previous neoplasias are risk factors for melanoma in older patients (60 years and over) J Eur Acad Dermatol Venereol. 2010;24:50–57. doi: 10.1111/j.1468-3083.2009.03353.x. [DOI] [PubMed] [Google Scholar]
- 26.Kiiski V, de Vries E, Flohil SC, et al. Risk factors for single and multiple basal cell carcinomas. Arch Dermatol. 2010;146:848–55. doi: 10.1001/archdermatol.2010.155. [DOI] [PubMed] [Google Scholar]
- 27.Grodstein F, Speizer FE, Hunter DJ. A prospective study of incident squamous cell carcinoma of the skin in the nurses' health study. J Natl Cancer Inst. 1995;87:1061–66. doi: 10.1093/jnci/87.14.1061. [DOI] [PubMed] [Google Scholar]
- 28.Karagas MR, Stukel TA, Greenberg ER, Baron JA, Mott LA, Stern RS. Risk of subsequent basal cell carcinoma and squamous cell carcinoma of the skin among patients with prior skin cancer. Skin Cancer Prevention Study Group. JAMA. 1992;267:3305–10. [PubMed] [Google Scholar]
- 29.Foote JA, Harris RB, Giuliano AR, et al. Predictors for cutaneous basal- and squamous-cell carcinoma among actinically damaged adults. Int J Cancer. 2001;95:7–11. doi: 10.1002/1097-0215(20010120)95:1<7::aid-ijc1001>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsay HM, Fryer AA, Hawley CM, Smith AG, Nicol DL, Harden PN. Factors associated with nonmelanoma skin cancer following renal transplantation in Queensland, Australia. J Am Acad Dermatol. 2003;49:397–406. doi: 10.1067/s0190-9622(03)00902-2. [DOI] [PubMed] [Google Scholar]
- 31.Odenbro A, Bellocco R, Boffetta P, Lindelof B, Adami J. Tobacco smoking, snuff dipping and the risk of cutaneous squamous cell carcinoma: a nationwide cohort study in Sweden. Br J Cancer. 2005;92:1326–28. doi: 10.1038/sj.bjc.6602475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–77. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 33.Yerger VB, Malone RE. Melanin and nicotine: a review of the literature. Nicotine Tob Res. 2006;8:487–98. doi: 10.1080/14622200600790039. [DOI] [PubMed] [Google Scholar]
- 34.Mills CM, Hill SA, Marks R. Transdermal nicotine suppresses cutaneous inflammation. Arch Dermatol. 1997;133:823–25. [PubMed] [Google Scholar]
- 35.Mills CM, Hill SA, Marks R. Altered inflammatory responses in smokers. BMJ. 1993;307:911. doi: 10.1136/bmj.307.6909.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ingram JR. Nicotine: does it have a role in the treatment of skin disease? Postgrad Med J. 2009;85:196–201. doi: 10.1136/pgmj.2008.073577. [DOI] [PubMed] [Google Scholar]
- 37.Balint K, Xiao M, Pinnix CC, et al. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest. 2005;115:3166–76. doi: 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–21. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama K. Growth and progression of melanoma and non-melanoma skin cancers regulated by ubiquitination. Pigment Cell Melanoma Res. 2010;23:338–51. doi: 10.1111/j.1755-148X.2010.00692.x. [DOI] [PubMed] [Google Scholar]
- 40.Panelos J, Massi D. Emerging role of Notch signaling in epidermal differentiation and skin cancer. Cancer Biol Ther. 2009;8:1986–93. doi: 10.4161/cbt.8.21.9921. [DOI] [PubMed] [Google Scholar]
- 41.Morita A. Tobacco smoke causes premature skin aging. J Dermatol Sci. 2007;48:169–75. doi: 10.1016/j.jdermsci.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 42.Vollmer RT. Solar elastosis in cutaneous melanoma. Am J Clin Pathol. 2007;128:260–64. doi: 10.1309/7MHX96XH3DTY32TQ. [DOI] [PubMed] [Google Scholar]
- 43.Grant WB. Skin aging from ultraviolet irradiance and smoking reduces risk of melanoma: epidemiological evidence. Anticancer Res. 2008;28:4003–08. [PubMed] [Google Scholar]
- 44.Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol. 2002;52:276–84. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 45.Tanner CM, Goldman SM, Aston DA, et al. Smoking and Parkinson’s disease in twins. Neurology. 2002;58:581–88. doi: 10.1212/wnl.58.4.581. [DOI] [PubMed] [Google Scholar]
- 46.O’Reilly EJ, Chen H, Gardener H, Gao X, Schwarzschild MA, Ascherio A. Smoking and Parkinson’s disease: using parental smoking as a proxy to explore causality. Am J Epidemiol. 2009;169:678–82. doi: 10.1093/aje/kwn388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertoni JM, Arlette JP, Fernandez HH, et al. Increased melanoma risk in Parkinson disease: a prospective clinicopathological study. Arch Neurol. 2010;67:347–52. doi: 10.1001/archneurol.2010.1. [DOI] [PubMed] [Google Scholar]
- 48.Bajaj A, Driver JA, Schernhammer ES. Parkinson’s disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control. 2010;21:697–707. doi: 10.1007/s10552-009-9497-6. [DOI] [PubMed] [Google Scholar]
- 49.Driver JA, Logroscino G, Buring JE, Gaziano JM, Kurth T. A prospective cohort study of cancer incidence following the diagnosis of Parkinson’s disease. Cancer Epidemiol Biomarkers Prev. 2007;16:1260–65. doi: 10.1158/1055-9965.EPI-07-0038. [DOI] [PubMed] [Google Scholar]
- 50.Pan T, Li X, Jankovic J. The association between Parkinson’s disease and melanoma. Int J Cancer. 2011;128:2251–60. doi: 10.1002/ijc.25912. [DOI] [PubMed] [Google Scholar]
- 51.Paisan-Ruiz C, Houlden H. Common pathogenic pathways in melanoma and Parkinson disease. Neurology. 2010;75:1653–55. doi: 10.1212/WNL.0b013e3181fb4466. [DOI] [PubMed] [Google Scholar]
- 52.Chen H, Schernhammer E, Schwarzschild MA, Ascherio A. A prospective study of night shift work, sleep duration, and risk of Parkinson’s disease. Am J Epidemiol. 2006;163:726–30. doi: 10.1093/aje/kwj096. [DOI] [PubMed] [Google Scholar]
- 53.Schernhammer ES, Razavi P, Li TY, Qureshi AA, Han J. Rotating night shifts and risk of skin cancer in the nurses’ health study. J Natl Cancer Inst. 2011;103:602–06. doi: 10.1093/jnci/djr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, Chen H, Gao X, et al. Telomere length and risk of Parkinson’s disease. Mov Disord. 2008;23:302–05. doi: 10.1002/mds.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han J, Qureshi AA, Prescott J, et al. A prospective study of telomere length and the risk of skin cancer. J Invest Dermatol. 2009;129:415–21. doi: 10.1038/jid.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramchandran K, Patel JD. Sex differences in susceptibility to carcinogens. Semin Oncol. 2009;36:516–23. doi: 10.1053/j.seminoncol.2009.09.005. [DOI] [PubMed] [Google Scholar]