Abstract
Background Previous studies suggest an association between obesity and oesophageal (OA) and oesophagogastric junction adenocarcinomas (OGJA). However, these studies have been limited in their ability to assess whether the effects of obesity vary by gender or by the presence of gastro-oesophageal reflux (GERD) symptoms.
Methods Individual participant data from 12 epidemiological studies (8 North American, 3 European and 1 Australian) comprising 1997 OA cases, 1900 OGJA cases and 11 159 control subjects were pooled. Logistic regression was used to estimate study-specific odds ratios (ORs) and 95% confidence intervals (CIs) for the association between body mass index (BMI, kg/m2) and the risk of OA and OGJA. Random-effects meta-analysis was used to combine these ORs. We also investigated effect modification and synergistic interaction of BMI with GERD symptoms and gender.
Results The association of OA and OGJA increased directly with increasing BMI (P for trend <0.001). Compared with individuals with a BMI <25, BMI ≥40 was associated with both OA (OR 4.76, 95% CI 2.96–7.66) and OGJA (OR 3.07, 95% CI 1.89–4.99). These associations were similar when stratified by gender and GERD symptoms. There was evidence for synergistic interaction between BMI and GERD symptoms in relation to OA/OGJA risk.
Conclusions These data indicate that BMI is directly associated with OA and OGJA risk in both men and women and in those with and without GERD symptoms. Disentangling the relationship between BMI and GERD will be important for understanding preventive efforts for OA and OGJA.
Keywords: Oesophageal neoplasms, aetiology, risk factors, gastro-oesophageal reflux, obesity, oesophagogastric junction
Background
The incidence of adenocarcinomas of the oesophagus (OA) has increased >650% in the USA over the past 35 years, the steepest increase of any cancer during this period.1,2 To a lesser extent, the incidence of the anatomically linked oesophagogastric junction adenocarcinoma (OGJA) has also risen.3 Five-year survival for these cancers continues to be low despite improvements in treatment modalities and supportive care.4,5 Over the past 2 decades, research has focused on identifying risk factors for OA and OGJA, with an ultimate goal of elucidating a strategy to reduce incidence and mortality from these cancers. Any potentially effective prevention programme will need to take into consideration that the incidence varies dramatically by race and gender, with White men comprising 80% of individuals with OA and OGJA.6,7
Consistently documented risk factors for these tumours include symptoms of gastro-oesophageal reflux (GERD)8,9 and male gender.6 Increasing body mass index (BMI) has also been associated with OA and OGJA tumours (reviewed in the article by Lagergren10), and perhaps GERD (reviewed in the articles by El-Serag11 and Friedenberg12). Higher BMI (kg/m2) may directly increase the propensity for GERD13,14; android obesity may increase intra-abdominal pressure, distort the lower oesophageal sphincter and increase the likelihood of hiatal hernia. Several recent lines of evidence also suggest that BMI may increase the risk of OA, its precursor lesion Barrett’s oesophagus, and OGJA independently of GERD.13–19 Potential indirect mechanisms of these associations include the idea that differences in adipose distribution between men and women may partly explain the much higher incidence of these cancers in men.20,21 Men typically acquire android fat patterns, characterized by central abdominal fat deposits, whereas women typically develop gynoid forms, with fat deposits on the hips and thighs21,22; android (visceral) fat is known to be more metabolically active20 relative to gynoid.23,24 Disentangling these relationships will be important for primary prevention of these tumours, but so far these efforts have been limited by small case numbers of participants in individual studies. The International Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON, http://beacon.tlvnet.net/) provided data from 12 studies to assess the effect of increasing BMI on OA and OGJA risk. The large size of the consortium also enabled the assessment of potential effect modification by GERD and gender.
Methods
Study population
This combined analysis included participants in OA and OGJA studies who contributed their data to the International BEACON. BEACON was formed in 2005 by an international group of investigators in collaboration with the US National Cancer Institute. The consortium consists of completed or ongoing case–control and cohort studies of OA, OGJA and/or Barrett’s oesophagus and has a primary aim to provide an open scientific forum for epidemiological research into the aetiology and prevention of these diseases by facilitating the sharing of data across population-based studies. Therefore, rather than relying on a meta-analytical approach with published risk estimates, which often have different variable definitions and statistical models, BEACON enables pooled analyses of individual participant data from population-based studies using a standard model, after harmonization of variable definitions and common confounders.
Data from 10 case–control studies, conducted between 1988 and 2005, and two cohort studies, with recruitment between 1964 and 1996, were available through BEACON at the time of analysis. Of the 12 studies, 8 were conducted in North America,25–31 3 in Europe18,32,33 and 1 in Australia.19 Detailed descriptions of case ascertainment procedures can be found in study-specific publications.18,19,23,25–30,32–34
Cases
The main outcomes were (i) OA, (ii) OGJA and (iii) OA and OGJA grouped together (all adenocarcinomas). For each study, determination of case participant eligibility was based on pathology reports and/or review of medical records. In both cases, determination of histology and site of tumour origin of OA vs OGJA was made based on radiology, surgery, or endoscopy reports and review of pathology or reports.
Control subjects
Control subjects were identified from the populations from which the cases arose. For the cohort studies, a nested case–control approach was used, where a random sample of control subjects for each case was selected. The case–control ratio was 1:4 for the National Institutes of Health–American Association of Retired Persons (formerly known as American Association of Retired Persons) cohort study and 1:8 for the Kaiser Permanente cohort.27
Inclusion criteria and the pooling strategy have been described elsewhere.35 In total, 4214 cases and 13 750 control subjects were available for pooling. Cases included 2138 OAs and 2076 OGJAs. We excluded participants for whom either height or weight was missing (422 control subjects 49 OA, 45 OGJA). The analysis was limited to non-Hispanic White participants because the BEACON pooled data set had too few African American (12 OA, 35 OGJA, 1226 control subjects), Hispanic (55 OA, 48 OGJA, 442 control subjects) or other-ethnicity participants (23 OA, 45 OGJA, 449 control subjects) for meaningful analysis. In addition, we excluded individuals for whom race was unknown (52 control subjects, 2 OA, 3 OGJA). Analyses were thus based on 3897 cases (1997 OA, 1900 OGJA) and 11 159 control subjects.
In Table 1, we present basic descriptive characteristics of the case and control populations for each study. The conduct of individual studies and contribution of data for consortial analyses in BEACON were approved by the relevant institutional review or research ethics boards for each study.
Table 1.
BEACON studies available for analysis of body mass index and adenocarcinomas of the oesophagus and oesophagogastric junction
| Name | Country of Study | Period of Recruitment | Cases |
Controls |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OA (n) | OGJA (n) | AA (n) | Age [mean (SD)] | Male (%) | BMI [mean (SD)] | n | Age [mean (SD)] | Male (%) | BMI [mean (SD)] | |||
| Australian Cancer Studya | Australia | 2001–05 | 344 | 403 | 747 | 63.5 (9.7) | 88.5 | 28.7 (5.0) | 1447 | 60.5 (11.7) | 65.8 | 26.9 (4.6) |
| Nova Scotia Barrett Esophagus Studya | Canada | 2001–03 | 55 | 55 | 64.2 (9.7) | 89.5 | 27.5 (5.6) | 99 | 54.7 (13.7) | 64.7 | 28.9 (5.9) | |
| Factors Influencing the Barrett’s Adenocarcinoma Relationship Studya | Ireland | 2002–04 | 130 | 92 | 222 | 64.2 (11.1) | 85.2 | 28.6 (4.8) | 259 | 63.0 (12.8) | 84.6 | 27.0 (3.9) |
| Swedish Esophageal Cancer Studya | Sweden | 1995–97 | 189 | 262 | 451 | 65.3 (9.9) | 86.0 | 25.6 (3.9) | 816 | 66.0 (10.0) | 82.8 | 23.9 (2.8) |
| United Kingdom Study of Esophageal Cancer in Womena | United Kingdom | 1993–96 | 72 | 72 | 65.3 (8.7) | 0.0 | 29.8 (6.9) | 72 | 65.9 (8.8) | 0.0 | 26.9 (4.8) | |
| Kaiser Permanente Multiphasic Health Checkup Studyb | United States | 1964–73 | 86 | 85 | 171 | 46.2 (11.2) | 80.6 | 26.2 (3.6) | 2021 | 47.5 (11.0) | 74.6 | 25.2 (3.7) |
| Larynx/Esophagus/Oral Cavity Studya | United States | 1983–90 | 129 | 154 | 283 | 61.1 (9.3) | 89.6 | 26.5 (4.4) | 694 | 59.8 (10.0) | 69.9 | 25.9 (4.0) |
| Los Angeles Multi-ethnic Studya | United States | 1992–97 | 168 | 205 | 373 | 60.9 (9.8) | 86.7 | 27.0 (5.2) | 834 | 58.7 (11.5) | 73.7 | 25.8 (4.4) |
| Nebraska Health Study IIa | United States | 1988–93 | 123 | 44 | 167 | 68.3 (12.4) | 86.6 | 26.4 (3.7) | 489 | 68.3 (17.6) | 56.6 | 25.2 (4.5) |
| NIH-AARP Studyb | United States | 1995–96 | 366 | 296 | 662 | 63.7 (5.0) | 90.9 | 28.2 (4.8) | 3083 | 62.1 (5.3) | 60.2 | 27.0 (4.7) |
| Population Health Studya | United States | 1986–89 | 58 | 113 | 171 | 62.5 (9.6) | 100.0 | 26.7 (3.8) | 722 | 61.3 (11.0) | 100.0 | 25.2 (3.2) |
| US Multi-Center Studya | United States | 1993–95 | 277 | 246 | 523 | 63.7 (10.9) | 84.5 | 26.1 (4.2) | 623 | 62.8 (10.7) | 79.9 | 24.9 (3.4) |
| Total | 1997 | 1900 | 3897 | 62.7 (10.3) | 86.3 | 27.3 (4.8) | 11 159 | 58.9 (12.0) | 71.9 | 26.0 (4.3) | ||
aCase–control study.
bCohort study with a nested case–control set selected for BEACON.
OA = oesophageal adenocarcinoma; OGJA = oesophagogastric junction adenocarcinoma; AA = all adenocarcinomas (OA and OGJA); BMI = body mass index; SD = standard deviation.
Study variables
BMI was based on self-reported adult height and weight for all studies, although the exposure date in relation to cancer diagnosis varied across studies. Consequently, we used usual adult weight.15,28,30,34 If usual adult weight was unavailable, we used weight 1 year,19,26,29 5 years32 or 20 years before interview.18 One study ascertained weight at age 20 years and maximum adult weight (excluding pregnancies), for which we used the latter weight, assuming it more accurately reflected usual adult weight.28 For the two nested case–control studies, we used weight at cohort entry.23,27 BMI was categorized into an ordinal variable with five groups based on WHO criteria36: BMI <25, BMI 25–29.9, BMI 30–34.9, BMI 35–39.9 and BMI ≥40. Additionally, we defined a combined obese group (BMI ≥30). Fewer than 1% of participants had a BMI <19, and these were included in the referent category of BMI <25.
GERD symptoms, where available,19,25,26,32 were defined as a history of reflux or heartburn 1 year before diagnosis for cases and 1 year before interview for control subjects. Heartburn symptoms solicited included ‘burning or aching pain behind the breastbone not due to heart problems’. Reflux symptoms solicited included ‘sour taste from acid, bile or contents of the stomach'.
Other covariables included in analyses were age (categorized as <50, 50–59, 60–69 and ≥70 years), gender, education (less than high school, high school or more), cigarette smoking (categorized as <15, 15 to <30, 30 to <45 and ≥45 pack-years derived from dividing the number of cigarettes smoked by 20 and multiplying by the total number of years smoked), regular alcohol consumption (yes/no) and, where available,15,18,26,32 Helicobacter pylori colonization (yes/no).
Statistical analyses
Using each study’s individual-level data and covariates, we estimated study-specific odds ratios (ORs) and 95% confidence intervals (95% CIs) for the association between BMI categories and adenocarcinoma outcomes using logistic regression models. We also estimated ORs and 95% CIs per unit increase in BMI as a continuous covariate. All models were adjusted for age, gender, education, cigarette smoking, GERD (where available) and study-specific variables, such as study centre,35 as applicable. Study-specific estimates were subsequently combined using random-effects meta-analytic models. The results from fixed-effects models were similar; however, we believe that random-effects models are more appropriate for the current analyses.37 To estimate heterogeneity, we computed the I2 statistic.38 The I2 statistic ranges from 0 to 100%, where I2 = 0 indicates no observed heterogeneity and larger values indicate increasing heterogeneity.
We also investigated the relationship between BMI and cancer using spline models39 to plot the relationship on a continuous scale. Restricted cubic spline models allow for easy visualization of non-linear relationships between an exposure and an outcome40,41—in this case, BMI and OA/OGJA. These analyses were adjusted for age (categorical), gender, pack-years of cigarette smoking (categorical), education (harmonized, dichotomous: less than high school, high school or more) and study site/centre (categorical) using the pooled data set of individual patient data. Results from spline models were plotted using a linear scale on the x-axis for BMI and a logarithmic (base 10) scale on the y-axis for the OR. Plots were constructed for OA and OGJA overall and also for subgroups defined by gender and GERD symptoms.
We assessed whether there was evidence for effect modification—i.e. whether the effect of single exposure (BMI) on cancer risk (OA/OGJA) varied over strata of a second variable (an effect modifier).42–44 The variables, age, gender and GERD symptoms were tested as potential effect modifiers of the association between BMI and cancer. We evaluated the strength of potential effect modification by addition of product terms to study-specific logistic regression models followed by random-effects meta-analysis.
We also assessed whether there was evidence of interaction (synergism or departure from additivity)42–44 i.e. whether the joint effect of two exposures (BMI and another) had greater effects on the risk of OA and OGJA than would be expected from the independent effects of each exposure. Dichotomous variables tested for departure from additivity with BMI (dichotomized at <27.5 and ≥27.5) were cigarette smoking, gender, alcohol, GERD symptoms and H. pylori colonization. For each combination of variables, we generated four exposure categories. These variables were modelled in the pooled data set using logistic regression adjusted for age (categorical), gender, BMI (continuous), heartburn or reflux (if unavailable for a study, all individuals were recoded to a missing category and were excluded from the heartburn/reflux/heartburn-or-reflux–BMI interaction models), education (harmonized, dichotomous: less than high school, high school or more) and study site/centre (categorical). The output from these models was used to estimate three interaction statistics: interaction contrast ratio (ICR), attributable proportion (AP) and synergy index (S). When the ICR and AP ≠ 0, and S ≠ 1, there is evidence for departure from additivity (interaction). ICR is the excess risk due to interaction relative to the risk without either exposure. AP is the proportion of disease attributable to interaction among individuals with both exposures. S is the ratio of the observed excess risk in individuals exposed to both factors relative to the expected excess risk assuming that both exposures are independent risk factors (i.e. under the assumption of no additive interaction). CIs for these metrics were estimated using the delta method.45 All analyses were conducted using STATA software version 11 (StataCorp LP, College Station, TX).
Results
Descriptive characteristics of case and control groups by study are shown in Table 1. The number of cases varied between studies from 72 to 747, and the mean ages were generally 63–65 years, although the age was much younger in the Kaiser Permanente Multiphasic Health Check-up Study because of its design (cohort). The UK study was of women only, and the Population Health Study was of men only, but all other studies were composed of, on average, 86% men and 14% women. The mean BMI for cases was generally higher than that for control subjects with overall means being 27.2 and 26.0, respectively.
Table 2 shows adjusted summary ORs for the associations between BMI and the risk of OA, OGJA and all adenocarcinomas among men and women combined. Compared with BMI <25, the increase in OA risk in individuals with BMI 25–29.9 was 1.54 (95% CI 1.26–1.88), a risk that increased to >2-fold in individuals with BMI 30–34.9 (OR 2.39, 95% CI 1.86–3.06) or BMI 35–39.9 (OR 2.79, 95% CI 1.89–4.12) (Table 2), and almost 5-fold in individuals with BMI ≥40 (OR 4.76, 95% CI 2.96–7.66). The patterns of association between BMI and these adenocarcinomas were similar in younger (≤65 years) and older (>65 years) individuals when stratified by age at diagnosis (data not shown). These results were unaltered when we repeated the analyses, adjusting for GERD, in the five studies with GERD information (data not shown). This monotonic increase in risk was also observed when BMI was evaluated in relation to OGJA and all adenocarcinomas. The associations were slightly stronger with OA than OGJA. Excluding individuals with potential cachexia (BMI <18.5) had little effect on the estimates attained.
Table 2.
Adjusted summary odds ratios and 95% confidence intervals for the association between body mass index and adenocarcinomas of the oesophagus and oesophagogastric junction among all subjects (men and women combined)
| OA |
OGJA |
All adenocarcinomas |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | Controls (n) | Cases (n) | OR | 95% CI | I2 | N | Controls (n) | Cases (n) | OR | 95% CI | I2 | N | Controls (n) | Cases (n) | OR | 95% CI | I2 | N | |||
| <25.0 | 4744 | 577 | Referent | 4686 | 663 | Referent | 4777 | 1240 | Referent | ||||||||||||
| 25.0–29.9 | 4232 | 862 | 1.54 | 1.26–1.88 | 55 | 12 | 4147 | 742 | 1.28 | 1.13–1.45 | 0 | 10 | 4232 | 1604 | 1.41 | 1.24–1.60 | 37 | 12 | |||
| 30.0–34.9 | 1109 | 331 | 2.39 | 1.86–3.06 | 42 | 12 | 1081 | 304 | 2.08 | 1.75–2.47 | 0 | 10 | 1109 | 635 | 2.23 | 1.83–2.71 | 41 | 12 | |||
| 35.0–39.9 | 273 | 86 | 2.79 | 1.89–4.12 | 23 | 11 | 266 | 85 | 2.36 | 1.75–3.17 | 0 | 9 | 273 | 171 | 2.40 | 1.89–3.04 | 0 | 11 | |||
| ≥40 | 94 | 41 | 4.76 | 2.96–7.66 | 0 | 9 | 91 | 28 | 3.07 | 1.89–4.99 | 0 | 8 | 99 | 69 | 3.65 | 2.50–5.34 | 0 | 10 | |||
| Continuous | 10 481 | 1897 | 1.09 | 1.06–1.12 | 76 | 12 | 10 295 | 1822 | 1.07 | 1.05–1.09 | 54 | 10 | 10 481 | 3719 | 1.08 | 1.06–1.10 | 75 | 12 | |||
Results were adjusted for age (categorical: <50, 50–59, 60–69, ≥70 years), gender, pack-years of smoking (categorical: <15, 15 to <30, 30 to <45, ≥45), education (study-specific) and other study-specific adjustment variables (e.g. study centre, where applicable). Summary odds ratios and 95% confidence intervals were obtained from random-effects models. All 12 studies were included for analysis unless otherwise specified.
I2 estimates variability in results across studies.
OA = oesophageal adenocarcinoma; OGJA = oesophagogastric junction adenocarcinoma; AA = all adenocarcinomas (OA and OGJA); BMI = body mass index; OR = odds ratio; N = number of studies in the analysis.
We evaluated the association between BMI and OA, OGJA and all adenocarcinomas, stratified by GERD symptoms, including heartburn and reflux (Table 3). The pattern and magnitude of associations between BMI and OA, OGJA or all adenocarcinomas were similar in the studies with GERD data available compared with those of all participants (data not shown). A history of GERD symptoms did not materially alter the patterns and magnitude of associations between BMI and cancer. Among individuals with a history of GERD symptoms, overweight status was associated with an ∼50% increase in OA risk relative to BMI <25. Risk increased linearly with increasing BMI to >2-fold in individuals with BMI 30–34.9 (OR 2.21, 95% CI 1.44–3.39) and 5-fold in individuals with BMI 35–39.9 (OR 5.84, 95% CI 2.72–12.55). A similar pattern and magnitude of association were also observed among individuals with no history of GERD symptoms. The pattern of association in GERD-stratified analyses was similar for OGJA and the combined grouping of all adenocarcinomas, although the magnitude of association was somewhat stronger in the BMI–OA than the BMI–OGJA analyses.
Table 3.
Adjusted odds ratios and 95% confidence intervals for the association between body mass index and risk of oesophageal adenocarcinoma, oesophagogastric junction adenocarcinoma and all adenocarcinomas, stratified by heartburn and gastro-oesophageal reflux
| No GERD |
GERD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | Controls (n) | Cases (n) | OR | 95% CI | I2 | Controls (n) | Cases (n) | OR | 95% CI | I2 |
| Oesophageal adenocarcinoma | ||||||||||
| <25.0 | 614 | 94 | Referent | 705 | 180 | Referent | ||||
| 25.0–29.9 | 532 | 95 | 1.12 | 0.80–1.58 | 0 | 739 | 282 | 1.48 | 1.07–2.05 | 40 |
| 30.0–34.9 | 136 | 37 | 1.85 | 0.91–3.73 | 48 | 224 | 129 | 2.21 | 1.44–3.39 | 37 |
| 35.0–39.9 | 47 | 15 | 2.08 | 1.00–4.30 | 0 | 62 | 36 | 2.95 | 1.15–7.59 | 49 |
| ≥40 | 10 | 4 | 6.45 a | 1.60–25.99 | 100 | 20 | 18 | 5.84 | 2.72–12.55 | 0 |
| Continuous | 1341 | 246 | 1.07 | 1.03–1.11 | 0 | 1750 | 645 | 1.08 | 1.03–1.14 | 71 |
| Oesophagogastric junctional adenocarcinoma | ||||||||||
| <25.0 | 614 | 128 | Referent | 705 | 172 | Referent | ||||
| 25.0–29.9 | 532 | 136 | 1.22 | 0.77–1.95 | 56 | 739 | 239 | 1.16 | 0.87–1.56 | 24 |
| 30.0–34.9 | 136 | 54 | 2.08 | 1.36–3.19 | 0 | 224 | 116 | 1.91 | 1.4–2.6 | 0 |
| 35.0–39.9 | 47 | 14 | 1.49 | 0.74–2.98 | 0 | 62 | 40 | 3.65 | 1.58–8.46 | 50 |
| ≥40 | 12 | 4 | 3.20 b | 0.89–11.52 | 0 | 20 | 11 | 2.64 | 1.16–5.99 | 0 |
| Continuous | 1341 | 337 | 1.06 | 1.03–1.09 | 0 | 1750 | 578 | 1.07 | 1.04–1.09 | 0 |
| All adenocarcinomas | ||||||||||
| <25.0 | 614 | 222 | Referent | 705 | 352 | Referent | ||||
| 25.0–29.9 | 532 | 231 | 1.20 | 0.92–1.58 | 17 | 739 | 521 | 1.33 | 1.02–1.74 | 44 |
| 30.0–34.9 | 136 | 91 | 1.88 | 1.32–2.67 | 0 | 224 | 245 | 2.09 | 1.58–2.77 | 13 |
| 35.0–39.9 | 47 | 29 | 1.68 | 0.97–2.93 | 0 | 62 | 76 | 2.98 | 1.5–5.93 | 45 |
| ≥40 | 12 | 8 | 3.74 b | 1.33–10.54 | 0 | 20 | 29 | 4.23 | 2.21–8.09 | 0 |
| Continuous | 1341 | 583 | 1.06 | 1.04–1.09 | 0 | 1750 | 1223 | 1.08 | 1.05–1.11 | 54 |
Results were adjusted for age (categorical: <50, 50–59, 60–69, ≥70 years), gender, pack-years of smoking (categorical: <15, 15 to <30, 30 to <45, ≥45), education (study-specific) and other study-specific adjustment variables (e.g. study centre, where applicable). Summary odds ratios and 95% confidence intervals were obtained from random-effects meta-analytic models. The four studies that had heartburn and reflux information available were included in these analyses.
aOnly one of the four studies with heartburn and reflux data was able to contribute a study-specific odds ratio to this summary estimate.
bOnly two of the four studies with heartburn and reflux data were able to contribute a study-specific odds ratio to this summary estimate.
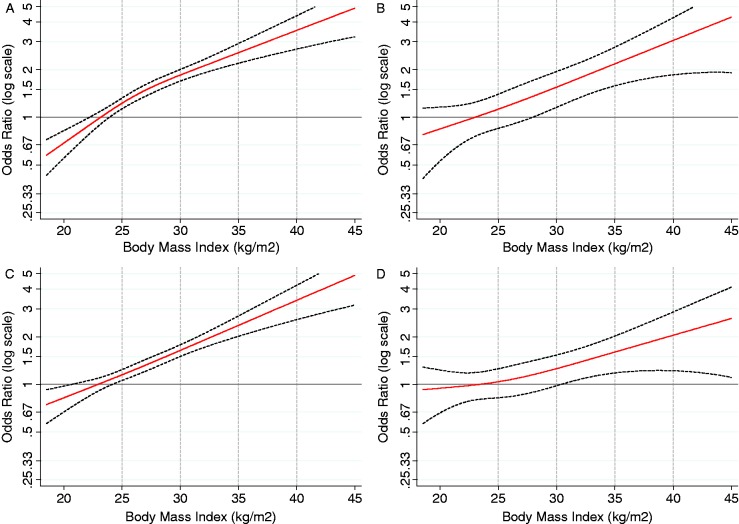
To determine whether gender was an effect modifier of the observed associations between the exposure, BMI and the outcome OA/OGJA, we conducted analyses stratified by gender (Table 4). Compared with men in the category BMI <25, those with BMI 25–29.9 were at greater than 60% increased risk of OA (OR 1.63, 95% CI 1.32–2.00), a risk that increased linearly with increasing BMI to >4-fold (OR 4.47, 95% CI 2.42–8.26) in men with BMI ≥40. These patterns of association were similar for OGJA and the combined group of all adenocarcinomas for men, although, again, associations were strongest for OA. This pattern of association between BMI and OA, and a weaker association with OGJA, were also observed among women (Figure 1).
Table 4.
Adjusted summary odds ratios and 95% confidence intervals for the association between body mass index and adenocarcinomas of the oesophagogastric junction stratified by gender
| OA |
OGJA |
All adenocarcinomas |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | Controls (n) | Cases (n) | OR | 95% CI | I2 | Controls (n) | Cases (n) | OR | 95% CI | I2 | Controls (n) | Cases (n) | OR | 95% CI | I2 |
| Males | |||||||||||||||
| <25.0 | 3171 | 477 | Referent | 3157 | 535 | Referent | 3171 | 1012 | Referent | ||||||
| 25.0–29.9 | 3310 | 779 | 1.63 | 1.32–2.00 | 51 | 3274 | 675 | 1.36 | 1.19–1.55 | 0 | 3310 | 1454 | 1.49 | 1.29–1.71 | 38 |
| 30.0–34.9 | 743 | 280 | 2.47 | 1.94–3.13 | 26 | 733 | 268 | 2.24 | 1.85–2.71 | 0 | 743 | 548 | 2.38 | 1.95–2.91 | 32 |
| 35.0–39.9 | 150 | 69 | 2.87 | 1.89–4.36 | 17 | 149 | 68 | 2.50 | 1.78–3.53 | 0 | 150 | 137 | 2.58 | 1.94–3.43 | 2 |
| ≥40 | 33 | 22 | 4.47 | 2.42–8.26 | 0 | 33 | 16 | 3.16 | 1.63–6.11 | 0 | 35 | 38 | 3.49 | 2.09–5.84 | 0 |
| Continuous | 5588 | 1306 | 1.09 | 1.06–1.13 | 76 | 5525 | 1320 | 1.08 | 1.06–1.11 | 51 | 5588 | 2626 | 1.09 | 1.06–1.11 | 75 |
| Females | |||||||||||||||
| <25.0 | 1369 | 94 | Referent | 1529 | 128 | Referent | 1567 | 227 | Referent | ||||||
| 25.0–29.9 | 794 | 80 | 1.24 | 0.67–2.29 | 56 | 873 | 67 | 1.04 | 0.67–1.61 | 26 | 910 | 147 | 1.06 | 0.81–1.38 | 0 |
| 30.0–34.9 | 331 | 49 | 2.66 | 1.59–4.46 | 17 | 296 | 36 | 1.71 | 1.06–2.77 | 0 | 362 | 85 | 1.74 | 1.24–2.43 | 0 |
| 35.0–39.9 | 110 | 11 | 1.38 | 0.57–3.35 | 0 | 114 | 15 | 1.80 | 0.92–3.52 | 0 | 115 | 29 | 1.83 | 1.06–3.15 | 0 |
| ≥40 | 46 | 13 | 5.88 | 2.28–15.1 | 0 | 51 | 9 | 3.08 | 1.25–7.56 | 0 | 57 | 23 | 3.55 | 1.87–6.75 | 0 |
| Continuous | 1522 | 228 | 1.07 | 1.04–1.10 | 13 | 1786 | 220 | 1.04 | 1.01–1.07 | 0 | 1878 | 457 | 1.05 | 1.03–1.07 | 0 |
Results were adjusted for age (categorical: <50, 50–59, 60–69, ≥70 years), pack-years of smoking (categorical: <15, 15 to <30, 30 to <45, ≥45), education (study-specific) and other study-specific adjustment variables (e.g. study centre, where applicable). Summary odds ratios and 95% confidence intervals were obtained from random-effects models.
I2 estimates variability in results across studies.
OA = oesophageal adenocarcinoma; OGJA = oesophagogastric junction adenocarcinoma; BMI = body mass index; OR = odds ratio.
Figure 1.
Restricted cubic spline models of the relationship between body mass index and adenocarcinomas of the oesophagus and oesophagogastric junction. (a) Oesophageal adenocarcinoma in men. (b) Oesophageal adenocarcinoma in women. (c) Oesophagogastric junction adenocarcinoma in men. (d) Oesophagogastric junction adenocarcinoma in women. Plots are restricted to body mass indexes 18.5–45 and odds ratios 0.25–5 for clarity and consistency
To explore the relationships between BMI and OA/OGJA in men and women further, and the lack of attenuation of the magnitude of the association in individuals with symptomatic GERD (heartburn and reflux), we stratified the spline models by gender and GERD. The relationship between increasing BMI and cancer risk was similar for men and women who reported symptomatic GERD (Supplementary Figure S1, available as Supplementary data at IJE online). Conversely, the relationship appeared attenuated in women compared with the relationship observed in men with no GERD symptoms (Supplementary Figure S2, available as Supplementary data at IJE online), although the analysis of women who did not report GERD was based on just 33 OA cases and 357 control subjects.
In analyses testing for interaction (synergism, departure from additivity), we found evidence for synergism between BMI and GERD with respect to OA risk (Table 5). Compared with the referent of no GERD symptoms and low BMI (<27.5), the OR for GERD symptoms and low BMI was 2.28 (1.83–2.84), for no GERD symptoms and high BMI (≥27.5) was 1.26 (0.91–1.74) and for GERD and high BMI was 3.18 (2.45–4.13). The excess risk attributable to the synergistic interaction of GERD and high BMI was estimated to be 0.64 (0.12–1.17), with an AP of OA due to interaction of 0.20 (0.05–0.35).
Table 5.
Interaction statistics for departure from additivity
| Departure from additivity |
|||
|---|---|---|---|
| Variables tested for interaction with body mass index | ICR (95% CI) (null hypothesis = 0) | AP (95% CI) (null hypothesis = 0) | S (95%CI) (null hypothesis = 1) |
| OA vs controls | |||
| Cigarette smoking | 0.22 (−0.30, 0.73) | 0.07 (−0.09, 0.22) | 1.11 (0.87, 1.40) |
| Alcohol | 0.05 (−0.30, 0.40) | 0.04 (−0.25, 0.34) | 1.31 (0.12, 13.74) |
| H. pylori (negative) | 0.06 (−0.77, 0.89) | 0.03 (−0.42, 0.49) | 1.08 (0.37, 3.20) |
| Heartburn | 0.44 (−0.16, 1.04) | 0.18 (−0.04, 0.39) | 1.42 (0.89, 2.26) |
| Reflux | 0.14 (−0.31, 0.58) | 0.08 (−0.16, 0.32) | 1.20 (0.64, 2.28) |
| Heartburn or refluxa | 0.64 (0.12, 1.17) | 0.20 (0.05, 0.35) | 1.42 (1.04, 1.94) |
| OGJA vs controls | |||
| Cigarette smoking | 0.25 (−0.28, 0.78) | 0.08 (−0.08, 0.23) | 1.12 (0.88, 1.44) |
| Alcohol | 0.02 (−0.30, 0.33) | 0.02 (−0.36, 0.40) | 0.90 (0.16, 4.97) |
| H. pylori (negative) | −0.37 (−0.97, 0.22) | −0.44 (−1.18, 0.30) | |
| Heartburn | 0.05 (−0.34, 0.45) | 0.04 (−0.22, 0.29) | 1.12 (0.48, 2.58) |
| Reflux | 0.29 (−0.01, 0.58) | 0.25 (0.00, 0.50) | |
| Heartburn or reflux | 0.30 (−0.01, 0.60) | 0.20 (0.00, 0.40) | 2.51 (0.42, 14.98) |
AP = attributable proportion due to interaction; ICR = interaction contrast ratio; OR = odds ratio; S = synergy index.
aThe excess risk attributable to the synergistic interaction of heartburn or reflux and high BMI ICR, attributable proportion (AP) of OA.
Discussion
In this large consortial analysis, we report strong linear relationships between increasing BMI and the risk of OA. There was no evidence of effect modification when stratified by GERD symptoms, which may suggest an indirect proinflammatory route of association between BMI and OA/OGJA exists, as well as direct mechanical effects of android fat. There was putative evidence that gender may modify the relationship between BMI and OA in individuals without a history of GERD symptoms, which also may be interpreted as further evidence for an indirect proinflammatory route of association emanating from highly metabolic visceral fat when direct inflammatory routes (GERD) of pathogenesis do not predominate. Lastly, we found evidence for synergistic effects of BMI and GERD; the risk of cancer in obese individuals with GERD was significantly higher than predicted under an additive model.
Our findings of a strong positive dose–response relationship between BMI and the risk of OA reiterate the fact that this cancer is the primary malignancy associated with obesity.46 We also found similar, although somewhat weaker, associations for OGJA, and this is consistent with the hypothesis that OGJA represents a heterogeneous set of tumours with less clear origin compared with OA.47 Our results are compatible with findings from a previous meta-analysis that used published ORs from 14 studies,48 6 of them included here,15,18,26,33,34,49 as well as findings reported by other studies not included in the present analysis.50–56 Importantly, our study extends these results through the use of pooled individual participant data and harmonized variables and statistical models while also enabling analyses of effect modification and interaction. Overall, our findings support the hypothesis that BMI is a risk factor for OA and OGJA, the predominant causal theory of which is that obesity increases abdominal pressure, which subsequently relaxes the lower oesophageal sphincter, exposing the lower oesophagus to gastric acid and increasing the risk of GERD.20,48,56–58 In support of this hypothesis, the prevalence of GERD has been shown to increase with increasing levels of BMI.8,12
The large size of this analysis presented us the unique opportunity to investigate potential mechanisms of these associations. When stratified by history of GERD symptoms, we found no difference in the pattern of associations between BMI and these adenocarcinomas. In addition, adjustment for GERD symptoms (ever/never) did not attenuate the ORs for associations between BMI and OA/OGJA. These observations do not dispute the idea that a mechanical effect of BMI that increases the propensity for GERD and thus the risk for cancer exists, as we did not adjust for severity or frequency of GERD symptoms. Moreover, we could not adjust for asymptomatic GERD, as ascertainment of such would obviously have required all individuals to have undergone ambulatory 24-h pH-metry. However, what it may suggest is that an indirect, possibly proinflammatory, carcinogenic pathway between BMI and OA risk may exist, in addition to the accepted mechanical pathway of oesophageal sphincter distortion, increased intra-gastric pressure and increased risk of hiatal hernia. Spline models stratified by GERD and gender provided further evidence for an indirect proinflammatory pathogenic mechanism of BMI on OA risk—in individuals without a history of GERD symptoms, increasing BMI was associated with OA risk in men but not women. This may suggest that when inflammatory routes of associations are not saturated by the direct effects of GERD, the indirect effect can be detected. Such may be detectable in men, but not women, given the fact that android fat patterning, with highly metabolic visceral adipose tissue,20 is common in men relative to the preferred gynoid fat patterning, with a much lower metabolic rate, of women.21,22 Obesity-related hormones may induce oesophageal inflammatory damage, promoting proliferation and malignant transformation.24,59,60 Although we lack central adiposity metrics to test the theory directly, the evidence we present is provocative. However, our findings do not preclude the possibility of collider-stratification bias61 resulting from the more complex and possibly less direct relationships among obesity, GERD and OA/OGJA. This could occur if the relationship between GERD and BMI is mediated by a higher BMI giving way to increased severity and/or duration of GERD, in addition to the previously posited metabolic carcinogenic effects that a higher BMI may confer.8,12,14,20,24,30,57–66 In addition, it should be noted that our findings contrast with those of at least four previous studies15,19,56,67 that found stronger associations between BMI and OA/OGJA in individuals with a history of GERD symptoms, with the magnitude of the association increasing with increasing duration and severity of symptoms.67 However, these studies, some of which are included in the current analyses, suffered small sample sizes, raising the possibility that inconsistent findings could be attributed, at least in part, to unstable risk estimates. Lastly, and supporting the idea of direct mechanical (distortion of the lower oesophageal sphincter, increased intra-gastric pressure, increased risk of herniation) and indirect metabolic effects on OA risk, was the observation of synergism between BMI and GERD symptoms, with an excess risk attributable to synergistic interaction of 0.64 (0.12–1.17). Such interaction was previously suggested in a paper by Whiteman et al.in an analysis of one of the studies included in this pooled analysis presented herein.19 As a whole, the evidence we present advocates for at least two pathways through which increased BMI can modify OA risk, which may also be related to the large gender disparity of these malignancies,6 given gender differences in adipose patterning. Although the stratified models suggest that the BMI–OA/OGJA relationships may vary by gender in some BMI categories, with stronger estimates observed in overweight and obese men, relative to equivalent estimates in women, it is important to keep in mind that, even in this large consortial analysis, the number of women available for analysis was limited. These limitations are also applicable to a previous meta-analysis48 and three other published studies68–70 that have made similar observations of differences by gender.6,20–22
Several limitations of this study should be considered when interpreting our findings. First, none of the studies included in these analyses collected data on fat distribution, including body shape, and few collected waist circumference at different ages during adulthood. Consequently, fat distribution could not be evaluated in our analyses. Future studies should identify and use improved measures of central obesity and other measures of the body habitus, including those that could be retrospectively documented.21,71 We have proposed that one of many ways this could be achieved is by retrospectively querying study participants about life-course changes in clothing sizes, particularly trouser waist size, at least for men, focusing on changes in adulthood.21 Results from two of three studies that evaluated waist circumference and BMI in relation to OA19,26,72 suggest that central obesity is a risk factor for OA, and a study of Barrett’s oesophagus, an OA precursor, suggests visceral fat may influence risk independent of BMI.73
A second potential limitation of our study is that the study-specific analyses are not adjusted for dietary intake, primarily because of differing ways these data were collected across studies. However, strong associations between BMI and OA/OGJA have been reported, regardless of whether adjustments are made for dietary intake.15,74
A third limitation is that our pooled analysis predominantly consists of case–control studies that lack the ability to determine the sequence of events between obesity and OA/OGJA, as BMI ascertainment among these studies was limited to ≥1 years before interview. However, restricting our analyses to individuals with a BMI >18.5 did not alter our findings. Moreover, the association between BMI and OA/OGJA was also found in the two prospective cohort studies. Furthermore, such differential recall in case–control studies would attenuate the magnitude of the risk for the association between BMI and OA/OGJA, thus assuaging any concerns that our findings are due to underestimates of past body weight in patients. Also related to the timing of case ascertainment relative to cancer onset is the potential limitation that data for this pooled analysis include case accrual over a 25-year period (1964–2006), during which time there have been rapid increases in the prevalence of obesity in all countries from which the included studies derive. However, we found no evidence of effects by calendar period, by visual inspection of forest plots and meta-regression of mid-year of recruitment.
In summary, this consortial analysis of pooled individual participant data has provided evidence that increasing BMI is associated with an increasing risk of OA and OGJA, and that these relationships are similar in those with and without a history of GERD symptoms. In addition, we provide tentative evidence for effect modification by gender in those without GERD symptoms and, lastly, evidence of synergistic interactions between BMI and GERD. Future studies should focus on elucidating the mechanisms that underlie these observations, specifically the multifaceted effects of obesity on the risk of OA.
Supplementary Data
Funding
This work was supported in part by extramural grants from the National Institute of Health for C.H. (K01CA104517), M.D.G. (U01CA057983 and P30ES10126), H.A.R. (U01CA057923), National Cancer Institute of Canada with funds from the Canadian Cancer Society for A.G.C. (14676), T.L.V. (U01CA57949 and K05CA124911), L.B. (R01CA59636, U54CA 116848 and K05CA136967), by the National Cancer Institute of Canada with funds from the Canadian Cancer Society for A.G.C. (14676), and by the Intramural Program of the National Institutes of Health. D.C.W. is supported in part by the Australian Research Council.
Conflict of interest: None declared.
KEY MESSAGES.
BMI is directly associated with OA risk in both men and women and in those with and without GERD symptoms.
Findings suggest an indirect proinflammatory route of association between BMI and OA exists, as well as direct mechanical effects of android fat.
Effects of BMI and GERD symptoms on OA risk may be synergistic.
References
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–46. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 2.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–87. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–53. [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Trivers KF, De Roos AJ, Gammon MD, et al. Demographic and lifestyle predictors of survival in patients with esophageal or gastric cancers. Clin Gastroenterol Hepatol. 2005;3:225–30. doi: 10.1016/s1542-3565(04)00613-5. [DOI] [PubMed] [Google Scholar]
- 6.Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174–82. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosetti C, Levi F, Ferlay J, et al. Trends on oesophageal cancer incidence and mortality in Europe. Int J Cancer. 2008;58:16–23. doi: 10.1002/ijc.23232. [DOI] [PubMed] [Google Scholar]
- 8.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Derakhshan MH, Malekzadeh R, Watabe H, et al. Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut. 2008;57:298–305. doi: 10.1136/gut.2007.137364. [DOI] [PubMed] [Google Scholar]
- 10.Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol. 2011;8:340–47. doi: 10.1038/nrgastro.2011.73. [DOI] [PubMed] [Google Scholar]
- 11.El-Serag H. The association between obesity and GERD: a review of the epidemiological evidence. Dig Dis Sci. 2008;53:2307–12. doi: 10.1007/s10620-008-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedenberg FK, Xanthopoulos M, Foster GD, Richter JE. The association between gastroesophageal reflux disease and obesity. Am J Gastroenterol. 2008;103:2111–22. doi: 10.1111/j.1572-0241.2008.01946.x. [DOI] [PubMed] [Google Scholar]
- 13.Cook MB, Greenwood DC, Hardie LJ, Wild CP, Forman D. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett's esophagus. Am J Gastroenterol. 2008;103:292–300. doi: 10.1111/j.1572-0241.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 14.Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:2619–28. doi: 10.1111/j.1572-0241.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- 15.Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–55. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 16.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. quiz 311. [DOI] [PubMed] [Google Scholar]
- 17.Edelstein ZR, Bronner MP, Rosen SN, Vaughan TL. Risk factors for Barrett’s esophagus among patients with gastroesophageal reflux disease: a community clinic-based case-control study. Am J Gastroenterol. 2009;104:834–42. doi: 10.1038/ajg.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883–90. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–80. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity. Geneva: World Health Organization, 2000. Report No 894. [PubMed] [Google Scholar]
- 21.Hoyo C, Gammon MD. In: Cancer Prevention and Management Through Exercise and Weight Control. Boca Raton, FL: Taylor & Francis; 2006. Obesity and overweight in relation to adenocarcinoma of the esophagus; pp. 269–88. [Google Scholar]
- 22.Bray GA. The underlying basis for obesity: relationship to cancer. J Nutr. 2002;132(Suppl 11):3451S–55S. doi: 10.1093/jn/132.11.3451S. [DOI] [PubMed] [Google Scholar]
- 23.Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer. 2008;44:465–71. doi: 10.1016/j.ejca.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 25.Gammon MD, Schoenberg JB, Ahsan H, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277–84. doi: 10.1093/jnci/89.17.1277. [DOI] [PubMed] [Google Scholar]
- 26.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States) Cancer Causes Control. 2001;12:721–32. doi: 10.1023/a:1011290704728. [DOI] [PubMed] [Google Scholar]
- 27.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352–58. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Tucker KL, Graubard BI, et al. Nutrient intakes and adenocarcinoma of the esophagus and distal stomach. Nutr Cancer. 2002;42:33–40. doi: 10.1207/S15327914NC421_5. [DOI] [PubMed] [Google Scholar]
- 29.Vaughan TL, Davis S, Kristal A, Thomas DB. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 1995;4:85–92. [PubMed] [Google Scholar]
- 30.Veugelers PJ, Porter GA, Guernsey DL, Casson AG. Obesity and lifestyle risk factors for gastroesophageal reflux disease, Barrett esophagus and esophageal adenocarcinoma. Dis Esophagus. 2006;19:321–28. doi: 10.1111/j.1442-2050.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 31.Brown LM, Swanson CA, Gridley G, et al. Adenocarcinoma of the esophagus: role of obesity and diet. J Nat Cancer Inst. 1995;87:104–09. doi: 10.1093/jnci/87.2.104. [DOI] [PubMed] [Google Scholar]
- 32.Anderson LA, Watson RG, Murphy SJ, et al. Risk factors for Barrett's oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol. 2007;13:1585–94. doi: 10.3748/wjg.v13.i10.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng KK, Sharp L, McKinney PA, et al. A case-control study of oesophageal adenocarcinoma in women: a preventable disease. Br J Cancer. 2000;83:127–32. doi: 10.1054/bjoc.2000.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown LM, Silverman DT, Pottern LM, et al. Adenocarcinoma of the esophagus and esophagogastric junction in white men in the United States: alcohol, tobacco, and socioeconomic factors. Cancer Causes Control. 1994;5:333–40. doi: 10.1007/BF01804984. [DOI] [PubMed] [Google Scholar]
- 35.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Nat Cancer Inst. 2010;102:1344–53. doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Obesity. Geneva: World Health Organization; 2009. http://www.who.int/topics/obesity/en/ (27 March 2009, date last accessed) [Google Scholar]
- 37.Poole C, Greenland S. Random-effects meta-analyses are not always conservative. Am J Epidemiol. 1999;150:469–75. doi: 10.1093/oxfordjournals.aje.a010035. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buis M. POSTRCSPLINE: Stata module. Stata J. 2009;9:643–47. [Google Scholar]
- 40.Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62:511–17. doi: 10.1016/j.jclinepi.2008.05.015. e1. [DOI] [PubMed] [Google Scholar]
- 41.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–57. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 42.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd edn. Philadelphia, PA: Wolters Kluwer Health/Lippincott Willams & Wilkins; 2008. ISBN/ISSN: 9780781755641. [Google Scholar]
- 43.VanderWeele TJ. On the distinction between interaction and effect modification. Epidemiology. 2009;20:863–71. doi: 10.1097/EDE.0b013e3181ba333c. [DOI] [PubMed] [Google Scholar]
- 44.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41:514–20. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–56. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 47.Mackay HJ, McInnes A, Paul J, et al. A phase II study of epirubicin, cisplatin and raltitrexed combination chemotherapy (ECT) in patients with advanced oesophageal and gastric adenocarcinoma. Ann Oncol. 2001;12:1407–10. doi: 10.1023/a:1012552823543. [DOI] [PubMed] [Google Scholar]
- 48.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872–78. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Ward MH, Graubard BI, et al. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr. 2002;75:137–44. doi: 10.1093/ajcn/75.1.137. [DOI] [PubMed] [Google Scholar]
- 50.Bollschweiler E, Wolfgarten E, Nowroth T, Rosendahl U, Monig SP, Holscher AH. Vitamin intake and risk of subtypes of esophageal cancer in Germany. J Cancer Res Clin Oncol. 2002;128:575–80. doi: 10.1007/s00432-002-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett's oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut. 2002;51:323–28. doi: 10.1136/gut.51.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tretli S, Robsahm TE. Height, weight and cancer of the oesophagus and stomach: a follow-up study in Norway. Eur J Cancer Prev. 1999;8:115–22. doi: 10.1097/00008469-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Zhang ZF, Kurtz RC, Sun M, et al. Adenocarcinomas of the esophagus and gastric cardia: medical conditions, tobacco, alcohol, and socioeconomic factors. Cancer Epidemiol Biomarkers Prev. 1996;5:761–68. [PubMed] [Google Scholar]
- 54.Merry AH, Schouten LJ, Goldbohm RA, van den Brandt PA. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut. 2007;56:1503–11. doi: 10.1136/gut.2006.116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacInnis RJ, English DR, Hopper JL, Giles GG. Body size and composition and the risk of gastric and oesophageal adenocarcinoma. Int J Cancer. 2006;118:2628–31. doi: 10.1002/ijc.21638. [DOI] [PubMed] [Google Scholar]
- 56.Zhai R, Chen F, Liu G, et al. Interactions among genetic variants in apoptosis pathway genes, reflux symptoms, body mass index, and smoking indicate two distinct etiologic patterns of esophageal adenocarcinoma. J Clin Oncol. 2010;28:2445–51. doi: 10.1200/JCO.2009.26.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandolfino JE, El-Serag HB, Zhang Q, Shah N, Ghosh SK, Kahrilas PJ. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–49. doi: 10.1053/j.gastro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 58.Barak N, Ehrenpreis ED, Harrison JR, Sitrin MD. Gastro-oesophageal reflux disease in obesity: pathophysiological and therapeutic considerations. Obes Rev. 2002;3:9–15. doi: 10.1046/j.1467-789x.2002.00049.x. [DOI] [PubMed] [Google Scholar]
- 59.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 60.Atherfold PA, Jankowski JA. Molecular biology of Barrett's cancer. Best Pract Res Clin Gastroenterol. 2006;20:813–27. doi: 10.1016/j.bpg.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Cole SR, Platt RW, Schisterman EF, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39:417–20. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Vries DR, van Herwaarden MA, Smout AJ, Samsom M. Gastroesophageal pressure gradients in gastroesophageal reflux disease: relations with hiatal hernia, body mass index, and esophageal acid exposure. Am J Gastroenterol. 2008;103:1349–54. doi: 10.1111/j.1572-0241.2008.01909.x. [DOI] [PubMed] [Google Scholar]
- 63.Jaffin BW, Knoepflmacher P, Greenstein R. High prevalence of asymptomatic esophageal motility disorders among morbidly obese patients. Obes Surg. 1999;9:390–95. doi: 10.1381/096089299765552990. [DOI] [PubMed] [Google Scholar]
- 64.O’Brien TF. Jr. Lower esophageal sphincter pressure (LESP) and esophageal function in obese humans. J Clin Gastroenterol. 1980;2:145–48. [PubMed] [Google Scholar]
- 65.Orlando RC. Overview of the mechanisms of gastroesophageal reflux. Am J Med. 2001;111(Suppl 8A):174S–77S. doi: 10.1016/s0002-9343(01)00828-2. [DOI] [PubMed] [Google Scholar]
- 66.Gao L, Weck MN, Rothenbacher D, Brenner H. Body mass index, chronic atrophic gastritis and heartburn: a population-based study among 8936 older adults from Germany. Aliment Pharmacol Ther. 2010;32:296–302. doi: 10.1111/j.1365-2036.2010.04334.x. [DOI] [PubMed] [Google Scholar]
- 67.Lagergren J, Ye W, Bergstrom R, Nyren O. Utility of endoscopic screening for upper gastrointestinal adenocarcinoma. JAMA. 2000;284:961–62. doi: 10.1001/jama.284.8.961. [DOI] [PubMed] [Google Scholar]
- 68.Lofdahl HE, Lu Y, Lagergren J. Sex-specific risk factor profile in oesophageal adenocarcinoma. Br J Cancer. 2008;99:1506–10. doi: 10.1038/sj.bjc.6604701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryan AM, Rowley SP, Fitzgerald AP, Ravi N, Reynolds JV. Adenocarcinoma of the oesophagus and gastric cardia: male preponderance in association with obesity. Eur J Cancer. 2006;42:1151–58. doi: 10.1016/j.ejca.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 70.Steffen A, Schulze MB, Pischon T, et al. Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18:2079–89. doi: 10.1158/1055-9965.EPI-09-0265. [DOI] [PubMed] [Google Scholar]
- 71.Steevens J, Schouten LJ, Driessen AL, et al. A prospective cohort study on overweight, smoking, alcohol consumption, and risk of Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2001;20:345–58. doi: 10.1158/1055-9965.EPI-10-0636. [DOI] [PubMed] [Google Scholar]
- 72.O’Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut. 2012;61:1261–68. doi: 10.1136/gutjnl-2011-300551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El-Serag HB, Kvapil P, Hacken-Bitar J, Kramer JR. Abdominal obesity and the risk of Barrett's esophagus. Am J Gastroenterol. 2005;100:2151–56. doi: 10.1111/j.1572-0241.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 74.Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404–13. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]