Abstract
An oral gene-based avian influenza vaccine would allow rapid development and simplified distribution, but efficacy has previously been difficult to achieve by the oral route. This study assessed protection against avian influenza virus challenge using a chimeric adenovirus vector expressing hemagglutinin and a double-stranded RNA adjuvant. Immunized ferrets and mice were protected upon lethal challenge. Further, ferrets immunized by the peroral route induced cross-clade neutralizing antibodies, and the antibodies were selective against hemagglutinin, not the vector. Similarly, experiments in mice demonstrated selective immune responses against HA with peroral delivery and the ability to circumvent preexisting vector immunity.
INTRODUCTION
Avian influenza vaccines currently in clinical development are designed for administration by injection and are often manufactured by the traditional egg-based technology that is used for annual influenza vaccines (1). The current mortality rate of avian influenza is approximately 60%. While human-to-human transmission has not been substantially documented, recent studies suggest that the development of mammalian-transmissible strains is not impossible (2, 3). An oral vaccine that could be delivered in pill form and is manufactured in cell culture has many potential advantages for controlling pandemic influenza, including faster vaccine production against evolving viral strains and easier distribution without the need for skilled medical personnel. One option for creating such a vaccine is to use a replication-deficient adenovirus (Ad) carrying an antigen of choice. Adenovirus-based vaccines can be manufactured rapidly, are less sensitive to changes in antigen sequence than influenza virus grown in eggs, and can have substantial long-term stability. Orally delivered adenoviral vaccines have a long safety record of use in the U.S. military; enterically coated wild-type adenovirus strains 4 and 7 were used from the 1970s until the late 1990s for peroral (p.o.) vaccination of basic trainees as protection against severe respiratory infection (reviewed by Tucker et al. [4]). In addition, oral administration of adenovirus-based vaccines has been shown to be effective even in the presence of preexisting systemic immunity (5).
Despite the attractiveness of using recombinant adenovirus (rAd) as an oral delivery vector, previous studies have reported inefficient vaccine performance (5–8). Antigen recognition within the intestinal microenvironment has been difficult to achieve but can potentially be improved by increasing the innate immunostimulatory response through the use of adjuvants (9). The adjuvant we have tested for this purpose is double-stranded RNA (dsRNA). Toll-like receptor 3 (TLR3) is one of the major receptors for pathogenic dsRNA and can be found on the endosomes of dendritic cells (10) and on intestinal epithelial cells (11). In this paper, we evaluate whether oral delivery of a rAd type 5 (rAd5) vector that expresses both the hemagglutinin (HA) gene of influenza virus and a dsRNA hairpin adjuvant induces immune responses and is protective in both mouse and ferret lethal-challenge models of influenza. Further we have investigated whether this technology can be used to deliver antigens without inducing vector immunity.
MATERIALS AND METHODS
Cell lines.
HEK293 (Molecular Medicine Bioservices, Carlsbad, CA) and HeLa (ATCC) cells were propagated in Dulbecco's modified Eagle medium (DMEM) plus GlutaMax (Invitrogen, Carlsbad, CA) supplemented with 10% bovine serum (BS) and antibiotics (10 units/ml penicillin, 10 μg/ml streptomycin).
Adenovirus propagation and characterization.
Adenovirus subtype 5 vectors with E1 and E3 deleted were produced using the AdEasy (Qbiogene, Carslbad, CA) kit as described in the manufacturer's handbook. The shuttle vector pShuttle-CMV (Qbiogene, Quebec, Canada), expressing HIV gp120 (NIH AIDS Reference and Reagent Program), codon-optimized HA (A/Indo/05/2005 (A/Indo), synthesized by CelTek, [Nashville, TN]), and Venezuelan equine encephalomyelitis (VEE) virus structural genes E3, E2, and 6K (12), was constructed by standard molecular methods. A separate vector expressing the dsRNA species luc1 under the cytomegalovirus (CMV) promoter was made by overlapping oligonucleotide primers GAAACGATATGGGCTGAATAC and a short 6-bp spacer between them that forms the loop of the hairpin. A shuttle vector expressing both luc1 and HA was also made. The shuttle vectors were linearized with PmeI and transferred into an adenoviral genome by homologous recombination in bacterial strain BJ5183-AD1 (Stratagene, La Jolla, CA). Recombinants were selected based on XbaI/SpeI restriction digests and kanamycin selection. The recombinant adenoviral constructs were then PacI digested and transfected into HEK293 cells by calcium-phosphate transfection (13). The next day, the cells were split into four 60-mm plates and grown for 5 to 10 days until viral plaques became apparent. Individual plaques were isolated and screened for viral growth in HEK293 cells. After plaque selection, the infected monolayers were harvested, freeze-thawed 4 times at −80°C and 37°C, and centrifuged, and the supernatant was harvested. One milliliter of this primary viral stock was used to infect 5 × 106 cells in a T-75 flask. The virus was further expanded in three T-175 flasks before the final quaternary infection using 6 to 40 T-175 flasks. Cells were harvested by centrifugation at 500 × g for 5 min and resuspended in 10 ml of PBS. A cell lysate was prepared by 4 cycles of freeze-thawing, and the supernatant was clarified by centrifugation at 1,500 × g for 10 min and then by filtration through a 0.4-μm syringe filter. The virus was then purified by ion-exchange chromatography using a ViraBind adenovirus purification kit (Cell Biolabs, Inc., San Diego, CA). When larger amounts of vector were required, as was the case for the ferret studies, the vectors were purified using the Adeno X kit from Clontech (Mountain View, CA). The vector was resuspended in 25 mM Tris, pH 7.5, 2.5 mM MgCl2, 1 M NaCl, and glycerol was added to 10% (vol/vol). The viral titer (infectious units/ml) was determined using a QuickTiter adenovirus immunoassay kit (Cell Biolabs, Inc.). Replication-competent adenovirus (RCA) was determined by infecting A549 cells with purified vectors and monitoring for cytopathic effects (CPE). An E1A PCR method was used as an additional level of confirmation (14).
Animal experiments.
Animal research was approved by the Institutional Animal Care and Use Committees (IACUC) at Vaxart and Southern Research Institute (Birmingham, AL). Six- to 7-week-old BALB/c mice were acquired from Taconic (NY) and vaccinated perorally (p.o.), similar to the method described by other investigators (15, 16). Briefly, 0.2 ml of 7.5% sodium bicarbonate was given by 24-gauge feeding tube (Fine Science Tools, Foster City, CA), followed less than a minute later with rAd in 0.2 to 0.3 ml of PBS. Intranasal (i.n). and intramuscular (i.m.) vaccinations were performed similarly to those described by Moore et al. (17). For the dosage, 1.0 × 107 IU per mouse was given unless specifically stated otherwise. Plasma samples were acquired by cheek pouch lancet (Medipoint, Mineola, NY) at several time points postvaccination. Lung washes and splenocytes were acquired postmortem. Lung wash samples were performed by inserting an 18-gauge needle into the trachea and washing 0.3 ml of PBS plus 1% bovine serum albumin (BSA) into and out of the lung. Any lung wash samples with visible red blood cell (RBC) contamination were removed from analysis. Five- to 8-month-old castrated male Fitch ferrets seronegative by hemagglutinin inhibition (HAI) for human circulating influenza and H5N1 (Triple F Farms, Sayre, PA) were fasted between 1 and 4 h to allow ample time for the gastric emptying that is necessary for visualizing landmarks. The bronchoscope was gently advanced through the oral cavity and down the esophagus to the esophageal gastric sphincter. Air insufflation was used to distend the esophagus, allowing passage of the bronchoscope. Air insufflation was also useful in the dilation and opening of the esophageal gastric sphincter to allow the bronchoscope to enter the stomach. When the bronchoscope reached the stomach, the stomach was insufflated with sufficient air to allow proper visualization of the gastric anatomy. Care was taken to avoid overfilling the stomach with gas. The endoscope was then reflected approximately 150o back upon itself (toward the animal's right axillary region) until the pyloric sphincter was visualized. The bronchoscope was then advanced through the sphincter and as far into the duodenum/proximal intestine as possible given the length of the bronchoscope. Insufflation with air and rinsing through the channel with distilled water were used as necessary to make visualization of the intestinal tract possible. Once the bronchoscope was fully inserted, the vaccine could be delivered through the channel. Ferrets were given 4 × 108 IU of rAd vectors administered in 1 ml of PBS at weeks 0 and 4. The channel was then rinsed with 1 ml PBS to ensure that the entire volume of test article was delivered. After delivery of the test article, the bronchoscope was slowly withdrawn from the animal, stopping in the stomach to deflate as necessary.
ELISAs.
Specific antibody titers to proteins were measured similarly to methods described previously (18). Briefly, microtiter plates (MaxiSorp: Nunc) were coated in 1× carbonate buffer (0.1 M at pH 9.6) with 1.0 μg/ml protein, either gp120 (Fitzgerald, Concord, MA) or influenza virus HA (Protein Sciences, Meridian, CT). The plates were incubated overnight at 4°C in a humidified chamber and then blocked in PBS plus 0.05% Tween 20 (PBST) plus 1% BSA solution for 1 h before washing. Plasma samples were serially diluted in PBST. After a 2-h incubation, the plates were washed with PBST at least 5 times. Antibodies were then added, either as a mixture of anti-mouse IgG1-horseradish peroxidase (HRP) and anti-mouse IgG2a-HRP (Bethyl Laboratories, Montgomery, TX) or, alternatively, anti-mouse IgA-HRP (Bethyl Laboratories) for mucosal samples. Each secondary antibody was used at a 1:5,000 dilution. The plates were washed at least 5 times after a 1-h incubation. Antigen-specific mouse antibodies were detected with 3,3′,5,5′-tetramethyl-benzidine (TMB) substrate (Rockland, Gilbertsville, PA) using a microplate reader. H2SO4 was used as a stop solution, and the plates were read at 450 nm on an Emax enzyme-linked immunosorbent assay (ELISA) plate reader. Ferret antibody responses to HA were measured by ELISA using a protocol similar to that used with mice, with the exception that the secondary antibody was anti-ferret IgG-HRP (Bethyl Laboratories). Average antibody titers were reported as the reciprocal dilution giving an absorbance value greater than the average background plus 2 standard deviations, unless otherwise stated.
Total IgA was measured with a kit from Bethyl Laboratories before normalizing and measuring specific IgA in the lung wash samples from mice. Any samples without measurable IgA or with visible blood contamination were discarded before specific IgA measurement. The mouse fecal IgA samples were normalized on a weight-per-volume basis before measuring the specific IgA-to-HA ratio as described previously (18). Briefly, samples were processed by adding PBS plus 1% BSA at 1 mg/ml to the fecal pellets and vortexing to mix them. After centrifuging and pelleting the solid material, the sample optical density (OD) values were measured with 20% and 5% fecal material supernatant in PBST using ELISA plates coated with HA at 1 μg/ml.
For measuring anti-Ad titers, a protocol was adopted from another publication (19). Briefly, 100 μl of adenovirus antigen (1 × 1010 particles/ml) was mixed with carbonate buffer, and the plates were coated with the mixture overnight at 4°C. Plates were washed four times with PBST and blocked with 3% BSA in PBS (blocking buffer) for 1 to 3 h at room temperature. Serum samples (1:100 and/or log dilutions) were diluted in blocking buffer, and 100 μl was added to each well. After an overnight incubation at 4°C, the plates were washed 4 times with wash buffer. Goat anti-mouse IgG-HRP antibody (Bethyl Laboratories) at a 1:4,000 dilution was added at 100 μl per well and incubated for 1 h at room temperature. The plates were washed 6 times, and TMB was added to develop them. Stop solution was added before reading the plates at 450 nm.
Neutralization assay.
This protocol was adapted from Scallan, et al. (20). Mouse serum or plasma was incubated at 56°C for 30 min to inactivate complement and then diluted in naïve mouse sera (Innovative Research, Southfield, MI) in 10-fold increments starting at a 1:10 dilution. Each dilution (50 μl) was then mixed with an equal volume of DMEM with Ad expressing β-galactosidase (1 × 104 IU), incubated for 1 h at 37°C, and added to HEK293 cells in 96-well tissue culture plates (4 × 104 cells/well). After a 1-h incubation at 37°C, 100 μl of DMEM with 20% (wt/vol) BS was added to each well. The plates were incubated overnight at 37°C. The next day, the medium was removed, and the wells were washed with 100 μl of PBS. The cells were lysed with 100 μl of reporter lysis buffer (Promega, Madison, WI). In order to aid cell lysis, the plates went through 2 rounds of freeze-thawing at 37°C and at −80°C. Then, 20 μl of o-nitrophenyl-β-d-galactopyranoside (4 mg/ml in PBS) was added to each well. The plates were allowed to develop at 37°C before the reaction was stopped by the addition of 20 μl of sodium bicarbonate. The optical density was read at 420 nm on a microplate reader. Neutralizing titers were determined as the highest dilution at which β-galactosidase expression was reduced by a minimum of 50%.
HAI and challenge studies.
HAI titers to both clade II (A/Indo/05/2005) and clade I (A/VN/1203/2004 [A/VN]) were measured in sera by the Southern Research Institute (SRI) at 4 and 7 weeks using protocols described previously (21). Wild-type A/Indo/05/2005, obtained by SRI from the CDC, is a biosafety level 3 (BSL3) select agent and is handled by SRI in appropriate BSL3+ facilities using approved protocols. SRI follows all applicable rules of the Occupational Safety and Health Administration (OSHA), Environmental Protection Agency (EPA), NIH Guidelines, CDC and NIH's current Biosafety in Microbiological and Biomedical Laboratories (BMBL) edition, CDC/USDA Select Agent and Toxin Program requirements, and the SRI Institutional Biosafety Committee (IBC)-approved recommendations and additional health and safety requirements when handling select agents. Mice and ferrets were challenged at 23 and at 8 weeks, respectively, with 10 times the 50% lethal dose (LD50) of A/Indo/05/2005 by administering the virus i.n.
Statistical analysis.
Where mentioned in the text, Student's t test (one tailed) was used to determine the significance, with the exceptions that Fisher's exact test was used to determine significance for challenge studies and the Mann-Whitney U test was used for determining whether anti-vector titers between groups were significant. P values of less than 0.05 were considered significant.
RESULTS
dsRNA enhances antibody responses to antigen produced by rAd.
In order to increase vaccine efficacy, we designed a dsRNA ligand (luc1) encoded on the same viral vector as the antigen. As an exogenously provided hairpin, the luc1 dsRNA was previously shown to induce type I interferons by stimulating TLR3 on appropriate expressing cells (22). The ligand luc1 was incorporated into Ad vectors using the CMV promoter to drive expression (rAd-HA-dsRNA) (Fig. 1A). To compare antibody responses, an Ad vector expressing the HA transgene, but without the expressed dsRNA (rAd5-HA), was used. Each of these rAd vectors encodes the influenza virus HA protein from clade 2, A/Indo/05/2005 avian influenza virus under the control of the CMV promoter. Mice were vaccinated with a single oral gavage administration of each rAd vector, and at 4 weeks postvaccination, the antibody responses to HA were measured by an anti-HA IgG ELISA. As a positive control, animals were vaccinated by i.m. injection of rAd5-HA. The results show that the antibody responses to HA were greater with the adenoviral vector that expressed dsRNA luc1 (rAd-HA-dsRNA), inducing a >1.5-log-unit improvement in antibody response over oral gavage of the rAd5-HA vector (Fig. 1B and C). Significantly, oral gavage of rAd-HA-dsRNA induced antibody responses similar to those of i.m.-injected rAd5-HA. When injected into muscle, the dsRNA-expressing vector did not improve antibody titers over rAd5-HA (Fig. 1B). These results suggest that rAd-HA-dsRNA has a benefit for p.o. administration, not muscle administration.
Fig 1.
Vaccination with dsRNA improves systemic antibody responses to transgene after p.o. administration of rAd. (A) A rAd vector was constructed to express dsRNA with the CMV promoter to drive expression (rAd-HA-dsRNA). (B) Antibody titers (IgG) against avian influenza virus HA following a single vaccination in mice with either rAd-HA-dsRNA or rAd5-HA vector with no adjuvant. The titers were measured at week 4. (C) Anti-HA IgG responses of rAd-HA-dsRNA and rAd5-HA after p.o. delivery (OD versus dilution). Dilutions between 50 and 4,050 had P values of <0.05 when comparing the vectors with or without adjuvant. The titers were measured at week 7. n = 6 for all groups except rAd-HA-dsRNA i.m., where n = 10. The error bars indicate standard errors.
Distal and local mucosal immune responses to transgene.
In order to determine whether mucosal IgA was being elicited to HA, fecal samples and lung washes were taken. Mice were vaccinated twice with the rAd-HA-dsRNA vector (4 weeks apart), followed by a boost 2 weeks later. After an additional 2 weeks, fecal samples were harvested and compared to samples from naïve animals for anti-IgA responses to HA. Several animals were found to have significant specific IgA to HA in their fecal samples compared to the unimmunized animals (Fig. 2A). At the end of the experiment, lung wash samples were taken. As a positive control for lung IgA, animals were vaccinated once by i.n. vaccination, because this method induces substantial local IgA. Plasma IgG results for the i.n. vaccination demonstrated a measured titer of 1.0 × 104 after 3 weeks compared to 1.0 × 105 for p.o. vaccination. The results show a substantial amount of antigen-specific IgA was induced for both i.n. and p.o. vaccination (Fig. 2B). There was no statistical difference between the two groups (P = 0.22). These results demonstrate that p.o. administration of rAd-HA-dsRNA can induce both distal and local mucosal responses to the transgene.
Fig 2.
Mucosal IgA responses to the HA transgene. (A) Anti-fecal IgA to HA. Animals were vaccinated once before measuring anti-IgA antibody responses to HA at week 4 using fecal samples normalized on a weight-per-volume basis. OD values were measured and plotted using 20%, 5%, and 1% fecal supernatants. n = 10 per group. (B) Anti-HA IgA responses in lung washes following vaccination. Mice were vaccinated a total of 3 times at 0, 4, and 10 weeks. The animals were euthanized 2 weeks later, and total IgA was measured in the lung wash samples before measuring specific IgA to HA using 50, 20, and 5 ng total IgA per sample. Samples without IgA were discarded. As a positive control, animals were i.n. vaccinated 3 weeks before harvest. n = 5 for p.o. vaccination, and n = 4 for i.n. vaccination of naïve mice. P = 0.22 between intranasal and oral delivery. ND = not determined. The error bars indicate standard errors.
Peroral delivery of the rAd-HA-dsRNA vector can elicit protection against lethal avian influenza challenge in mice.
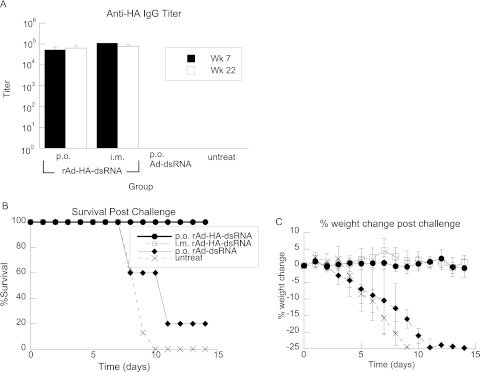
The ability to elicit protective immunity in animals was tested with two different animal models of oral vaccine delivery. In the first model, mice were gavaged with 1 × 107 IU of rAd-HA-dsRNA at weeks 4 and 8 and challenged at week 23 with 1 × 106 50% egg infective doses (EID50) (approximately 10 times the LD50) of A/Indo/05/2005. As a control for nonspecific innate immune effects, a group of mice was administered the vector expressing the adjuvant without antigen (Ad-dsRNA). Antibody responses to HA were measured at weeks 7 and 22, and the geometric mean titers (GMT) were plotted (Fig. 3A). All rAd-HA-dsRNA-immunized mice were protected from death and sickness, whereas the control vector-treated and untreated mice all lost significant amounts of weight, and all but one animal perished (Fig. 3B and C).
Fig 3.
rAd expressing dsRNA and HA elicits protective immune responses in mice. (A) IgG antibody GMT against HA from animals immunized at weeks (Wk) 0 and 4. Perorally and i.m.-immunized animals showed significant antibody responses to HA that did not diminish over 22 weeks. (B) After 23 weeks, animals were challenged with A/Indo/05/2005 at 10 times the LD50; the rAd-HA-dsRNA-immunized animals showed an increased ability to survive lethal challenge compared to animals immunized with a control vector that expresses only dsRNA or untreated animals. (C) rAd-HA-dsRNA-vaccinated animals maintained their weight, whereas the control vector-treated and untreated animals got sick and lost significant weight. The symbols are the same as in panel B. n = 6 per vaccinated group, n = 5 for the control group, and n = 8 for the untreated group. rAd-HA-dsRNA-immunized animals were significantly healthy (no weight loss or death at day 13 postchallenge) compared to control and untreated groups by Fisher's exact test (P = 0.002 and 0.0003, respectively). The error bars indicate standard errors.
Peroral delivery of the rAd-HA-dsRNA vector can elicit cross-clade neutralizing antibody responses in ferrets, but protection against homologous challenge correlates with total IgG antibody response.
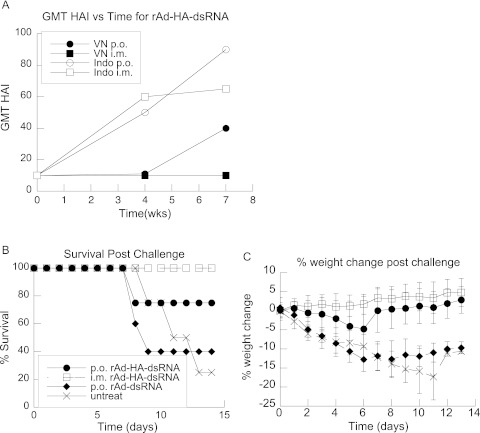
The ability to elicit protective immunity against avian influenza was tested by vaccinating ferrets, an accepted model for human influenza virus infection (23, 24). In order to simulate enteric release of Ad from a pill, an endoscope was used to deliver the vector through the acidic environment of the stomach to the duodenum. A group of ferrets were also vaccinated i.m. with rAd-HA-dsRNA as a control for delivery. As a control for nonspecific innate immune effects, a group of animals were given a rAd vector that expressed the adjuvant without the antigen. Animals were administered the rAd vectors at weeks 0 and 4. The results showed substantial anti-HA antibody responses in groups administered the rAd vectors both p.o. and i.m. at week 7 (Table 1). HAI titers were measured to determine the ability of plasma samples to neutralize both homologous and heterologous avian influenza virus strains. The results from the i.m. and p.o. groups were similar at week 4 (Fig. 4A), but the HAI in the p.o. group was higher by week 7 (Fig. 4A and Table 1), with p.o. vaccination showing improved HAI to homologous virus, as well as significant cross-clade antibody responses to A/VN. There were no measurable HAI responses in the control vector or unvaccinated animals (data not shown).
Table 1.
Ferret survival after homologous challenge with influenza virus A/Indo/5/05 correlates with total antibody titer
| Route | Total Aba titer | HAI/Indob | HAI/VNb | Survivalc |
|---|---|---|---|---|
| p.o. | 12,800 | 640 | 320 | Yes |
| p.o. | 3,200 | 120 | 15 | Yes |
| p.o. | 3,200 | 160 | 160 | Yes |
| p.o. | 12,800 | 1,280 | 480 | Yes |
| p.o. | 200 | 10 | 10 | Yes |
| p.o. | 6,400 | 240 | 10 | Yes |
| p.o. | 1 | 10 | 10 | No |
| p.o. | 1 | 10 | 15 | No |
| i.m. | 12,800 | 10 | 10 | Yes |
| i.m. | 12,800 | 1,280 | 10 | Yes |
| i.m. | 12,800 | 480 | 10 | Yes |
| i.m. | 6,400 | 640 | 10 | Yes |
| i.m. | 12,800 | 10 | 10 | Yes |
| i.m. | 12,800 | 80 | 10 | Yes |
| i.m. | 6,400 | 10 | 10 | Yes |
| i.m. | 6,400 | 10 | 10 | Yes |
Total anti-HA antibody (Ab) was measured using an ELISA specific for A/Ind5/05 HA.
HAI titers were measured using either the homologous influenza virus strain (A/Ind/5/05) or the heterologous strain A/Vietnam/1203/2004. HAI data at 7 weeks are shown.
Survival was monitored for 14 days post-i.n. challenge with 10 LD50 of A/Ind 5/2005.
Fig 4.
rAd expressing dsRNA and HA elicits HAI responses and protects ferrets against lethal avian influenza virus challenge. (A) HAI GMT of vaccinated animals. Perorally vaccinated animals showed significant HAI titers to both influenza A/Vietnam (VN p.o.) and A/Indo (Indo p.o.), whereas i.m.-vaccinated animals showed HAI titers only against A/Indo (Indo i.m.). Immunizations were performed at weeks 0 and 4. Control-treated and all untreated animals had HAI titers of 10, the background of the assay (data not shown). (B) After week 8, animals were challenged with A/Indo/05/2005 at 10 times the LD50, and survival and weight were monitored for 14 days. Vaccinated animals showed an increased ability to survive lethal challenge compared to control vector-treated (diamond) or untreated animals. (C) Surviving vector-vaccinated animals maintained their weight, whereas the control vector-treated and untreated animals became sick and lost significant weight. The symbols are the same as in panel B. n = 8 per vaccinated group, and n = 4 for the untreated group. rAd-HA-dsRNA-immunized animals were significantly healthy (no weight loss at day 13 postchallenge) compared to control groups by Fisher's exact test (P < 0.05). The error bars indicate standard errors.
At week 8, vaccinated and unvaccinated ferrets were challenged with 1 × 105.25 EID50, 10 times the LD50 of A/Indo/05/2005. The health of the animals was monitored daily for 14 days by measuring weight. Vaccinated animals that produced an HA antibody titer above 200, including those that had no significant HAI titers against the challenge strain, survived influenza virus challenge and remained healthy (Table 1 and Fig. 4B). Animals that had no detectable antibody titers to HA became sick and lost significant weight (Fig. 4C). The majority of the control vector-vaccinated and unvaccinated animals did not survive (Fig. 4B), and no animals from these groups regained their prechallenge weight (Fig. 4C). In contrast, rAd-HA-dsRNA-vaccinated animals were all healthy, with the exception of the 2 animals that had antibody titers of less than 200 (Table 1). These results demonstrate that a rAd vector coexpressing a dsRNA adjuvant and HA can protect against lethal challenge with a homologous strain in a large-animal model of avian influenza.
Peroral rAd performance is not hindered by preexisting Ad immunity.
We performed the following experiment to explore whether oral immunization could circumvent vector immunity. Mice were vaccinated with an Ad5 vector carrying the HIV gp120 antigen (rAd-gp120) either by the p.o. route [with poly(I·C) as a dsRNA analogue] or by i.m. vaccination, and the antibody titers to the vector were measured by an anti-Ad5 ELISA 3 weeks later. Animals vaccinated with rAd-gp120 once by i.m. injection had significant antibody titers to Ad5, whereas 11 out of 12 p.o.-vaccinated animals had no detectable antibody response to Ad5 (Table 2). The mice were immunized 4 weeks later with rAd carrying HA from influenza virus A/PR8/34 under the CMV promoter (rAd-CMV-HA). A group of age-matched controls were vaccinated only once with rAd-CMV-HA. IgG anti-HA titers were compared between animals that were vaccinated twice (representing prior exposure to Ad) and animals vaccinated once with rAd-CMV-HA only (representing no prior exposure to Ad5). HA antibody responses to A/PR8/34 were measured by ELISA. The ELISA results showed that animals immunized twice by i.m. injection had a 98% decrease in HA titers compared to naïve animals given only rAd5-CMV-HA (Table 2). In contrast, perorally immunized animals that were previously vaccinated by either the p.o. or i.m route were able to induce antibody titers comparable to those of naïve animals given a single p.o. vaccination. Anti-vector IgG titers were measured at the end of the experiment (week 8) and were found to be highest in the muscle-injected groups, with the double-injected group having average titers of 1:100,000. An anti-Ad neutralizing antibody assay was performed to evaluate the level of vector neutralizing antibodies in the plasma of these animals. The p.o.-vaccinated animals (groups A and E) had no measurable neutralization titers after one or two vaccinations, whereas in groups B, D, and F, where animals were vaccinated either one or two times by i.m. injection, the animals had neutralization titers between 1:10 and 1:100.
Table 2.
Oral vaccination with Ad plus adjuvant permits successful readministration
| Parametera | Value for group: |
|||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| 1st vaccination (rAd-GP120) route | p.o. | i.m. | p.o. | i.m. | NAb | NA |
| Anti-vector titer | <100 | 1,000–10,000 | <100 | 1,000–10,000 | NA | NA |
| 2nd vaccination (rAd-CMV-HA) route | p.o. | p.o. | i.m. | i.m. | p.o. | i.m. |
| Anti-vector titer (avg) | 100 | 10,000 | 10,000 | 100,000 | <100 | 6.4e3 |
| Anti-vector neutralization (±SD) | <10 | 38.5 ± 48 | NDc | 65 ± 49 | <10 | 80 ± 44 |
| Anti-HA titer (±SD) | 692 ± 721 | 1,050 ± 464 | 6,750 ± 4,182 | 167 ± 147 | 550 ± 409 | 9,145 ± 4,182 |
| % HA titer compared to naive | 126 | 190 | 71 | 2 | 100 | 100 |
Mice were vaccinated with 1 × 107 IU of Ad-GP120 vector and the exogenous adjuvant on day 1. Four weeks later, the mice were vaccinated with 1 × 107 IU of rAd-CMV-HA. Anti-vector and anti-HA responses were measured 3 weeks post-vaccination 1 and 2.
NA, not applicable.
ND, not done.
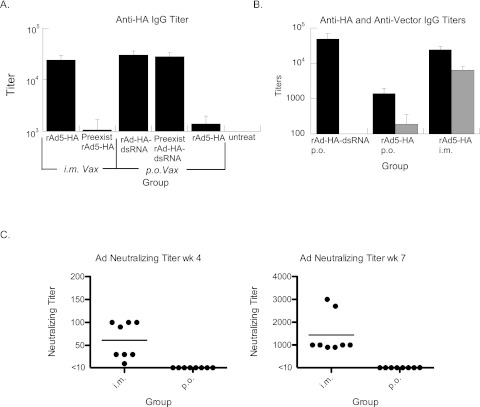
A subsequent experiment was performed to verify that these effects were also applicable to the use of the rAd-HA-dsRNA vector. Animals were either preimmunized p.o. with rAd expressing an irrelevant antigen with the adjuvant (rAd-VEE-dsRNA) or not preimmunized, and 4 weeks later, the rAd-HA-dsRNA vector was given p.o. to the mice. Similarly, a group of animals were preimmunized by i.m. injection (with rAd-VEE-dsRNA) and then 4 weeks later were immunized i.m. with rAd5-HA and compared to mice that were not preimmunized. Groups of animals given rAd5-HA (without adjuvant) p.o. and untreated animals were also included in the experiment. The results show that the i.m. vaccination is significantly affected by prior Ad exposure, whereas p.o. vaccination with rAd-HA-dsRNA is not (Fig. 5A).
Fig 5.
Peroral administration elicits a substantial antibody response to transgene but no measurable response to vector. (A) Vectors expressing HA were given to mice that were either preimmunized 4 weeks before using 1 × 107 IU rAd-VEE-dsRNA (preexist) or not preimmunized. p.o.-preimmunized animals were vaccinated (Vax) p.o., and i.m.-preimmunized animals were vaccinated i.m. Antibody titers to HA were measured by ELISA 4 weeks after the last vector was given. As a control, the rAd5-HA vector (without dsRNA adjuvant) was given perorally to show the effects of the adjuvant on p.o. vaccine performance. (B) rAd5-HA-dsRNA (p.o.) or rAd5-HA (p.o. and i.m.) were administered, and antibody titers to both HA (black) and vector (gray) were measured 3 weeks later. Antivector responses were significantly higher in the i.m.-immunized group. n = 6. P < 0.01 by Mann-Whitney U test for anti-vector response comparing either p.o. group to the i.m. group. The error bars indicate standard errors. (C) Neutralizing antibody titers to Ad5 were measured in ferrets following vaccinations at weeks 0 and 4. P.o. vaccination induced undetectable (less than 1:10) neutralizing antibody titers to Ad5 in all animals at week 4 and in 7 of 8 animals at week 7. In contrast, i.m.-vaccinated animals had substantial neutralizing titers to Ad5 at weeks 4 and 7. P < 0.01 by the Mann-Whitney U test. Individual results for each animal are shown as dots. The bars represent the average neutralizing titers. Figure 4A shows the average HAI responses for the same animals. n = 8.
Based on the results in Fig. 5A, we also examined the antibody titers to Ad5 in animals vaccinated with either Ad-HA-dsRNA or Ad5-HA by p.o. delivery and compared them to those for i.m. delivery of Ad5-HA. Boosting may be required to elicit protective immune responses to avian influenza in humans, and a substantial anti-vector response might hinder the response to antigen. We found that p.o. immunization of mice with the rAd-HA-dsRNA vector elicited strong anti-HA IgG titers with limited antibody titers to Ad5 (Fig. 5B). Peroral immunization with rAd5-HA induced a generally low anti-vector response, with 5 out of 6 animals with low to no titers to vector and 1 high-titer outlier. In contrast, i.m. immunization with the Ad5-HA vector elicited substantial responses to both the vector and the transgene in 6 out of 6 animals after a single administration. In order to track the immune specificities of the different approaches, we calculated the ratio between the anti-HA and anti-Ad5 IgG antibody responses. For p.o. rAd-HA-dsRNA-treated mice, this ratio was approximately 2,000:1, whereas the ratio was 5.7:1 after i.m. administration of Ad5-HA. We observed that HAI antibody titers to A/Indo in the ferret challenge study did not increase after the second vaccination for i.m.-vaccinated animals (Fig. 4A). We measured the Ad5 neutralizing titers in the sera of these ferrets and determined that the i.m.-vaccinated animals had substantial neutralizing titers at weeks 4 and 7 (Fig. 5C). In contrast, the p.o.-vaccinated ferrets had no detectable neutralizing antibody responses to Ad5 at week 4 (less than 1 in 10), and 7 of 8 had no detectable neutralizing antibody response at week 7, with one animal showing a titer of 10 (Fig. 5C). In vaccinated ferrets, the calculated selectivity ratio of the A/Indo HAI titer to the anti-Ad5 neutralizing titer was approximately 100:1 for p.o. vaccination versus 0.2:1 for i.m. vaccination. These results indicate that oral administration of our Ad vaccine vectors is extremely selective in generating immune responses to the transgene.
DISCUSSION
This study shows for the first time that animals can be protected against lethal avian influenza virus challenge by two different oral immunization models, a mouse gavage model and a ferret endoscope model. Problems with current avian influenza vaccines under development include reliance on eggs for manufacturing the vaccine and reliance on qualified medical personnel for administration. An oral Ad vaccine delivered in a pill could be more easily distributed and grown to high titers in cell culture without eggs. A key limitation in the past was that oral rAd vaccination was not efficient enough in large animals to provide meaningful antibody titers to the payload antigen. Our approach relies on an adjuvant (dsRNA) that significantly improves antibody responses following p.o. delivery of rAd. Other key limitation for developing an oral vaccine platform are the differences in gastrointestinal (GI) transit time and pH between humans and animal models. Due to these differences, oral dosage forms are not so easily evaluated in animals using a formulation suitable for humans. We have already formulated the rAd-HA-dsRNA vector in a solid dosage form (capsule) and tested in vitro release characteristics to demonstrate that the enteric coated capsules retain Ad activity and are stable for lengthy periods at room temperature, which are requirements for a successful oral vaccine (C. D. Scallan and G. Trager, unpublished results). Future work will involve testing these capsules in animals, as well as formulations predicted to be more suited to the GI environment of experimental animals.
Human and ferret vaccination against H5 avian influenza virus has been performed with traditional influenza vaccine technology and found to require more antigen and to have much poorer immunogenicity (25, 26). The use of adjuvants is one way to improve the efficacy of these vaccines. In an avian influenza virus ferret challenge study by Baras et al., an injected protein vaccination containing HA protein plus the adjuvant AS04 was able to protect ferrets for 5 days, at which point the animals were sacrificed to measure the influenza virus titers in the lungs (27). Approximately 67% of the animals in this study had HAI titers against the strain-matched influenza virus, and these animals had reduced virion titers in the lungs on day 5, closely matching the HAI results. In our studies, 88% of ferrets immunized with rAd with adjuvant were protected against challenge for the 14-day study duration, where protection correlated, not with HAI, but with total HA antibody titers. The lack of correlation between HAI titers and influenza virus protection is an important observation, as the assay is typically used to predict protection. However, reports of protection in the absence of HAI titers are not unusual (23). This non-HAI neutralizing protection could be the result of a number of alternate immune responses that are not measured by the titer assay, such as IgA in the lungs or systemic T cells, which were not measured by HAI titers. In support of this theory, we have observed mucosal anti-HA IgA in the lungs of treated mice (Fig. 2), and we have detected systemic T cell immunity to avian influenza virus in the same animals (data not shown). In addition, in vitro neutralizing assays are not always predictive of in vivo virus neutralization, due in part to the limitations in the ability of the assay to fully model viral inactivation in animals (20).
The high incidence of Ad infections in human populations leads to preexisting immunity that can reduce the efficacy of traditional adenoviral vector-based vaccines (5, 28) (Table 2). Injected Ad vectors have been used successfully to protect against avian and annual influenza virus challenge in mice (29–31) and naïve ferrets (Fig. 4B), but preexisting immunity in human populations could severely hinder the general use of injectable Ad vector-based vaccines. It is unlikely the survival levels for i.m.-vaccinated ferrets in this study could be achieved in i.m.-vaccinated animals with preexisting Ad immunity, as witnessed by the lack of boosting previously seen in preimmunized animals (Fig. 4C). In an effort to avoid preexisting immunity, several different Ad vectors are being explored as alternatives to rAd5 (32). The advantage of using a vector with low seroprevalence is the potential ability to avoid neutralizing antibodies against the vector. Similarly, oral rAd5 vaccination may be able to overcome the problem of preexisting immunity because it does not appear to be affected by the presence of Ad5 anti-vector immune responses (5). A recent study using intranasal delivery also showed some ability to generate humoral immunity to influenza virus in spite of preexisting Ad immunity (33). In our study, we found that i.m. vaccination induced substantial serum antibody responses to transgenes but also elicited significant Ad5 antibody responses in mice and in ferrets. In contrast, peroral delivery of our vector induced substantial anti-transgene antibody titers but no measurable anti-vector antibody responses after a single administration. After a second p.o. administration, low antibody titers were observed in the plasma, but vaccine performance was not hindered. This selective advantage may permit vector reuse with greater efficiency in larger animals, as well. Our oral vector may generate this selective response in part because there is typically poor early immune recognition of the vector in the gut environment but improved recognition of the transgene when the dsRNA is being made. Because adjuvant and antigen are made within the same cell at essentially the same time, immune recognition may be tightly focused on the antigen. Indeed, some studies have suggested preferential loading of protein from engulfed apoptotic cells when TLR3 is stimulated on endosomes (34). Because our vector is nonreplicating, Ad capsid proteins are not being made when the adjuvant is available, which may also account for the preferential antigen response. A replicating Ad vector is not likely to have this selective advantage. Consistent with this idea, the military Ad7 vaccine using a replicating (wild-type) virus was able to induce substantial protective antibody responses against Ad, approaching 86% efficacy (35).
We have shown that dsRNA can function as an adjuvant for p.o. vaccination and that it is capable of increasing antibody responses to levels seen with i.m.-injected rAd. dsRNA adjuvants have been shown to improve humoral immune responses to protein antigens following i.n. vaccination (36). Similarly, the use of TLR3 ligands to stimulate targeted dendritic cells led to improvements in the numbers of polyfunctional T cells generated against a peptide antigen (37). A study using expressed dsRNA in a DNA vaccine format showed no improvement in humoral immunity following repeated injection but did show a 2-fold increase in cellular immune responses (38). We saw no improvement in the antibody immune response to HA following i.m. vaccination. We did observe a greater than 10-fold improvement in anti-HA IgG titers after oral vaccination with the rAd-HA-dsRNA vector compared to the rAd5-HA vector, which lacks adjuvant. Ad has been shown to stimulate type I interferons directly through TLR-independent and -dependent mechanisms (39, 40). Following i.m. injection, the recognition of antigens expressed by Ad is very efficient, and high-titer antibody and T cell responses are obtained to vector as well as expressed antigen. In contrast, traditional oral Ad vectors are inefficient in eliciting these responses (8, 41). Perhaps this is imparted through a general suppression of innate immune responses in the gut environment in order to avoid deleterious immune responses to food or to the commensal bacteria. The environment of the gastrointestinal tract has a complex set of TLRs and inhibitory mechanisms in place in order to prevent commensal bacteria from causing abnormal inflammation, but this receptor system can still respond to pathogens when they cause damage (42, 43). A recent study by Contractor and colleagues has shown that Peyer's patch dendritic cells are poor inducers of type I interferons in response to TLR9 ligands and whole influenza virus (44). Our approach may be able to function in part by compensating for the poor inductive ability in the gut, thus improving antibody titers to encoded antigens.
In conclusion, a recombinant Ad-based vaccine coexpressing a dsRNA adjuvant can protect against avian influenza in small and large animals. Further, antibody responses elicited by this kind of vaccine are selective to the transgene but not the vector, which may allow vector reuse. We have produced capsules of the rAd-based vaccine for a human clinical trial, which should be completed shortly.
ACKNOWLEDGMENTS
We thank Rich Whitley, Rubin Donis, and Wendy Peters for reviewing and commenting on the manuscript.
Funding for this work was supported by National Institutes of Health grant R44 AI074216 (to Sean N. Tucker) and funding from Vaxart, Inc.
Footnotes
Published ahead of print 14 November 2012
REFERENCES
- 1. WHO 2006. R & D for avian/pandemic influenza vaccines by IFPMA Influenza International Supply Task Force (IVS ITF) members. http://www.who.int/vaccine_research/diseases/influenza/IFPMA_Avian-Pandemic_Influenza_Vaccine_R&D.pdf
- 2. Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336: 1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486: 420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tucker SN, Tingley DW, Scallan CD. 2008. Oral adenoviral-based vaccines: historical perspective and future opportunity. Expert Rev. Vaccines 7: 25–31 [DOI] [PubMed] [Google Scholar]
- 5. Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. 2003. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 77: 10780–10789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng X, Ming X, Croyle MA. 2003. PEGylated adenoviruses for gene delivery to the intestinal epithelium by the oral route. Pharm. Res. 20: 1444–1451 [DOI] [PubMed] [Google Scholar]
- 7. Mutwiri G, Watts T, Lew L, Beskorwayne T, Papp Z, Baca-Estrada ME, Griebel P. 1999. Ileal and jejunal Peyer's patches play distinct roles in mucosal immunity of sheep. Immunology 97: 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lubeck MD, Davis AR, Chengalvala M, Natuk RJ, Morin JE, Molnar-Kimber K, Mason BB, Bhat BM, Mizutani S, Hung PP. 1989. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc. Natl. Acad. Sci. U. S. A. 86: 6763–6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pizza M, Giuliani MM, Fontana MR, Monaci E, Douce G, Dougan G, Mills KH, Rappuoli R, Del Giudice G. 2001. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19: 2534–2541 [DOI] [PubMed] [Google Scholar]
- 10. Nishiya T, Kajita E, Miwa S, Defranco AL. 2005. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J. Biol. Chem. 280: 37107–37117 [DOI] [PubMed] [Google Scholar]
- 11. Cario E, Podolsky DK. 2000. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68: 7010–7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phillpotts RJ, O'Brien L, Appleton RE, Carr S, Bennett A. 2005. Intranasal immunisation with defective adenovirus serotype 5 expressing the Venezuelan equine encephalitis virus E2 glycoprotein protects against airborne challenge with virulent virus. Vaccine 23: 1615–1623 [DOI] [PubMed] [Google Scholar]
- 13. Jordan M, Schallhorn A, Wurm FM. 1996. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 24: 596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki E, Murata T, Watanabe S, Kujime Y, Hirose M, Pan J, Yamazaki T, Ugai H, Yokoyama KK. 2004. A simple method for the simultaneous detection of E1A and E1B in adenovirus stocks. Oncol. Rep. 11: 173–178 [PubMed] [Google Scholar]
- 15. Kong Q, Richter L, Yang YF, Arntzen CJ, Mason HS, Thanavala Y. 2001. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc. Natl. Acad. Sci. U. S. A. 98: 11539–11544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharpe S, Fooks A, Lee J, Hayes K, Clegg C, Cranage M. 2002. Single oral immunization with replication deficient recombinant adenovirus elicits long-lived transgene-specific cellular and humoral immune responses. Virology 293: 210–216 [DOI] [PubMed] [Google Scholar]
- 17. Moore AC, Kong WP, Chakrabarti BK, Nabel GJ. 2002. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J. Virol. 76: 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tucker SN, Lin K, Stevens S, Scollay R, Bennett MJ, Olson DC. 2003. Systemic and mucosal antibody responses following retroductal gene transfer to the salivary gland. Mol. Ther. 8: 392–399 [DOI] [PubMed] [Google Scholar]
- 19. Croyle MA, Chirmule N, Zhang Y, Wilson JM. 2001. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J. Virol. 75: 4792–4801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S, Couto LB, Pierce GF. 2006. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 107: 1810–1817 [DOI] [PubMed] [Google Scholar]
- 21. Holman DH, Wang D, Raja NU, Luo M, Moore KM, Woraratanadharm J, Mytle N, Dong JY. 2008. Multi-antigen vaccines based on complex adenovirus vectors induce protective immune responses against H5N1 avian influenza viruses. Vaccine 26: 2627–2639 [DOI] [PubMed] [Google Scholar]
- 22. Kariko K, Bhuyan P, Capodici J, Weissman D. 2004. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J. Immunol. 172: 6545–6549 [DOI] [PubMed] [Google Scholar]
- 23. Govorkova EA, Rehg JE, Krauss S, Yen HL, Guan Y, Peiris M, Nguyen TD, Hanh TH, Puthavathana P, Long HT, Buranathai C, Lim W, Webster RG, Hoffmann E. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 79: 2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith H, Sweet C. 1988. Lessons for human influenza from pathogenicity studies with ferrets. Rev. Infect. Dis. 10: 56–75 [DOI] [PubMed] [Google Scholar]
- 25. Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, Höschler K, Zambon MC. 2006. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 367: 1657–1664 [DOI] [PubMed] [Google Scholar]
- 26. Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. 2006. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 354: 1343–1351 [DOI] [PubMed] [Google Scholar]
- 27. Baras B, Stittelaar KJ, Simon JH, Thoolen RJ, Mossman SP, Pistoor FH, van Amerongen G, Wettendorff MA, Hanon E, Osterhaus AD. 2008. Cross-protection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PLoS One 3: e1401 doi:10.1371/journal.pone.0001401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, Robbins PD, Gambotto A. 2004. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 11: 351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao W, Soloff AC, Lu X, Montecalvo A, Nguyen DC, Matsuoka Y, Robbins PD, Swayne DE, Donis RO, Katz JM, Barratt-Boyes SM, Gambotto A. 2006. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 80: 1959–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoelscher MA, Garg S, Bangari DS, Belser JA, Lu X, Stephenson I, Bright RA, Katz JM, Mittal SK, Sambhara S. 2006. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet 367: 475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang M, Harp JA, Wesley RD. 2002. Recombinant adenovirus encoding the HA gene from swine H3N2 influenza virus partially protects mice from challenge with heterologous virus: A/HK/1/68 (H3N2). Arch. Virol. 147: 2125–2141 [DOI] [PubMed] [Google Scholar]
- 32. Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, Liu J, Thorner AR, Swanson PE, Gorgone DA, Lifton MA, Lemckert AA, Holterman L, Chen B, Dilraj A, Carville A, Mansfield KG, Goudsmit J, Barouch DH. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441: 239–243 [DOI] [PubMed] [Google Scholar]
- 33. Pandey A, Singh N, Vemula SV, Couetil L, Katz JM, Donis R, Sambhara S, Mittal SK. 2012. Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine. PLoS One 7: e33428 doi:10.1371/journal.pone.0033428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljeström P, Reis e Sousa C. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433: 887–892 [DOI] [PubMed] [Google Scholar]
- 35. Top FH, Jr, Buescher EL, Bancroft WH, Russell PK. 1971. Immunization with live types 7 and 4 adenovirus vaccines. II. Antibody response and protective effect against acute respiratory disease due to adenovirus type 7. J. Infect. Dis. 124: 155–160 [DOI] [PubMed] [Google Scholar]
- 36. Knopf HL, Blacklow NR, Glassman MI, Cline WL, Wong VG. 1971. Antibody in tears following intranasal vaccination with inactivated virus. II. Enhancement of tear antibody production by the use of polyinosinic: polycytidilic acid (poly I:C). Invest. Ophthalmol. 10: 750–759 [PubMed] [Google Scholar]
- 37. Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, Schlesinger SJ, Colonna M, Steinman RM. 2008. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc. Natl. Acad. Sci. U. S. A. 105: 2574–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li D, Liu Y, Zhang Y, Xu J, Hong K, Sun M, Shao Y. 2007. Adjuvant effects of plasmid-generated hairpin RNA molecules on DNA vaccination. Vaccine 25: 6992–7000 [DOI] [PubMed] [Google Scholar]
- 39. Nociari M, Ocheretina O, Schoggins JW, Falck-Pedersen E. 2007. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 81: 4145–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu J, Huang X, Yang Y. 2007. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 81: 3170–3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mutwiri G, Bateman C, Baca-Estrada ME, Snider M, Griebel P. 2000. Induction of immune responses in newborn lambs following enteric immunization with a human adenovirus vaccine vector. Vaccine 19: 1284–1293 [DOI] [PubMed] [Google Scholar]
- 42. Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, Zhou Y, Hu B, Arditi M, Abreu MT. 2003. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170: 1406–1415 [DOI] [PubMed] [Google Scholar]
- 43. Shibolet O, Podolsky DK. 2007. TLRs in the gut IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am. J. Physiol. Gastrointest. Liver Physiol. 292: G1469–G1473 [DOI] [PubMed] [Google Scholar]
- 44. Contractor N, Louten J, Kim L, Biron CA, Kelsall BL. 2007. Cutting edge: Peyer's patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFbeta, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J. Immunol. 179: 2690–2694 [DOI] [PubMed] [Google Scholar]