Abstract
HIV-exposed but uninfected (HEU) infants born to HIV-infected mothers from areas in the world with a high burden of infectious disease suffer higher infectious morbidity and mortality than their HIV unexposed uninfected (HUU) peers. Vaccination provides protection from infection. The possibility exists that altered response to vaccination contributes to the higher rate of infection in HEU than in HUU infants. While short-term, cross-sectional studies support this notion, it is unclear whether or not HEU infants develop long-term protective immune responses following the WHO extended program on immunization (EPI). Vaccine-specific antibody responses were compared between HEU and HUU infants from 2 weeks until 2 years of age in a longitudinal South African cohort. Total IgG and antibodies specific for Bordetella pertussis, Haemophilus influenzae type b (Hib), tetanus toxoid, hepatitis B virus (HepB), and measles virus were measured at multiple time points throughout the first 2 years of life. Prevaccine antibodies (maternal antibodies passively acquired) specific for tetanus were lower in HEU than in HUU infants, while prevaccine antibodies to HepB were higher in HEU than in HUU infants. Both groups responded similarly to tetanus, Hib, and HepB vaccination. HEU demonstrated stronger pertussis vaccine responses, developing protective titers 1 year earlier than HUU patients, and maintained higher anti-tetanus titers at 24 months of age. Vaccine-induced antibodies to measles virus were similar in both groups at all time points. Our results suggest that the current EPI vaccination program as practiced in South Africa leads to the development of vaccine-specific antibody responses that are equivalent in HEU and HUU infants. However, our data also suggest that a large fraction of both HEU and HUU South African infants have antibody titers for several infectious threats that remain below the level of protection for much of their first 2 years of life.
INTRODUCTION
Vaccination is essential to combat infectious mortality and morbidity in children under 5 years of age (1). Despite the availability of effective vaccines, 6 million children die from infectious diseases annually, mainly in low- to middle-income countries where HIV is often prevalent (2, 3). While a lack of access to vaccines surely is the most important contributor to the high number of vaccine-preventable deaths in these regions, it is unclear if vaccination is equally protective in all children. Globally, more than 2 million babies are born to HIV-infected mothers every year (4). Programs for vertical transmission prevention of HIV (VTP) have reduced vertical infection to well below 10% (5), therefore nearly 2 million HIV-exposed but uninfected (HEU) infants are born annually. Recent evidence indicates that HEU infants are at a higher risk of infectious morbidity and mortality than their HIV-unexposed, uninfected (HUU) peers (6–12). The underlying reason(s) for this phenomenon are still unclear but are possibly multifactorial; severity of maternal HIV disease (13), avoidance of breastfeeding (14–16), and differences in microbial exposures (17) have all been postulated. Furthermore, exposure to HIV itself (18), as well as to antiretroviral drugs for VTP, may also directly impact the HEU infant's immune system (19, 20). Suboptimal response to vaccination thus has been suggested to contribute to the increased infectious burden of HEU. This notion has been supported by several studies, which documented low vaccine-specific antibody titers in HIV-infected mothers and attenuated vaccine-specific antibody levels in HEU compared to HUU infants (21–24). These differences have been ascribed to a compromise in maternally transferred antibodies, differing antibody half lives (24), and altered responses to vaccination (21, 22). To date, however, studies have only investigated vaccine responses in either short-term cohorts or in cross-sectional analysis (21–24), thereby not addressing long-term vaccine-induced immunity in HEU infants (14). A longitudinal analysis of HEU responses to vaccination is required to better understand how the rapidly expanding population of HEU infants responds to childhood vaccination. To this end, we established a birth cohort study in South Africa, a country with an antenatal HIV prevalence of 30% (25), and monitored HEU and HUU infants from 2 weeks up to 2 years of life (12), evaluating their vaccine-specific immune responses. Based on the published literature (21, 22, 24), we expected to find lower prevaccine-specific antibody titers in the HEU than in the HUU infants, followed by a higher level of response to certain vaccines in HEU than in HUU infants after vaccination. However, given the lack of data, we were not able to predict HEU infants' immune response to booster doses and the longevity of the resulting immune response. Our study aimed to provide this missing information.
MATERIALS AND METHODS
Cohort composition.
A prospective cohort study commenced in March 2009 in Cape Town, South Africa, to evaluate immune function in HEU and HUU infants over the first 2 years of life (12). The research ethics committees of Stellenbosch University and the University of British Columbia both approved the study. Infants of mothers with known HIV infection status were recruited at birth from the Tygerberg Academic Hospital (TAH) labor ward and evaluated at 0.5, 1.5, 3, 6, 12, 18, and 24 months. HIV infection status of the mothers was confirmed on presentation at TAH using serological HIV testing algorithms according to the South African national protocol (26). Infants received their vaccinations at public health clinics according to the then-applicable Expanded Program for Immunization (EPI). These included oral polio vaccine and Bacille Calmette-Guerin (BCG) vaccine at birth; diphtheria, tetanus toxoid (TT), cellular pertussis vaccines (DTP), Haemophilus influenzae type b (Hib) vaccine, hepatitis B, and oral polio vaccines (OPV) at 6, 10, and 14 weeks; measles vaccine at 9 and 18 months; and DTP as well as OPV booster doses at 18 months. At each visit, unblinded medical professionals assessed receipt of immunizations in each infant by evaluating the infant immunization card.
Laboratory assays.
A venous blood sample was collected from the infants at study visits and immediately transported to the laboratory, where plasma was frozen at −80°C for batch analysis of specific antibody levels. The HIV-negative status of both HEU and HUU infants was confirmed by HIV DNA PCR with an Amplicore HIV-1 DNA test kit, version 1.5 (Roche Diagnostics), at 0.5 and 1.5 months of life and using Cobas AmpliPrep/Cobas TaqMan HIV-1 test, v2.0, at 3 and 24 months of life.
Quantitative determination of specific IgG levels present in plasma to Bordetella pertussis, TT, Hib, hepatitis B virus, and measles virus were performed by standard commercial enzyme-linked immunosorbent assays (ELISAs) conducted according to the manufacturers' instructions. Specific IgG levels to B. pertussis, TT, and measles virus were evaluated using Serion ELISA classic kits (ESR120G, ESR108G, and ESR102G; Serion Immunodiagnostica GmbH, Würzburg, Germany). Hib-specific IgG was measured using VaccZyme human anti-Hib ELISA kits (MK016 and MK012; The Binding Site Ltd., Birmingham, England). HBsAg-specific IgG was measured using a human anti-hepatitis B surface antigen quantitative ELISA kit (4220-AHB; Alpha Diagnostic International Inc., San Antonio, TX). As recommended by the manufacturer, vaccine-specific IgG levels were expressed using the following units: anti-B. pertussis IgG in FDA units per milliliter, anti-TT IgG in international units per milliliter, anti-measles virus IgG and anti-HBsAg IgG in milli-international units per milliliter, and anti-Hib IgG in milligrams per milliliter. Quantitative determination of total IgG was evaluated at each visit using GenWay human IgG ELISA kits (GWB-A04A50; Genway Biotech, San Diego, CA); total IgG levels were measured in milligrams per milliliter. Antibody titers of 30 FDA U/ml were deemed protective for pertussis, as defined by the manufacturer. For measles virus, 200 mIU/ml was the cutoff for protection established by the manufacturer. The minimum protective antibody titers for tetanus of 0.1 IU/ml (27, 28), for hepatitis B virus (HepB) of 10 mIU/ml (28, 29), and for Hib of 1 mg/ml (28, 30) were considered protective.
Statistical analysis.
Statistical analysis of the antibody levels was performed using Microsoft Excel software (2007). Antibody responses were log transformed and subjected to unpaired, two-tailed Student's t tests for comparison of mean specific antibody levels between HEU and HUU infants at each time point. For comparison of longitudinal antibody responses we did not apply a correction for multiple comparisons, as these comparisons were not independent of one another. Statistical analysis of the baseline cohort characteristics was performed using R version 2.13.1 (R Foundation for Statistical Computing, Vienna, Austria); continuous variables were analyzed using the Student t test and the chi-square categorical variable, and two-sided alpha was set at 0.05.
RESULTS
Participant characteristics.
Fifty-nine infants were enrolled at 2 weeks; four were identified as HIV infected by HIV DNA PCR testing at 2 weeks of age and were excluded from analysis. Thus, 28 HUU infants and 27 HEU infants were retained at the first clinical visit; 17 HEU and 20 HUU infants were retained throughout the 2-year study. All HEU infants were confirmed to remain HIV uninfected by HIV DNA PCR at 1.5, 3, and 24 months of age. There was no significant difference in sex, birth weight, gestational age, or number retained at follow-up at 2 years between the two groups (Table 1). All HEU infants but one were exclusively formula fed; all but one HUU infant initiated breastfeeding at birth and continued breastfeeding with a median duration of exclusive breastfeeding of 12 weeks. Detailed descriptions of participant characteristics for this cohort were provided elsewhere (12).
Table 1.
Baseline infant characteristics by HIV exposure status
| Parametera | Infant exposure type |
P value | ||
|---|---|---|---|---|
| Total (n = 55) | HEU (n = 27) | HUU (n = 28) | ||
| No. (%) male | 22 (40) | 7 (26) | 15 (54) | 0.35 |
| Mean birth weight (95% CI), in g | 2,966 (2,857–3,075) | 2,945 (2,866–3,024) | 2,986 (2,830–3,142) | 0.7 |
| Mean gestational age (95% CI), in wk | 37.8 (37.1–38.4) | 37.7 (36.7–38.7) | 37.9 (37.0–38.7) | 0.8 |
| No. (%) of infants in follow-up at 24 mo | 37 (67) | 17 (63) | 20 (71) | 0.7 |
CI, confidence interval.
Specific antibody levels.
All HEU and HUU subjects were vaccinated within the same time frame relative to blood draws for serological analysis. None of the infants received DTP/Hib or HepB vaccination prior to the 0.5- and 1.5-month blood draw, thus antibody levels at these time points are reflective of transplacental maternal antibody transfer. Infants at the 6-month blood draw were also still measles vaccine naïve, as this vaccine was not administered until 9 months of age.
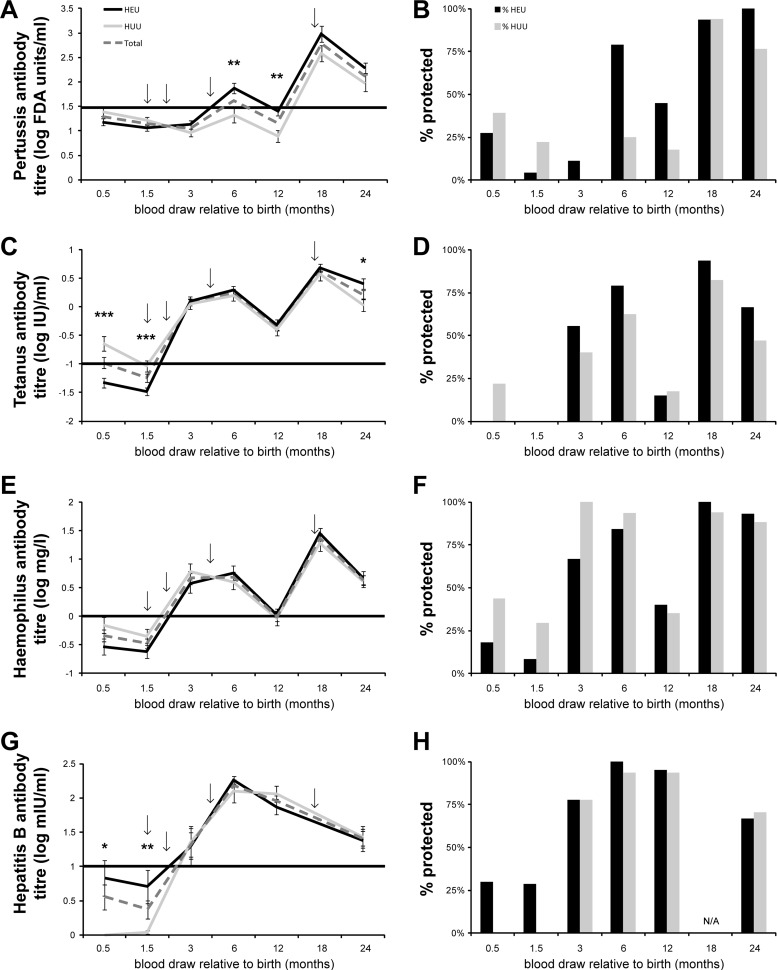
Prevaccination, at 0.5 and 1.5 months, HEU infants had overall lower antibody-specific levels for tetanus, pertussis, and Hib (Fig. 1A, C, E, and G). However, prevaccine, only anti-tetanus levels were statistically lower in HEU (P < 0.025). Conversely, HepB antibody levels were higher in HEU infants prior to vaccination (P < 0.025), although only one-quarter of HEU infants had levels considered to be protective.
Fig 1.
(A, C, E, and G) Specific antibody levels were measured in HEU (black line) and HUU (grey line) infants at 0.5, 1.5, 3, 6, 12, 18, and 24 months of age for all vaccines except hepatitis B, for which the 18-month time point was omitted (N/A) due to insufficient sample. The total response of the two groups combined is shown with the dashed line. The vaccine antibody level is indicated on the y axis. Arrows indicate the time of vaccination. The horizontal black line indicates the minimal antibody level associated with protection. Error bars indicate standard errors of the means (SEM). *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B, D, F, and H) Individual antibody titers were compared to established minimum protective antibody levels. The proportion of subjects whose titers exceeded the minimum level for protection are shown for HEU (black bar) and HUU (gray bar) infants.
Vaccine-specific titers of HEU and HUU infants reached similar levels after 2 vaccine doses as measured at the 3-month blood draw. However, at that time point (3 months) the average antibody titers reached protective levels in >50% of recipients only for tetanus, Hib, and HepB, while <20% of infants had protective anti-pertussis antibody levels (Fig. 1B, D, F, and H). Of note, HEU infants had significantly higher titers of anti-pertussis antibody after the third dose (by 6 months). As a result, a higher proportion of HEU than HUU infants reached protective levels at 6 months of age; protective anti-pertussis titers in HUU were not achieved until 18 months of age (after the fourth vaccine dose). Notably, for either group, once protective levels were reached, antibodies were maintained at or above average protective titers through 2 years of age.
Individual titers were analyzed within each group to determine the proportion of subjects with protective antibody titers. Prior to vaccination, more than 25% of infants in both HEU and HUU groups had protective levels of anti-pertussis antibodies (Fig. 1B). More than one-quarter of HEU subjects were also protected from HepB before vaccination, whereas no HUU infants had protective anti-HepB titers prior to vaccination (Fig. 1H). In contrast, the proportion of HUU infants protected from Hib was greater than that of the HEU group (Fig. 1F). Very few infants in either group had protective titers of anti-tetanus antibodies prior to their first vaccination (Fig. 1D).
On average, protective levels of anti-measles virus antibody were not reached by either the HEU or HUU group until after the first vaccine dose had been given at 9 months (Fig. 2A). When measured at 2 years, anti-measles virus antibody titers remained unchanged following administration of the second dose (administered at 18 months). The average levels of anti-measles virus antibody were nearly indistinguishable between the HEU and HUU groups at all time points throughout the first 2 years of life. Analyzing the individual subjects' anti-measles virus antibody revealed fewer than one-quarter of subjects with protective prevaccination titers in both groups at 2 weeks, and this dropped further by 6 weeks. The highest proportion of protected subjects was found at 12 months in HUU infants and at 18 months in HEU infants, with approximately three-quarters of subjects protected. Interestingly, the number of subjects deemed protected decreased at 2 years only in the HUU group, with only about half maintaining protective measles virus-specific antibody titers (Fig. 2B).
Fig 2.
(A) Specific antibody levels were measured in HEU (black line) and HUU (gray line) infants at 0.5, 1.5, 12, 18, and 24 months of age. The total response of the two groups combined is shown with the dashed line. Arrows indicate the time of vaccination. The horizontal black line indicates the minimal antibody level associated with protection. The vaccine antibody level is indicated on the y axis. Error bars indicate SEM. (B) Individual antibody titers were compared to minimum protective antibody levels. The proportion of subjects whose titers exceeded the minimum level for protection were shown for HEU (black bar) and HUU (gray bar) infants.
Total immunoglobulin.
Total IgG levels were measured at all time points in order to determine whether detected differences in vaccine-specific IgG were due to nonspecific differences in total IgG (e.g., hypergammaglobulinemia) (31). Equivalent total IgG levels, with age-appropriate physiological hypogammaglobulinemia at 1.5 and 3 months, were observed in HEU and HUU infants (Fig. 3).
Fig 3.
Total IgG levels were measured in HEU (gray bar) and HUU (black bar) infants at 0.5, 1.5, 3, 6, and 12 months of age. The total IgG level is indicated on the y axis. Error bars indicate SEM. P < 0.05 indicated significance.
DISCUSSION
In this prospective, longitudinal comparison of antibody response to vaccines administered over the first 24 months, we identified robust serological responses to the EPI vaccines in both groups. With the exception of an earlier response to the pertussis vaccine and a higher anti-tetanus titer at 24 months in HEU than HUU infants, the vaccine responses were comparable overall in magnitude between HEU and HUU infants. This suggests that HEU infants mount a quantitative response to EPI vaccines similar to that of HUU infants over the first 2 years of life.
The diminished baseline prevaccine antibody levels for tetanus (significant) and Hib (not significant) we detected in HEU infants could be due to lower levels of vaccine-specific antibodies in the mother, placental dysfunction resulting in decreased antibody transfer, or decreased half-life of transferred maternal antibodies (21, 22, 24, 32). On the other hand, the higher levels of prevaccine HepB antibodies observed in HEU infants likely reflected maternal HepB infection in HIV-infected mothers; indeed, high variability in the prevaccine HepB antibody levels were observed in our HEU cohort, suggesting that individual differences, such as maternal HepB infection, are involved. This, however, was not formally evaluated.
HEU infants developed significantly higher anti-pertussis titers than HUU infants at 6 and 12 months of age, extending the previous observation of Jones et al., who showed increased response at 4 months of age (22). Anti-B. pertussis titers had already reached protective levels in HEU infants by 6 months after receiving only 3 doses in total. HEU infants therefore may be protected from pertussis infection sooner than HUU infants, who attained protective titers of anti-B. pertussis antibody after the 18-month booster. This could relate to decreased maternal antibody interference during vaccine priming secondary to a lower level of placentally transferred antibodies in HEU than HUU infants (33).
Overall, the period of suboptimal protection from pertussis in infancy was unexpectedly long for both HEU and HUU infants, 6 to 12 months, requiring 3 to 4 doses of vaccine. Since the vaccine antibody response to Hib, tetanus, hepatitis B, and measles virus reached protective levels after 1 or, at most, 2 doses, an overall lower general immune response to vaccination in our subjects seems unlikely to be the cause for the suboptimal response to pertussis vaccination. The most critical period for severe pertussis is early in life; the prolonged gap in protective levels of antibodies of South African infants thus appears worthy of further investigation.
The prospective, longitudinal nature of our study, and especially the extension up to 24 months of age, provide the much-needed insight into long-term vaccine-induced protection in this highly vulnerable population. However, there are several limitations to our study. First and foremost, our sample size was small. However, it should be noted that our results confirm previous cross-sectional reports on vaccine responses in HEU infants (21, 22), and in their aggregate they strongly support our overall conclusion. Second, the feeding mode differed significantly between HUU and HEU infants, as formula feeding of HEU infants was prevalent in the Western Cape during our study. Breast- versus bottle feeding is known to influence vaccine responses. Third, we did not determine maternal antibody titers to the measured vaccine antigens, which clearly could have differed between HIV-infected and uninfected mothers and influenced the vaccine response of their respective infants. However, the aim of our study was to define the long-term vaccine response of HEU infants compared to HUU infants, not the cause of the differences. Differences in feeding preference reflect the reality of feeding patterns of HEU infants in South Africa (and elsewhere) and, along with differences in maternal antibody titers, may have contributed to differences in vaccine responses between HEU and HUU infants. Lastly, we did not collect information about the clinical status of HIV-infected mothers, who might have influenced their infant's response to vaccination, thus we cannot comment on the impact of maternal HIV disease on HEU infant vaccine responses.
For comparison of antibody responses, we did not apply a correction for multiple comparisons, as these comparisons were not independent of one another; this must be considered when interpreting our results. We reasoned that a given vaccine-specific antibody response would be interrelated when comparing time points for reasons such as interference from preexisting serum antibody levels and preestablished B cell memory largely dictating the strength of booster responses. Furthermore, general immune attributes that impact an individual's vaccine response likely apply to other vaccines. Lastly, we did not perform functional studies regarding antibody responses following immunization. This is important, as, for example, a study comparing responses to the 7-valent pneumococcal vaccine in HEU and HUU infants showed similar responses in HEU and HUU infants for 6 of 7 serotypes and an even better response than HUU infants to one serotype (6B). However, upon evaluation of qualitative function by the opsonophagocytic killing assay, HEU infants in fact required higher concentrations of antibody for 50% killing activity against one of the serotypes (19F) (34). Thus, we cannot exclude qualitative differences between HEU and HUU infants to the EPI vaccines we tested.
In conclusion, aside from and in spite of selected differences of prevaccination antibody levels, HEU infants responded quantitatively at least as well as HUU infants to current standard EPI vaccination.
ACKNOWLEDGMENTS
This work was supported in part by the National Institute of Allergy and Infectious Diseases, NIH, grant number N01 AI50023, British Columbia Children's Hospital Foundation, The Martha Piper Fund, and the Peter Wall Institute for Advanced Studies. T.R.K. is supported in part by a Burroughs Wellcome Career Award in the Biomedical Sciences and a Michael Smith Foundation for Health Research Career Investigator Award. B.A.R. is supported in full by the Michael Smith Foundation for Health Research [ST-SGS-02657(09--1)CLIN]. A.L.S. is supported by a CIHR Canada-HOPE Scholarship. D.P.S. is the Sauder Family Professor of Pediatric Infectious Diseases. Laboratory analyses in South Africa were funded through the National Health Laboratory Service Research Trust, grant number TY94171; National Health Laboratory Service, K Fund numbers KNC97 and KNC103; the Poliomyelitis Research Foundation, short-term research grant number 10/02; and the Harry Crossley Foundation, numbers 5415 and 5762. Student bursaries were awarded by the Poliomyelitis Research Foundation, numbers 10/31 and 11/37.
Footnotes
Published ahead of print 31 October 2012
REFERENCES
- 1. Wood N, Siegrist CA. 2011. Neonatal immunization: where do we stand? Curr. Opin. Infect. Dis. 24:190–195 [DOI] [PubMed] [Google Scholar]
- 2. Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375:1969–1987 [DOI] [PubMed] [Google Scholar]
- 3. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. 2006. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367:1747–1757 [DOI] [PubMed] [Google Scholar]
- 4. WHO 2009. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report. WHO, Geneva, Switzerland [Google Scholar]
- 5. UNAIDS 2011. How to get to zero: faster. Smarter. Better. UNAIDS World AIDS Day Report 2011. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland [Google Scholar]
- 6. Epalza C, Goetghebuer T, Hainaut M, Prayez F, Barlow P, Dediste A, Marchant A, Levy J. 2010. High incidence of invasive group B streptococcal infections in HIV-exposed uninfected infants. Pediatrics 126:e631–e638 doi:10.1542/peds.2010-0183 [DOI] [PubMed] [Google Scholar]
- 7. Filteau S. 2009. The HIV-exposed, uninfected African child. Trop. Med. Int. Health 14:276–287 [DOI] [PubMed] [Google Scholar]
- 8. Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, Mutasa K, Ruff A, Ward B. 2011. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr. Infect. Dis. J. 30:45–51 [DOI] [PubMed] [Google Scholar]
- 9. Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, Moulton LH, Salama P, Ward BJ. 2007. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr. Infect. Dis. J. 26:519–526 [DOI] [PubMed] [Google Scholar]
- 10. Mussi-Pinhata MM, Freimanis L, Yamamoto AY, Korelitz J, Pinto JA, Cruz ML, Losso MH, Read JS. 2007. Infectious disease morbidity among young HIV-1-exposed but uninfected infants in Latin American and Caribbean countries: the National Institute of Child Health and Human Development International Site Development Initiative Perinatal Study. Pediatrics 119:e694–e704 doi:10.1542/peds.2006-1856 [DOI] [PubMed] [Google Scholar]
- 11. Singh HK, Gupte N, Kinikar A, Bharadwaj R, Sastry J, Suryavanshi N, Nayak U, Tripathy S, Paranjape R, Jamkar A, Bollinger RC, Gupta A, India Study Team S. 2011. High rates of all-cause and gastroenteritis-related hospitalization morbidity and mortality among HIV-exposed indian infants. BMC Infect. Dis. 11:193 doi:10.1186/1471-2334-11-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slogrove A, Reikie B, Naidoo S, De Beer C, Ho K, Cotton M, Bettinger J, Speert D, Esser M, Kollmann T. 3 May 2012. HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. J. Trop. Pediatr. [Epub ahead of print.] doi:10.1093/tropej/fms019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuhn L, Sinkala M, Semrau K, Kankasa C, Kasonde P, Mwiya M, Hu CC, Tsai WY, Thea DM, Aldrovandi GM. 2010. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin. Infect. Dis. 50:437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Souza TL, Fernandes RC, Medina-Acosta E. 2011. Maternal HIV infection and antibody responses in uninfected infants. JAMA 305:1964–1965 [DOI] [PubMed] [Google Scholar]
- 15. Kafulafula G, Hoover DR, Taha TE, Thigpen M, Li Q, Fowler MG, Kumwenda NI, Nkanaunena K, Mipando L, Mofenson LM. 2010. Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1-uninfected children born to HIV-infected women in Malawi. J. Acquir. Immune Defic. Syndr. 53:6–13 [DOI] [PubMed] [Google Scholar]
- 16. Onyango-Makumbi C, Bagenda D, Mwatha A, Omer SB, Musoke P, Mmiro F, Zwerski SL, Kateera BA, Musisi M, Fowler MG, Jackson JB, Guay LA. 25 September 2009. Early weaning of HIV-exposed uninfected infants and risk of serious gastroenteritis: findings from two perinatal HIV prevention trials in Kampala, Uganda. J. Acquir. Immune Defic. Syndr. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McNally LM, Jeena PM, Lalloo U, Nyamande K, Gajee K, Sturm AW, Goldblatt D, Tomkins AM, Coovadia HM. 2005. Probable mother to infant transmission of Pneumocystis jiroveci from an HIV-infected woman to her HIV-uninfected infant. AIDS 19:1548–1549 [DOI] [PubMed] [Google Scholar]
- 18. Hygino J, Lima PG, Filho RG, Silva AA, Saramago CS, Andrade RM, Andrade DM, Andrade AF, Brindeiro R, Tanuri A, Bento CA. 2008. Altered immunological reactivity in HIV-1-exposed uninfected neonates. Clin. Immunol. 127:340–347 [DOI] [PubMed] [Google Scholar]
- 19. Faye A, Pornprasert S, Mary JY, Dolcini G, Derrien M, Barre-Sinoussi F, Chaouat G, Menu E. 2007. Characterization of the main placental cytokine profiles from HIV-1-infected pregnant women treated with anti-retroviral drugs in France. Clin. Exp. Immunol. 149:430–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heidari S, Mofenson L, Cotton MF, Marlink R, Cahn P, Katabira E. 2011. Antiretroviral drugs for preventing mother-to-child transmission of HIV: a review of potential effects on HIV-exposed but uninfected children. J. Acquir. Immune Defic. Syndr. 57:290–296 [DOI] [PubMed] [Google Scholar]
- 21. Abramczuk BM, Mazzola TN, Moreno YM, Zorzeto TQ, Quintilio W, Wolf PS, Blotta MH, Morcillo AM, da Silva MT, Vilela MM. 2011. Impaired humoral response to vaccines among HIV-exposed uninfected infants. Clin. Vaccine Immunol. 18:1406–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. 2011. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 305:576–584 [DOI] [PubMed] [Google Scholar]
- 23. Madhi SA, Kuwanda L, Saarinen L, Cutland C, Mothupi R, Kayhty H, Klugman KP. 2005. Immunogenicity and effectiveness of Haemophilus influenzae type b conjugate vaccine in HIV infected and uninfected African children. Vaccine 23:5517–5525 [DOI] [PubMed] [Google Scholar]
- 24. Scott S, Moss WJ, Cousens S, Beeler JA, Audet SA, Mugala N, Quinn TC, Griffin DE, Cutts FT. 2007. The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin. Infect. Dis. 45:1417–1424 [DOI] [PubMed] [Google Scholar]
- 25. National Department of Health SA 2010. The National Antenatal Sentinel HIV and Syphilis Prevalence Survey, South Africa. South African National Department of Health, Pretoria, South Africa: [Google Scholar]
- 26. South African National AIDS Council 2010. Clinical guidelines: PMTCT (prevention of mother-to-child transmission). In Motsoaledi A. (ed), SANDoHealth AIDS. Department of Health, Republic of South Africa, Pretoria, South Africa [Google Scholar]
- 27. McComb JA. 1964. The prophylactic dose of homologous tetanus antitoxin. N. Engl. J. Med. 270:175–178 [DOI] [PubMed] [Google Scholar]
- 28. Plotkin SA. 2008. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47:401–409 [DOI] [PubMed] [Google Scholar]
- 29. Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. 1999. What level of hepatitis B antibody is protective? J. Infect. Dis. 179:489–492 [DOI] [PubMed] [Google Scholar]
- 30. Kayhty H, Peltola H, Karanko V, Makela PH. 1983. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J. Infect. Dis. 147:1100. [DOI] [PubMed] [Google Scholar]
- 31. Silva MT, Centeville M, Tani SM, Toro AA, Rossi C, Vilela MM. 2001. Serum immunoglobulins in children perinatally exposed to human immunodeficiency virus. J. Pediatr. 77:209–218 [DOI] [PubMed] [Google Scholar]
- 32. Miyamoto M, Pessoa SD, Ono E, Machado DM, Salomao R, Succi RC, Pahwa S, de Moraes-Pinto MI. 2010. Low CD4+ T-cell levels and B-cell apoptosis in vertically HIV-exposed noninfected children and adolescents. J. Trop. Pediatr. 56:427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siegrist CA. 2003. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21:3406–3412 [DOI] [PubMed] [Google Scholar]
- 34. Madhi SA, Adrian P, Cotton MF, McIntyre JA, Jean-Philippe P, Meadows S, Nachman S, Kayhty H, Klugman KP, Violari A. 2010. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J. Infect. Dis. 202:355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]