Abstract
We measured the vaccination response to the new H1N1 virus in relation to the lymphocyte count prior to vaccination in pediatric cancer patients. The absolute lymphocyte count above the lower normal limits (LNL) for age prior to vaccination predicts the response to influenza vaccination in pediatric cancer patients treated with chemotherapy.
TEXT
The efficacy of vaccination in (pediatric) cancer patients is still a topic of debate. Most chemotherapeutic treatments lead to an impairment of the cellular immune response, which is the main risk factor for viral infections. These infections can cause severe morbidity in children with cancer (1, 2, 3). In April of 2009, a new swine-origin influenza A H1N1 virus was detected in humans and was subsequently declared as an influenza pandemic by the World Health Organization (WHO) (4). According to the national and international guidelines, vaccination was recommended for immunocompromised children (5). Vaccination against this new influenza A H1N1 virus, which is a neoantigen, allowed us to assess immunologic determinants in pediatric cancer patients that predict a protective response following vaccination. We studied the vaccination response to this new swine-origin influenza A H1N1 virus in pediatric cancer patients in relation with absolute counts of the lymphocytes and its subpopulations.
Children with cancer being treated with chemotherapy, or within 6 months after the end of chemotherapy, were vaccinated twice (3-week interval) with an intramuscular injection with an inactivated split-virion preparation of the A/California/07/2009(H1N1)v-like strain (X-179A), which contained 7.5 μg of hemagglutinin per dose of 0.5 ml. Patients with a known PCR-proven H1N1 influenza infection before the first vaccination were excluded. Blood sampling was done before vaccination and 3 weeks following the second vaccination. Antibody levels specific for A/California/07/2009(H1N1) were determined by serum hemagglutination inhibition (HI) assay before vaccination and 3 weeks after vaccination. A protective response was defined as achieving a HI antibody titer of ≥1:40 following vaccination. Prior to the first influenza vaccination (day −10 to day −1), blood samples were collected from the patients to analyze the total white blood count (TWBC) and the absolute lymphocyte count. Three weeks after vaccination, an additional 3 ml of heparin blood was obtained from a random subset of patients for analysis of lymphocyte subpopulations using standard flow cytometrical immunophenotyping. Lower normal limits (LNL) for the absolute lymphocyte counts according to age were defined as 1,700/mm3 for age 2 to 4 years, 1,100/mm3 for age 5 to 9 years, and 1,000/mm3 for age 10 years to adult (6). A comparison between the vaccination response and categorical factors was studied using the two-sided Fisher exact test. A comparison between the vaccination response and continuous variables was studied using the using the Mann-Whitney U test.
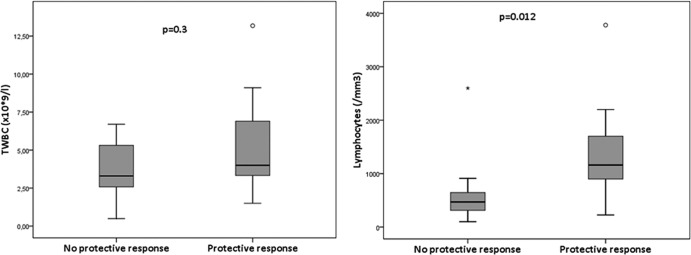
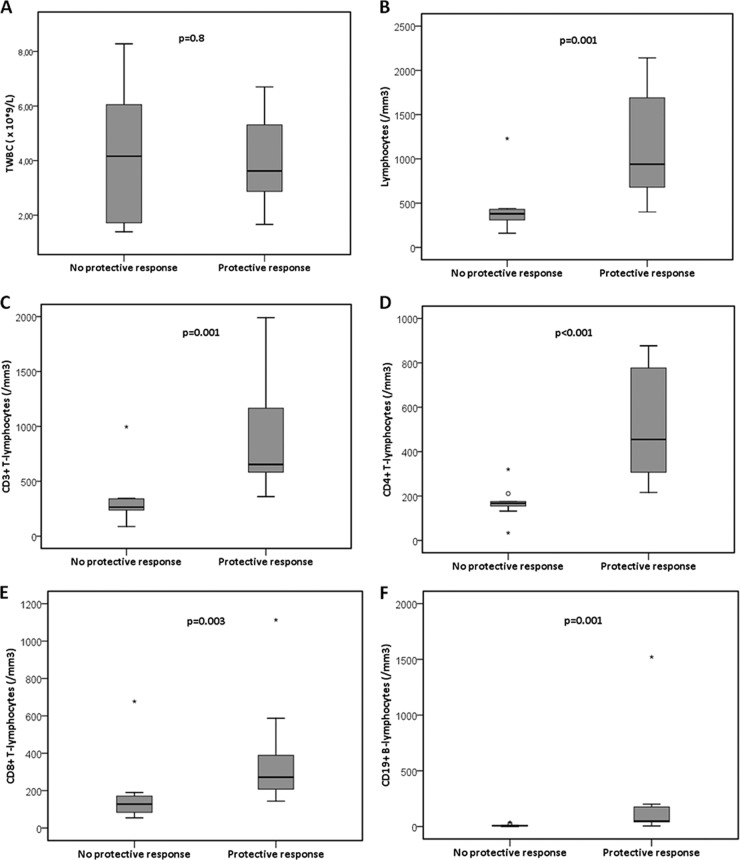
Of the 41 included patients, 2 patients did not receive the vaccinations, and 6 patients did not receive the second vaccination for different reasons. Thirty-three patients were analyzed further (15 female and 18 male; median age, 6 years [range, 2 to 17 years]). The patients suffered from acute lymphoblastic leukemia (ALL) (n = 19), acute myeloid leukemia (AML) (n = 2), Langerhans cell histiocytosis (LCH) (n = 1), atypical teratoid rabdoid tumor (ATRT) (n = 1), medulloblastoma (n = 3), neuroblastoma (n = 1), non-Hodgkin lymphoma (NHL) (n = 1), optical glioma (n = 3), rhabdomyosarcoma (n = 1), and Wilms' tumor (n = 1). Six of the 33 patients were treated with oseltamivir phosphate because of fever of unknown origin, later proven to be H1N1 negative by PCR. Twenty-nine patients were vaccinated during chemotherapy, four patients within 6 months after chemotherapy. Analysis of the immune response was possible in 31 patients. Two patients were excluded from the analysis of the immune response to vaccination: one patient had a HI antibody titer of ≥320 before the first vaccination without clinical symptoms, and one patient developed a PCR-proven H1N1 directly after the first vaccination dose. Of the 31 patients, 20 (65%) showed a rise in titer after vaccination, ranging from 1:20 to 1:640. Of these 31 patients, 18 (58%) had a protective immune response (>1:40). Twenty patients were treated for hematological malignancies, and 11 patients were treated for solid tumors. There was no significant difference in the vaccination response between the two diagnosis groups. We were able to obtain values of the TWBC and the absolute lymphocyte count from 28 patients in the period 10 days to 1 day prior to the first vaccination. The relation between these two parameters and the vaccination response is shown in Fig. 1. There is a significant difference in the absolute lymphocyte count prior to the first vaccination between the patients with a protective versus no protective vaccination response (P = 0.012). Absolute lymphocyte counts for above the LNL for age were seen in 13 of 28 patients (46%). In 12 of these 13 patients (92%), a protective response to vaccination was seen. In the 15 patients with absolute lymphocyte counts below the LNL for age, only 5 (33%) had a protective response to vaccination (P = 0.002). We were able to obtain samples for lymphocyte subpopulations in 22 patients. In Fig. 2, the relation between the total white blood cell count (TWBC) and the lymphocyte subpopulations and the response to vaccination is shown. There is a significant relation between the response to vaccination and levels of all the different lymphocyte subpopulations (P < 0.001 for the CD4+ T cells, and P = 0.001 for the other subpopulations) (Fig. 2). We found that if the absolute CD4+ T cell count is less than 200/mm3, no protective vaccination response can be observed.
Fig 1.
Relation between the total white blood count and the absolute lymphocyte count prior to the first vaccination and the response to vaccination. The relation between the total white blood count and the absolute lymphocyte count was studied using the Mann-Whitney U test.
Fig 2.
Relation between the total white blood count (A) and the lymphocyte subpopulations (B to F) and the response to vaccination. The relation between the absolute values of the lymphocyte subpopulations was studied using the Mann-Whitney U test.
Vaccination of immunocompromised pediatric cancer patients with inactivated split-virion preparation of influenza vaccine is considered to be safe (1–3). In our study, 58% of the patients had a protective response to vaccination. This is similar to data on vaccination response in pediatric cancer patients from previous influenza vaccinations (3). In addition, it is also similar to well-controlled HIV-infected patients, who showed a protective response in 60% (7). An important finding of our study was that an absolute lymphocyte count above the lower normal limit for age prior to vaccination led to a protective response to vaccination. This result gives us a predictive tool to determine at what moment vaccination in pediatric cancer patients will be effective. Shahin and colleagues failed to show a significant influence of a prevaccination lymphocyte count (above 1,000/mm3 versus 1,000/mm3 or less) on seroresponse rates in children with solid tumors (8). Yen and colleagues obtained results similar to those in our study. They showed that patients with absolute lymphocyte counts less than 1,500/mm3 during the vaccination period had a nonseroresponse (9). Assessment of absolute counts of lymphocyte subpopulations after vaccination showed that a protective response to vaccination was not observed in patients with an absolute CD4+ T lymphocyte count of less than 200/mm3. CD4+ T lymphocyte counts were only determined after vaccination, thus it could be possible that CD4+ T lymphocytes were slightly different at the time of vaccination. The crucial role of CD4+ T cells for the generation of an effective antibody response to influenza vaccination has been described previously (7). In HIV-infected individuals, suboptimal influenza vaccine responses with CD4+ T cell counts <200/mm3 have been reported repeatedly (10). The role of CD4+ T cells for an effective antibody response against the new H1N1 vaccine in HIV-infected individuals has also been reported (11). H1N1 vaccination has been clearly effective in the healthy adult population, with a protective vaccination response in more than 95% (12), as well as in healthy infants and children (92.5%) (13). The best approach to measure immunogenicity to influenza vaccination is a much-debated topic, especially in oncology patients (2). It should be appreciated that hemagglutination (HA) inhibition measures only part of the host response, and it has become clear that the neutralizing and protective antibodies to the H1N1 influenza strain in many patients following natural infection were often not hemagglutination inhibition positive, as they target the hemagglutinin stalk and not the hemagglutinin globular head (14).
In summary, the absolute lymphocyte count above the LNL in pediatric cancer patients predicts a protective response to vaccination. These findings have important implications for the establishment of a response to vaccination in pediatric cancer patients during treatment with chemotherapy.
ACKNOWLEDGMENT
We have no potential conflicts of interest, and no financial support was received for this study.
Footnotes
Published ahead of print 21 November 2012
REFERENCES
- 1. Chisholm JC, Devine T, Charlett A, Pinkerton CR, Zoambon M. 2001. Response to influenza immunization during treatment for cancer. Arch. Dis. Child. 84:496–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pollyea D, Brown JMY, Horning SJ. 2010. Utility of influenza vaccination for oncology patients. J. Clin. Oncol. 28:2481–2490 [DOI] [PubMed] [Google Scholar]
- 3. Goossen GM, Kremer LC, van de Wetering MD. 2009. Influenza vaccination in children being treated with chemotherapy for cancer. Cochrane Database Syst. Rev. doi:10.1002/14651858.CD006484.pub2 [DOI] [PubMed] [Google Scholar]
- 4. Halasa NB. 2010. Update on the 2009 pandemic influenza H1N1 in children. Curr. Opin. Pediatr. 22:83–87 [DOI] [PubMed] [Google Scholar]
- 5. CDC 2009. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 58:1–8 [PubMed] [Google Scholar]
- 6. Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, Hooijkaas H, van Dongen JJ. 1997. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J. Pediatr. 130:388–393 [DOI] [PubMed] [Google Scholar]
- 7. Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert AK, Brauer V, Banzhoff A, Rappuoli R, Del Giudice G, Castellino F. 2009. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc. Natl. Acad. Sci. U. S. A. doi:10.1073/pnas.0813390106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shahin K, Lina B, Billaud G, Pedone C, Faure-Conter C. 2012. Successful H1N1 influenza vaccination of children receiving chemotherapy for solid tumors. J. Pediatr. Hematol. Oncol. 34:e228–e231 [DOI] [PubMed] [Google Scholar]
- 9. Yen TY, Jou ST, Yang YL, Chang HH, Lu MY, Lin DT, Lin KH, Huang LM, Chang LY. 2011. Immune response to 2009 pandemic H1N1 influenza virus A monovalent vaccine in children with cancer. Pediatr. Blood Cancer 57:1154–1158 [DOI] [PubMed] [Google Scholar]
- 10. Yamanaka H, Teruya K, Tanaka M, Kikuchi Y, Takahashi T, Kimura S, Oka S, HIV/Influenza Vaccine Study Team 2005. Efficacy and immunologic responses to influenza vaccine in HIV-1-infected patients. J. Acquir. Immune Defic. Syndr. 39:167–173 [PubMed] [Google Scholar]
- 11. Tebas P, Frank I, Lewis M, Quinn J, Zifchak L, Thomas A, Kenney T, Kappes R, Wagner W, Maffei K, Sullivan K, Center for AIDS Research and Clinical Trials Unit of the University of Pennsylvania 2010. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS 24:2187–2192 [DOI] [PubMed] [Google Scholar]
- 12. Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, Dawson G, Hu W, Leggio C, Washington D, Basser RL. 2009. Response to a monovalent 2009 influenza A (H1N1) vaccine. N. Engl. J. Med. 361:2405–2413 [DOI] [PubMed] [Google Scholar]
- 13. Nolan TN, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, Nissen M, Marshall H, Booy R, Heron L, Hartel G, Lai M, Basser R, Gittleson C, Greenberg M. 2010. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in infants and children. JAMA 303:37–46 [DOI] [PubMed] [Google Scholar]
- 14. Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O'Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]