Abstract
Patients treated with erythropoietin-based erythropoiesis-stimulating agents (ESAs) can develop a rare but life-threatening condition called antibody-mediated pure red cell aplasia (amPRCA). The antibody characteristics in a nephrology patient with amPRCA include high antibody concentrations with neutralizing activity and a mixed IgG subclass including anti-ESA IgG4 antibodies. In contrast, anti-ESA IgG4 antibody is generally not detected in baseline samples and antibody-positive non-PRCA patients. Therefore, we validated a highly sensitive immunoassay on the ImmunoCAP 100 instrument to quantitate anti-ESA IgG4 antibodies using a human recombinant anti-epoetin alfa (EPO) IgG4 antibody as a calibrator. The biotinylated ESA was applied to a streptavidin ImmunoCAP, and bound anti-ESA IgG4 antibodies were detected using a β-galactosidase-conjugated mouse anti-human IgG4 antibody. The validated assay was used to detect anti-ESA IgG4 in amPRCA and non-PRCA patients. The immunoassay detected 15 ng/ml of human anti-EPO IgG4 antibody in the presence of a 200 M excess of human anti-ESA IgG1, IgG2, or IgM antibody and tolerated 2 μg/ml of soluble erythropoietin. All patient samples with confirmed amPRCA had measurable anti-ESA IgG4 antibodies. In addition, 94% (17/18) of non-PRCA patient samples were antibody negative or had below 15 ng/ml of anti-ESA IgG4 antibodies. This novel immunoassay can measure low-nanogram quantities of human anti-ESA IgG4 antibodies in the presence of other anti-ESA antibodies. An increased concentration of anti-ESA IgG4 antibody is associated with the development of amPRCA. We propose that the measurement of anti-ESA specific IgG4 antibodies may facilitate early detection of amPRCA in patients receiving all ESAs structurally related to human erythropoietin.
INTRODUCTION
Testing for anti-erythropoiesis-stimulating agent (anti-ESA) antibodies is critical to monitor ESA safety and efficacy during clinical development and in a postmarket setting (1). A variety of analytical immunoassay methods to detect and characterize antidrug antibodies (ADAs) have been described. Each screening method offers its own unique advantages and disadvantages (2). The most commonly used immunoassay methods in the industry for detection of binding antibodies (BAbs) are the enzyme-linked immunosorbent assay (ELISA), radioimmunoprecipitation assay (RIPA), electrochemiluminescence (ECL) assay, and surface plasmon resonance immunoassay (SPRIA), all of which have been demonstrated to detect the pathogenic antibodies in patients who develop antibody-mediated pure red cell aplasia (amPRCA) (3). These immunological antibody tests along with a bioassay to confirm neutralizing antibodies (NAbs) in an antibody-positive sample constitute one of a battery of methods to differentially diagnose the development of amPRCA from other causes of PRCA (4).
Although ESAs are generally well tolerated, rare cases of amPRCA have been reported (5, 6). The antibody response to ESAs structurally related to erythropoietin in patients who develop amPRCA has been previously characterized using a SPRIA and has been demonstrated to be a mixed IgG response in which IgG1 and IgG4 are predominant (6, 7). Of most importance, these antibodies cross-react and neutralize the endogenous erythropoietin and all recombinant erythropoietin-based ESAs. As a result of this broad cross-reactivity, patients with amPRCA develop resistance to endogenous erythropoietin and all recombinant erythropoietin-based ESAs. Therefore, after confirmation of amPRCA, it is recommended that treatment with any erythropoietin-based ESA should be immediately discontinued (8). An anti-ESA IgG1 antibody response appears in some antibody-positive non-PRCA patients but is also present with the detection of IgG4 in patients who develop amPRCA (3, 9). Although the IgG1 response is considered to precede the IgG4 response, the switch is driven by the repeated and prolonged exposure to the ESA. This is also well illustrated by the analysis of antibody to grass pollen and bee venom in novice beekeepers (10). The long-term administration of biological therapeutics such as beta interferon (IFN-β) 1b to multiple sclerosis patients (11) and factor VIII to hemophilia A patients (12) results in the development of IgG4 ADA. The development of anti-ESA IgG4 antibodies against erythropoietin-based ESAs is best studied in the nephrology patient population and has been shown to be coincident with amPRCA (3, 6, 9).
In general, serum concentrations of the IgG subclasses are not evenly distributed. The serum concentration ranges in normal adults for IgG1, IgG2, and IgG3 are 3.8 to 9.3 mg/ml, 2.4 to 7.0 mg/ml, and 0.22 to 1.76 mg/ml, respectively. The total IgG4 antibody is the least abundant in serum (4% of total IgG), with a normal range of 0.04 to 0.86 mg/ml in human serum (13). The appearance of drug-specific IgG antibodies generally corresponds with the maturation of a secondary antibody response upon repeated exposure and generally elicits a mixed IgG subclass response (14). The prevalence of the IgG subclasses can be antigen specific, and the chronic exposure to a protein has been shown to cause development of an IgG4 isotype restriction (15).
In the case of the antibody response to ESAs, the greatest analytic challenge with the current immunological methods is the ability to measure the low abundance of anti-ESA-specific IgG4 antibodies in the presence of much higher concentrations of the other ESA-specific IgG subclasses. The only published method to detect, but not quantitate, the anti-ESA antibody isotype is the SPRIA methodology (7). The challenge is that the more predominant isotypes such as IgG1 and IgG2 saturate the ESA-coated surface, making it difficult to detect the less abundant anti-IgG4 antibodies.
In this paper, we discuss the development of a highly sensitive and specific immunoassay for the measurement of anti-ESA IgG4 antibodies using the ImmunoCAP technology. The technology has existed for more than 50 years and has been used successfully to detect specific IgE antibodies to allergens (16) and more recently to detect antigen-specific IgG4 antibodies (17). The advantage of this technology is the large binding capacity, allowing the quantitation of low-level antigen-specific antibody isotypes such as IgG4 and IgE in a pool of other specific antibodies. Here, we have utilized this technology to develop a sensitive and specific immunoassay to measure the anti-ESA IgG4 antibody in patients administered an erythropoietin-based ESA.
MATERIALS AND METHODS
Sample selection and classification.
Sixty normal human serum samples and pooled normal human serum (PNHS) were obtained from Bioreclamation (Hicksville, NY) for assay validation. A total of 25 human serum samples were compiled from clinical studies (n = 6) and postmarketed safety samples from patients treated with an ESA (n = 19), of which 8 patient samples from the postmarket setting were classified as having amPRCA. All specimens from patients treated with an ESA were provided by Amgen Inc. (Thousand Oaks, CA). Patients were classified into 2 groups: antibody-mediated PRCA (amPRCA) patients and non-PRCA patients. Patients positive at 1 or more time points for neutralizing antibodies and diagnosed as having erythroid precursor deficiency by bone marrow biopsy were classified as having amPRCA. Patients with diagnosed PRCA by bone biopsy but antibody negative were not included in this analysis. Patients who had received and responded to ESA treatment and who tested antibody positive in the immunoassay but negative throughout ESA treatment for neutralizing antibodies were classified as non-PRCA patients. All patients provided consent to Amgen, Inc., for serum sample collection and testing for anti-ESA antibodies.
ImmunoCAP 100 method.
The anti-ESA IgG4 antibodies were measured using the ImmunoCAP 100 (Phadia AB, Uppsala, Sweden). In this method, streptavidin-conjugated ImmunoCAPs (catalog number R0121) were coated with 50 μl of 10-μg/ml biotinylated epoetin alfa (EPO) (Amgen, Thousand Oaks, CA). The addition of biotinylated EPO and subsequent incubations and washing steps were performed on the instrument by use of manual instrument programming instructions. The test serum samples were diluted 1:10 using sample diluent (catalog number 10-9498-0), incubated for 1 h, and washed with Phadia washing solution (catalog number 10-9422-01), and then β-galactosidase-conjugated anti-human IgG4-specific conjugate (catalog number 10-9465-02) was added to each ImmunoCAP. The reaction mixture was incubated for 1 h and washed, and then development solution (catalog number 10-9478-01) was added and incubated for 1 h. Finally, a stop solution (catalog number 10-9479-01) was added to each ImmunoCAP, and the chemiluminescence was read by the instrument and reported in response units (RU). The human IgG4 concentrations in the test samples were extrapolated from the calibrator curve generated using the GraphPad Prism v. 5.0 software.
Human antibody calibrator.
A panel of recombinant human anti-ESA antibodies was developed by Amgen (Thousand Oaks, CA) and used in the validation. The recombinant human antibodies included 8C10 IgG1 (8C10G1), 8C10 IgG2 (8C10G2), 8C10 IgG4 (8C10G4), and 11D12 monomeric IgM. The human anti-ESA antibody 8C10G4 was used as the calibrator to generate the standard curve and quality control (QC) standards in the assay. The details of the antibody generation and characterization were previously described (18).
Validation of the ImmunoCAP 100 assay.
Assay validation parameters followed published recommendations for antidrug antibody immunoassays (19).
ACP.
The assay cut point (ACP) is defined as the signal level that differentiates samples that are negative from those that are potentially positive. A total of 60 normal human serum samples were tested in duplicate to generate the ACP. The ACP was established by calculating the upper bound of a one-sided 95% reference interval for the distribution of donor raw chemiluminescence response units (mean RU + 1.645 standard deviation).
Sensitivity and precision.
The anti-ESA IgG4 antibody standard curve was analyzed over multiple days. The data from 13 individual dose-response curves with concentrations ranging from 100 to 0.005 μg/ml were analyzed using a 4-parameter logistic nonlinear regression model in GraphPad Prism v. 5.0, generating the mean standard curve. Intersection of the curve at the ACP value yielded the assay sensitivity. The precision of each concentration on the curve was then calculated as a percent coefficient of variation (CV).
LLRD.
Pilot experiments to determine the lower limit of reliable detection (LLRD) were performed by spiking human anti-ESA IgG4 antibody into 6 individual human serum samples at various concentrations to determine the recovery above the ACP. Based on the pilot experiment, a more rigorous spiking experiment was then performed with 30 individual serum samples to confirm 100% recovery above the ACP.
Specificity.
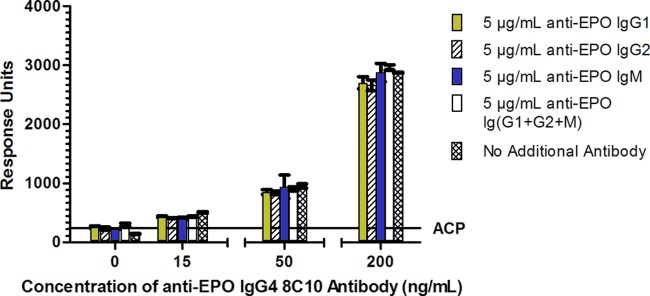
A range of anti-ESA specific antibody isotypes can be present in a patient sample. To replicate what may be found in an antibody-positive patient, 5 μg/ml of each anti-ESA antibody 8C10G1, 8C10G2, and 11D12 IgM or a cocktail of the three antibodies was spiked into PNHS containing 0, 15, 50, and 200 ng/ml of specific human anti-ESA antibody 8C10G4.
Drug tolerance.
The ESA dose administered to patients can vary, and high serum levels can interfere with the measurement of the anti-ESA IgG4 antibodies. Therefore, the method was evaluated to detect anti-ESA IgG4 antibody in the presence of soluble ESA. PNHS was spiked with 250 ng/ml and 15 ng/ml of human anti-ESA 8C10G4 antibody in the presence of various concentrations of ESA.
Statistical analysis.
The Wilcoxon two-sample exact test was applied to compare the anti-IgG4 concentrations between amPRCA and non-PRCA patients. Since this is a nonparametric test, the P value for this test was reported and the magnitude of the difference and the confidence interval of the difference were not reported.
RESULTS
Validation.
During assay validation, there were three key parameters that were critical to assess the feasibility of this assay: adequate sensitivity, the ability to measure the specific anti-ESA IgG4 in the presence of other anti-ESA-specific isotypes, and tolerance to soluble ESA. To determine these parameters, the ACP was first established. Based on 60 human samples, the mean ACP plus 1.645 standard deviations was calculated to be 243.75 response units. The human anti-ESA IgG4 antibody calibrator 8C10G4 was then spiked into PNHS from 100 to 0.005 μg/ml of anti-ESA IgG4 antibody and assayed in duplicate on 13 different days. A plot of the human 8C10G4 antibody concentration against response unit is shown in Fig. 1. At each antibody concentration on the curve, the precision was less than 25% CV (data not shown). It is also important to note that the human anti-ESA IgG4 calibrator was compared with the generic ImmunoCAP human IgG4 antibody calibrator, and both demonstrated parallelism (data not shown). The assay sensitivity, based on the antibody concentration intersecting the ACP, was determined to be 7 ng/ml of 8C10G4. At a spiked concentration of 15 ng/ml of human antibody 8C10G4 in 30 individual serum samples, all 30 spiked samples recovered above the ACP, confirming that the assay can reliably detect 15 ng/ml of anti-ESA IgG4 antibodies (data not shown). Since the antibody characteristics observed in patients with amPRCA present with a mixed IgG subclass response, the measurement of ESA-specific IgG4 antibody in the presence of excess ESA-specific IgG1, IgG2, and IgM antibodies was confirmed. The data presented in Fig. 2 show that despite the presence of the excess of other anti-ESA specific antibodies, the assay is capable of detecting 15 ng/ml of anti-ESA IgG4 antibody, even in the presence of 5 μg/ml of each of the ESA-specific IgG1, IgG2, and IgM mixed isotype. Therefore, the assay is highly specific and sensitive. Finally, soluble ESA in the serum sample can interfere with antibody detection in other assays. As shown in Fig. 3, we have demonstrated that 250 ng/ml of human antibody 8C10G4 can be detected in the presence of 10 μg/ml of excess ESA. Validation experiments also demonstrated the reproducible detection of 15 ng/ml of human antibody 8C10G4 in the presence of 2 μg/ml of soluble drug (data not shown).
Fig 1.
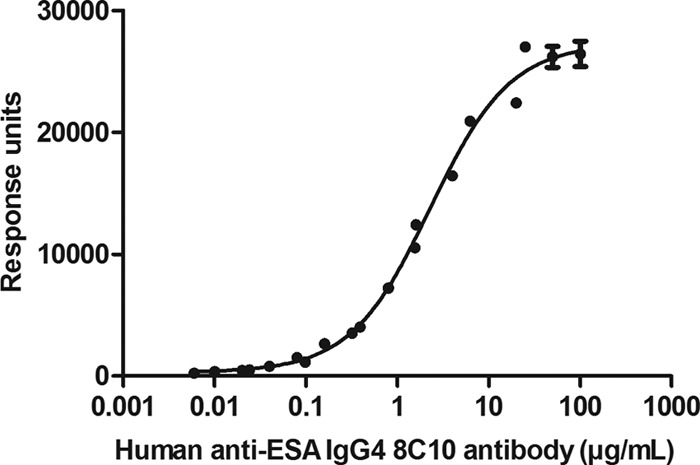
Graph representing the signal (in response units) relative to the PNHS spiked with 0.0025 to 100 μg/ml of human anti-ESA antibody 8C10G4. A total of 13 curves were analyzed in duplicate. The graph was constructed using a 4-parameter logistic nonlinear regression model in GraphPad Prism v. 5.0. The response unit per concentration is reported with the standard error of the mean (SEM).
Fig 2.
Graphical representation of the measurement of 0, 15, 50, and 200 ng/ml of human antibody 8C10G4 in the presence of 5 μg/ml of anti-ESA antibodies IgG1, IgG2, and IgM as well as a cocktail containing 5 μg/ml each of IgG1, IgG2, and IgM.
Fig 3.
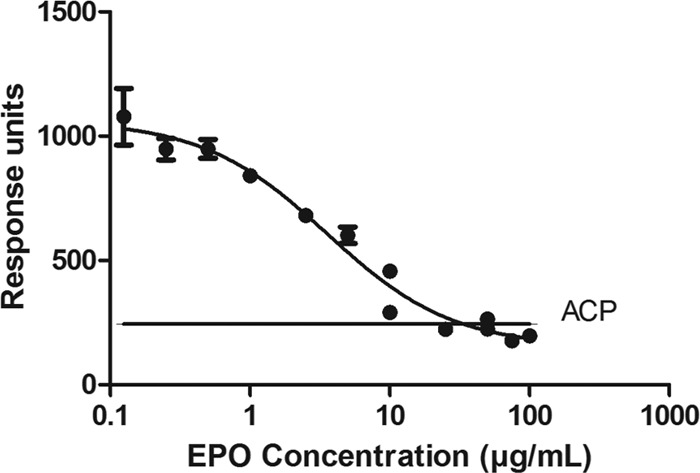
Pooled normal human serum samples containing 250 ng/ml of human anti-ESA antibody 8C10G4 spiked with 0 to 100 μg/ml of epoetin alfa. The response unit readout is displayed on the y axis, and the concentration of epoetin alfa (in μg/ml) is displayed on a logarithmic scale on the x axis. The solid line indicates the ACP (243.75).
Clinical sample analysis.
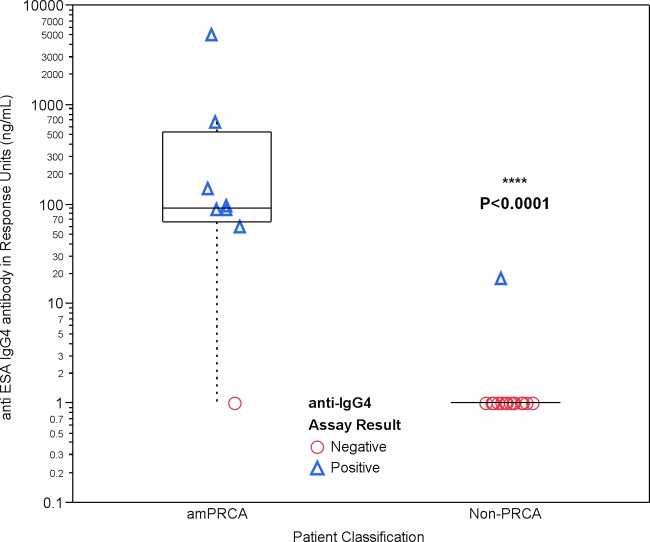
To evaluate the capability of the ImmunoCAP 100 assay to detect anti-ESA IgG4 antibody in the clinical patient population, a subset of serum samples previously tested and characterized in the SPRIA were analyzed using the ImmunoCAP 100 assay. The results from both methods are reported in Table 1. Eight patient samples had confirmed amPRCA. Six of 6 patient samples (100%) classified as having amPRCA had a confirmed anti-ESA IgG4 antibody in the SPRIA and also had measurable anti-ESA IgG4 antibody in the ImmunoCAP 100 assays, with the lowest concentration measured at 89 ng/ml of anti-ESA IgG4 antibody. In addition, one amPRCA patient without a confirmed isotype by SPRIA (sample 16) had 59 ng/ml of anti-ESA IgG4 antibody by ImmunoCAP 100; one amPRCA patient with a confirmed anti-ESA IgG1 antibody by SPRIA (sample 17) did not have measurable anti-ESA IgG4. In the non-PRCA patient population, all 17 patient samples tested anti-ESA IgG4 negative except one (sample 18), which scored IgG1 positive by SPRIA but had low-level anti-ESA IgG4 antibody (18 ng/ml) by the ImmunoCAP 100 assay. Despite the low level of measurable IgG4 in this one sample from the non-PRCA patient samples, statistical analysis of the median anti-ESA IgG4 antibody concentrations in the non-PRCA and amPRCA patient populations resulted in a statistically significant (P < 0.0001) difference (Fig. 4).
Table 1.
Results from the SPRIA and ImmunoCAP 100 anti-ESA IgG4 assays and a cell-based assay for neutralizing antibodiesa
| Sample | SPRIA |
ImmunoCAP 100 |
Patient classification based on NAb assay | |||
|---|---|---|---|---|---|---|
| Result | Isotype(s) | Anti-ESA Ab (ng/ml) | Result | Anti-ESA IgG4 Ab (ng/ml) | ||
| 1 | Positive | IgG1, IgG2, IgG3, IgG4 | >10,000 | Positive | 145 | amPRCA |
| 2 | Negative | Negative | Non-PRCA | |||
| 3 | Positive | IgG1, IgG2, IgG4 | 6,030 | Positive | 89 | amPRCA |
| 4 | Positive | IgG1, IgG2, IgG4 | 620 | Positive | 89 | amPRCA |
| 5 | Positive | IgG1, IgG2, IgG3, IgG4 | >10,000 | Positive | 669 | amPRCA |
| 6 | Negative | Negative | Non-PRCA | |||
| 7 | Positive | IgG1 | 600 | Negative | Non-PRCA | |
| 8 | Positive | IgG1 (IgG4b) | 1,730 | Positive | 95 | amPRCA |
| 9 | Negative | Negative | Non-PRCA | |||
| 10 | Negative | Negative | Non-PRCA | |||
| 11 | Negative | Negative | Non-PRCA | |||
| 12 | Negative | Negative | Non-PRCA | |||
| 13 | Positive | Unable to determine | 340 | Negative | Non-PRCA | |
| 14 | Negative | Negative | Non-PRCA | |||
| 15 | Negative | Negative | Non-PRCA | |||
| 16 | Positive | Unable to determine | 520 | Positive | 59 | amPRCA |
| 17 | Positive | IgG1 | 6,860 | Negative | amPRCA | |
| 18 | Positive | IgG1 | 840 | Positive | 18 | Non-PRCA |
| 19 | Positive | IgG1 | 3,690 | Negative | Non-PRCA | |
| 20 | Positive | IgM | 920 | Negative | Non-PRCA | |
| 21 | Positive | IgG1 | 1,210 | Negative | Non-PRCA | |
| 22 | Positive | IgM | 1.94 | Negative | Non-PRCA | |
| 23 | Positive | IgG3 | 700 | Negative | Non-PRCA | |
| 24 | Positive | IgG1 | 0.93 | Negative | Non-PRCA | |
| 25 | Positive | IgG1, IgG2, IgG3, IgG4 | >10,000 | Positive | 4,978 | amPRCA |
All patient samples that had an anti-ESA concentration of greater than 250 ng/ml by SPRIA were further characterized for isotype (IgG1, IgG2, IgG3, IgG4, and IgM) and tested in a cell-based bioassay to determine if neutralizing antibodies were present. All patient samples were tested in the ImmunoCAP anti-ESA IgG4 assay. All samples that that had less than 15 ng/ml of anti-ESA IgG4 were reported as negative.
This patient tested anti-ESA IgG4 antibody positive at a subsequent time point.
Fig 4.
Graphical representation of a Wilcoxon two-sample test, showing the distribution of the anti-ESA IgG4 concentration (in ng/ml) displayed on the y axis and the patient classification of amPRCA and non-PRCA patients on the x axis.
DISCUSSION
The diagnosis of amPRCA, although rare, has been characterized by the development of a high anti-ESA antibody concentration with neutralizing capacity and a mixed IgG subclass, including IgG4 (3). Despite this knowledge, longitudinal sampling from the early onset up to months prior to amPRCA has not been well documented. In the postmarket setting, samples are collected and sent for anti-ESA antibody testing after other causes of PRCA have been ruled out (20), resulting in a gap in our understanding of the time course between onset and full-blown amPRCA.
The analysis of samples from amPRCA and non-PRCA patients that tested antibody positive in the SPRIA was of most interest. We have previously shown that there is a strong statistical correlation between the anti-ESA antibody concentration and patients with amPRCA (3) and the presence of anti-ESA IgG4 and anti-ESA neutralizing antibody activity in amPRCA patients (9). Here, we have demonstrated a strong correlation between the anti-ESA IgG4 antibody concentration and patients with amPRCA. The data presented here demonstrate that using the ImmunoCAP technology, we can measure the concentration of anti-ESA specific IgG4 antibodies in a serum sample. Utilizing the human anti-ESA IgG4 antibody 8C10G4 as a calibrator, the quantitation of the anti-ESA-specific antibody in a serum sample can measure 15 ng/ml of anti-ESA IgG4 antibody. All samples below the 15-ng/ml concentration are considered anti-ESA IgG4 negative. We believe that the high surface capacity of the streptavidin ImmunoCAP coated with biotinylated epoetin alfa provides low-nanogram sensitivity to measure anti-ESA IgG4 antibody in the presence of a 200 M excess of ESA-specific IgG1 and IgG2 antibodies. This large surface binding capacity also allows for the detection of 15 ng/ml of anti-ESA IgG4 antibody in the presence of 2 μg/ml of excess soluble ESA.
The ImmunoCAP 100 assay proved to be more sensitive than the SPRIA, detecting more anti-ESA IgG4 antibody in both patient populations than SPRIA. In the amPRCA population (n = 8), 6 patient samples with detectable IgG4 by SPRIA all had measurable anti-ESA IgG4 greater than or equal to 89 ng/ml. In addition, anti-ESA IgG4 antibody was measured in a patient sample (sample 16) with confirmed amPRCA but no confirmed isotype by SPRIA. Based on the low antibody concentration in the SPRIA, this was not unexpected due to the lack of sensitivity around the SPRIA isotype confirmation assay and the high specificity and sensitivity of the ImmunoCAP assay. One additional patient sample (sample 17) with confirmed amPRCA demonstrated a high antibody concentration by SPRIA and a confirmed anti-ESA IgG1 antibody. The analysis for anti-ESA IgG4 antibody was negative. An investigation of the patient history revealed that this patient was treated with an ESA for correction of anemia associated with ribavirin and pegylated alfa interferon 2B treatment for a hepatitis C virus (HCV) infection. The prevalence of amPRCA in this patient population has been reported recently in the literature (21). There appears to be a predominance of anti-ESA IgG1 antibody and a lack of IgG4 observed in this patient population. Therefore, the measurement of anti-ESA IgG4 in this patient population may be different than what has been observed in the nephrology setting. Until more information is available, the application of this anti-ESA IgG4 assay in this patient population may not be appropriate. It is worth noting that antibodies to ESAs structurally different from human erythropoietin, such as peginesatide (also known as Hematide), a mimetic of erythropoietin for treatment of anemia in chronic kidney disease (CKD) patients, would not cross-react with erythropoietin-based ESAs and therefore would not be detected in this assay (22).
All non-PRCA patient samples that were antibody negative (n = 8) or antibody positive (n = 17) by SPRIA (Table 1) also scored negative for anti-ESA IgG4 antibody in the ImmunoCAP 100 assay, except for one patient sample (sample 18). This sample was borderline positive for anti-ESA IgG4, just above the 15-ng/ml assay limit. It would have been interesting to repeat the analysis to confirm the reproducibility of the analysis, but additional sample volume was not available. In spite of the predominant IgG1 detected by the SPRIA, this patient received an ESA for chronic kidney disease and did not exhibit a reduced response to the ESA treatment.
In summary, the long-term administration of an erythropoietin-based ESA can result in a rare but life-threatening condition called antibody-mediated pure red cell aplasia. With the utilization of the ImmunoCAP technology, we have shown that the anti-ESA IgG4 antibody concentration is associated with amPRCA. With the availability of a human anti-ESA IgG4 antibody as a calibrator, we have developed an immunoassay with wide dynamic range and assay sensitivity down to 15 ng/ml of anti-ESA specific IgG4 antibody in human serum. Since the total and drug-specific IgG4 antibody is generally a minor component of the total IgG concentration, we achieved the reproducible measurement of anti-ESA IgG4 in the presence of more prevalent anti-ESA specific IgG1 and IgG2 antibody as well as soluble ESA. Most importantly, the lack of anti-ESA IgG4 antibody in the non-PRCA clinical patient population and the measurement of ≥89 ng/ml of anti-ESA IgG4 in the amPRCA population were very encouraging. Despite these positive results, a more robust analysis of both non-PRCA and amPRCA patients across different patient populations is needed. In this study, we did not assess if the anti-ESA IgG4 antibody population is actually involved in the neutralization of ESAs, and therefore further investigation is needed. It is hoped that with a more extensive data set, this highly sensitive and specific ImmunoCAP assay can provide a positive predictive anti-ESA IgG4 concentration that can be used to facilitate the early detection of amPRCA.
ACKNOWLEDGMENTS
We thank Lei Zhou for performing the statistical analysis. We thank Steve Swanson and Troy Barger for reviewing the manuscript and providing valuable feedback.
Footnotes
Published ahead of print 31 October 2012
REFERENCES
- 1. Koren E, Zuckerman LA, Mire-Sluis AR. 2002. Immune responses to therapeutic proteins in humans—clinical significance, assessment and prediction. Curr. Pharm. Biotechnol. 3:349–360 [DOI] [PubMed] [Google Scholar]
- 2. Thorpe R, Swanson SJ. 2005. Current methods for detecting antibodies against erythropoietin and other recombinant proteins. Clin. Diagn. Lab. Immunol. 12:28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barger TE, Kuck AJ, Chirmule N, Swanson SJ, Mytych DT. 2012. Detection of anti-ESA antibodies in human samples from PRCA and non-PRCA patients: an immunoassay platform comparison. Nephrol. Dial. Transplant. 27:688–693 [DOI] [PubMed] [Google Scholar]
- 4. Casadevall N, Cournoyer D, Marsh J, Messner H, Pallister C, Parker-Williams J, Rossert J. 2004. Recommendations on haematological criteria for the diagnosis of epoetin-induced pure red cell aplasia. Eur. J. Haematol. 73:389–396 [DOI] [PubMed] [Google Scholar]
- 5. Schellekens H, Jiskoot W. 2006. Eprex-associated pure red cell aplasia and leachates. Nat. Biotechnol. 24:613–614 [DOI] [PubMed] [Google Scholar]
- 6. Swanson SJ, Ferbas J, Mayeux P, Casadevall N. 2004. Evaluation of methods to detect and characterize antibodies against recombinant human erythropoietin. Nephron Clin. Pract. 96:c88–c95 [DOI] [PubMed] [Google Scholar]
- 7. Mytych DT, La S, Barger T, Ferbas J, Swanson SJ. 2009. The development and validation of a sensitive, dual-flow cell, SPR-based biosensor immunoassay for the detection, semi-quantitation, and characterization of antibodies to darbepoetin alfa and epoetin alfa in human serum. J. Pharm. Biomed. Anal. 49:415–426 [DOI] [PubMed] [Google Scholar]
- 8. Eckardt KU, Casadevall N. 2003. Pure red-cell aplasia due to anti-erythropoietin antibodies. Nephrol. Dial. Transplant. 18:865–869 [DOI] [PubMed] [Google Scholar]
- 9. Barger T, Wrona D, Goletz T, Mytych DT. 2012. A detailed examination of the antibody prevalence and characteristics of anti-ESA antibodies. Nephrol. Dial. Transplant. 27:3892–3899 [DOI] [PubMed] [Google Scholar]
- 10. Aalberse RC, van der Gaag R, van Leeuwen J. 1983. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J. Immunol. 130:722–726 [PubMed] [Google Scholar]
- 11. Deisenhammer F, Reindl M, Berger T. 2001. Immunoglobulin subclasses in patients with neutralizing and nonneutralizing antibodies against IFN-beta1b. J. Interferon Cytokine Res. 21:167–171 [DOI] [PubMed] [Google Scholar]
- 12. van Helden PM, van den Berg HM, Gouw SC, Kaijen PH, Zuurveld MG, Mauser-Bunschoten EP, Aalberse RC, Vidarsson G, Voorberg J. 2008. IgG subclasses of anti-FVIII antibodies during immune tolerance induction in patients with hemophilia A. CORD Conf. Proc. 142:644–652 [DOI] [PubMed] [Google Scholar]
- 13. French MM. 1986. Serum IgG subclasses in normal adults. Monogr. Allergy 19:100–107 [PubMed] [Google Scholar]
- 14. Stavnezer JJ. 2000. Molecular processes that regulate class switching. Curr. Top. Microbiol. Immunol. 245:127–168 [DOI] [PubMed] [Google Scholar]
- 15. Shakib FF. 1986. The IgG4 subclass. Monogr. Allergy 19:223–226 [PubMed] [Google Scholar]
- 16. Maloney JM, Rudengren M, Ahlstedt S, Bock SA, Sampson HA. 2008. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J. Allergy Clin. Immunol. 122:145–151 [DOI] [PubMed] [Google Scholar]
- 17. Ito K, Futamura M, Moverare R, Tanaka A, Kawabe T, Sakamoto T, Borres MP. 2012. The usefulness of casein-specific IgE and IgG4 antibodies in cow's milk allergic children. Clin. Mol. Allergy 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mytych DT, Barger TE, King C, Grauer S, Haldankar R, Hsu E, Wu MM, Shiwalkar M, Sanchez S, Kuck A, Civoli F, Sun J, Swanson SJ. 2012. Development and characterization of a human antibody reference panel against erythropoietin suitable for the standardization of ESA immunogenicity testing. J. Immunol. Methods 382:129–141 [DOI] [PubMed] [Google Scholar]
- 19. Mire-Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M, Parish T, Scott G, Shankar G, Shores E, Swanson SJ, Taniguchi G, Wierda D, Zuckerman LA. 2004. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J. Immunol. Methods 289:1–16 [DOI] [PubMed] [Google Scholar]
- 20. Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian J-J, Martin-Dupont P, Michaud P, Papo T, Ugo V, Teyssandier I, Varet B, Mayeux P. 2002. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med. 346:469–475 [DOI] [PubMed] [Google Scholar]
- 21. Miura Y, Kami M, Yotsuya R, Toda N, Komatsu T. 2008. Pure red-cell aplasia associated with pegylated interferon-alpha-2b plus ribavirin. CORD Conf. Proc. 83:758–759 [DOI] [PubMed] [Google Scholar]
- 22. Woodburn KW, Fan Q, Winslow S, Chen MJ, Mortensen RB, Casadevall N, Stead RB, Schatz PJ. 2007. Hematide is immunologically distinct from erythropoietin and corrects anemia induced by antierythropoietin antibodies in a rat pure red cell aplasia model. Exp. Hematol. 35:1201–1208 [DOI] [PubMed] [Google Scholar]