Abstract
There is a constant need for improved adjuvants to augment the induction of immune responses against tumor-associated antigens (TAA) during immunotherapy. Previous studies have established that listeriolysin O (LLO), a cholesterol-dependent cytolysin derived from Listeria monocytogenes, exhibits multifaceted effects to boost the stimulation of immune responses to a variety of antigens. However, the direct ability of LLO as an adjuvant and whether it acts as a pathogen-associated molecular pattern (PAMP) have not been demonstrated. In this paper, we show that a detoxified, nonhemolytic form of LLO (dtLLO) is an effective adjuvant in tumor immunotherapy and may activate innate and cellular immune responses by acting as a PAMP. Our investigation of the adjuvant activity demonstrates that dtLLO, either fused to or administered as a mixture with a human papillomavirus type 16 (HPV-16) E7 recombinant protein, can augment antitumor immune responses and facilitate tumor eradication. Further mechanistic studies using bone marrow-derived dendritic cells suggest that dtLLO acts as a PAMP by stimulating production of proinflammatory cytokines and inducing maturation of antigen-presenting cells (APC). We propose that dtLLO is an effective adjuvant for tumor immunotherapy, and likely for other therapeutic settings.
INTRODUCTION
The Gram-positive facultative intracellular pathogen Listeria monocytogenes expresses a highly conserved pore-forming toxin known as listeriolysin O (LLO). LLO is a member of a large family of cholesterol-dependent cytolysins (CDCs) found in several bacterial pathogens (1). In L. monocytogenes, LLO is the primary virulence factor and is essential for its pathogenesis (2). During intracellular infection, the pore-forming activity of LLO allows L. monocytogenes to escape from phagocytic or endocytic vacuoles into the host cell cytosol, where the bacteria are able to multiply proficiently (3, 4). CDCs have no known protein receptors, although cholesterol is a prerequisite for membrane pore formation. However, LLO and other CDCs are potent signaling molecules that trigger a variety of cellular responses. Stimulation of cells with LLO results in a multifaceted response involving production of cytokines (5), influx of calcium signaling (6), epigenetic modifications (7), alteration of immunosuppression (8, 9), and induction of apoptosis in T lymphocytes and dendritic cells (10). It has been suggested that the mechanism of signaling by LLO and other bacterial cytolysins is through their ability to act as pathogen-associated molecular patterns (PAMPs) and to interact with pathogen recognition receptors (PRRs) such as Toll-like receptor 4 (TLR4) (11).
In accordance with the PAMP hypothesis, LLO has been reported to improve antigen presentation in the context of major histocompatibility complex (MHC) class I molecules and to enhance T cell-mediated immune responses when genetically fused to (12–14), mixed with (15), or conjugated to (16) antigens. The adjuvant properties of LLO have been demonstrated not only in the context of L. monocytogenes (17) but also in various vaccine platforms, such as modified vaccinia virus Ankara (MVA) or plasmid DNA (pDNA), and as a protein carrier for anti-idiotypic immune therapy of non-Hodgkin's lymphoma (12, 13, 17–20), suggesting broad applicability in immunotherapeutic strategies. Although there are sufficient data supporting LLO as a potent immune activator, how it exerts these effects is unknown. A direct measure of PAMP-like activity by LLO in cellular assays or in vivo is not feasible due to the toxicity associated with its pore-forming, cytolytic activity. To overcome this obstacle, we constructed a detoxified, nonhemolytic form of LLO (dtLLO), with mutations in three amino acids that are crucial for its binding to cholesterol (21), and then tested its ability to act as an adjuvant to the human papillomavirus type 16 (HPV-16) E7 protein in a mouse model of HPV-associated cancer (22). The study presented here demonstrates that dtLLO is a novel protein adjuvant in cancer immunotherapy that can be administered as either a dtLLO-antigen fusion protein or a mixture with HPV-16 E7 protein. Furthermore, we provide evidence that this adjuvant effect is due to dtLLO stimulating the synthesis of proinflammatory cytokines and inducing maturation of antigen-presenting cells (APC) in a PAMP-like manner.
MATERIALS AND METHODS
Mice and cell lines.
Six- to 8-week-old C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). The Tlr4−/− strain B6.B10ScN-tlr4lps-del/JthJ was purchased from Jackson Laboratories. The C57BL/6-syngeneic TC-1 tumor cell line is immortalized with HPV-16 E6 and E7 and transformed with the c-Ha-ras oncogene (22). TC-1 tumor cells express low levels of E6 and E7 and are highly tumorigenic. TC-1 cells were grown in RPMI 1640 medium with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 100 mM nonessential amino acids, 1 mM sodium pyruvate, and 50 mM 2-mercaptoethanol (2-ME) at 37°C with 5% CO2.
Construction of dtLLO, E7, and dtLLO-E7 expression vectors.
The hly gene that encodes LLO was amplified from L. monocytogenes 10403s by PCR and cloned into the pET29b plasmid by use of the NdeI and BamHI restriction sites upstream of a region encoding an in-frame C-terminal 6×His tag. The cholesterol binding domain (CBD) was modified by site-directed mutagenesis using the primers indicated below. An internal NheI restriction site in the hly gene was utilized for cloning of the mutated C-terminal region containing the CBD. Briefly, separate PCRs amplified two overlapping segments containing the mutated CBD. One PCR amplified the fragment between the NheI site (bold letters) in the hly gene and the CBD by using primers DTLLOF1 (GCTAGCTCATTTCACATCGT) and DTLLOR1 (TCTTGCAGCTTCCCAAGCTAAACCAGTCGCTTCTTTAGCGTAAACATTAATATT), which introduced mutations into the CBD (represented by underlined regions). The fragment between the CBD and the BamHI site (bold letters) was amplified in another PCR by using primers DTLLOF2 (GAAGCGACTGGTTTAGCTTGGGAAGCTGCAAGAACGGTAATTGATGACCGGAAC) and DTLLOR2 (GGATCCTTATTAGTGGTGGTGGTGGTGGTGTTCGATTGG), which introduced the same mutations into the CBD. These 2 PCR products were annealed, and the product was used as the template in a PCR using primers DTLLOF1 and DTLLOR2. The fragment between the NheI and BamHI sites in the pET29b-LLO plasmid, containing the original CBD sequence, was replaced with the resulting overlapping PCR fragment containing the mutated CBD sequence.
To create a dtLLO-E7 fusion protein expression plasmid, the C-terminal region of dtLLO was joined to E7 by splicing by overlap extension PCR (SOE PCR) using the following primers: DTLLOF1 (GCTAGCTCATTTCACATCGT), DTLLO-E7R (CATGCAATGTAGGTGTATCTCCATGTTCGATTGGATTATCTACTTTATTAC), DTLLOE7F (GTAATAAAGTAGATAATCCAATCGAACATGGAGATACACCTACATTGCATG), and E7-His R (CTCACTCGAGGTGGTGGTGGTGGTGGTGTGGTTTGTGAGAACAGATGG). The DNA fragment containing the C-terminal region of dtLLO fused to E7 was cloned into the pET29b-DTLLO plasmid at the NheI and XhoI restriction sites (indicated in bold) to create pET29b-dtLLOE7 (23).
Purification of dtLLO, E7, and dtLLO-E7 proteins.
For animal studies, pET29b constructs encoding E7, dtLLO, and dtLLO-E7 were transformed into Escherichia coli BL21(DE3) and grown in LB selection medium containing kanamycin. For in vitro mechanistic studies, the same constructs were transformed into the BL21(DE3) IpxM strain, which has greatly reduced lipopolysaccharide (LPS) pyrogenicity, prior to purification (24). All purified proteins contained a histidine motif at the amino terminus to allow for purification over a Ni-nitrilotriacetic acid (Ni-NTA) column (Qiagen) according to the manufacturer's instructions. The purity of each protein preparation was verified by SDS-PAGE followed by Coomassie blue staining. Subsequently, contaminating endotoxins were removed from each purified protein preparation by use of a Norgen Proteospin endotoxin removal maxikit per the manufacturer's instructions (Norgen Biotek Corporation). Endotoxin removal was confirmed by Western blotting with anti-LPS core antibody (HyCult Biotechnology).
Assay of hemolytic activity.
The hemolytic activity of dtLLO was examined using sheep red blood cells (SRBC) as described previously (25). Briefly, purified preparations of dtLLO and wild-type LLO (LLO WT) were diluted 10-fold in phosphate-buffered saline (PBS)–cysteine acidic buffer (pH 5.5) or neutral PBS (7.4). After activation for 30 min, PBS-washed intact SRBC were added to a series of LLO WT or dtLLO dilutions. Following incubation at 37°C for 45 min, samples were centrifuged and supernatants were analyzed for hemoglobin absorption at 570 nm. The number of hemolytic units was defined as the dilution of the sample at which 50% of the SRBC had been lysed.
Tumor regression study.
All animal experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee of the University of Pennsylvania. TC-1 tumor cells (2 × 105) were injected subcutaneously (s.c.) into the flanks of C57BL/6 mice (8 mice/group) on day 0. After tumors were allowed to establish for 3 days, protein vaccines were administered s.c. as follows: 50 μg of E7, 200 μg of LLO, 250 μg of LLO-E7, or 50 μg of E7 plus 200 μg of dtLLO per mouse. Mice were immunized again on day 10, and tumor growth was observed every 3 days by use of calipers spanning the shortest and longest surface diameters. The mean of these two measurements was calculated as the mean tumor diameter in millimeters (W). Tumors with a diameter of <3 mm could not be measured and were classified as a tumor-free size. Mice were sacrificed when the tumor diameter reached >20 mm. Data are shown as tumor volumes, calculated as 0.5 × W3, and also as percentages of tumor-free mice for each vaccine group at various time points after tumor challenge.
Intracellular cytokine production by E7-specific CTLs.
C57BL/6 mice were immunized s.c. with E7, dtLLO, dtLLO plus E7, or dtLLO-E7 and boosted 7 days later. Splenocytes were harvested 9 days after the boost and processed for E7-specific intracellular cytokine staining. Briefly, 106 splenocytes were stimulated with an E7-specific cytotoxic T lymphocyte (CTL) epitope peptide (5 μg/ml) plus interleukin-2 (IL-2) (5 U/ml), along with GolgiStop, for 5 h. Subsequent to stimulation, cells were processed for four-color flow cytometry by staining initially for CD8 (antibody 53-6.7 conjugated to fluorescein isothiocyanate [FITC]), CD62L-selectin (CD62L) (MEL-14 antibody conjugated to allophycocyanin), and CD11b (peridinin chlorophyll protein [PerCP]-Cy5.5-conjugated antibody). Cells were then fixed and permeabilized, followed by intracellular staining for gamma interferon (IFN-γ) (phycoerythrin [PE]-conjugated antibody). Analysis was performed using a FACSCalibur flow cytometer with CellQuest software (Becton, Dickinson, Mountain View, CA).
E7-specific TILs.
C57BL/6 mice were immunized s.c. with E7, dtLLO, dtLLO plus E7, or dtLLO-E7 and boosted 7 days later. Three-color flow cytometry for CD8 (FITC-conjugated 53-6.7 antibody), CD62L (allophycocyanin-conjugated MEL-14 antibody), and PE-conjugated E7 H-2Db tetramer was performed using a FACSCalibur flow cytometer with CellQuest software (Becton, Dickinson, Mountain View, CA). Splenocytes and tumor-infiltrating lymphocytes (TILs) were harvested 9 days after the boost and were stained at room temperature with H-2Db tetramers loaded with the E7 peptide (RAHYNIVTF). Tumors were manually separated, and TILs were isolated from whole-tumor preparations as described previously (17). Tetramers were provided by the National Institute of Allergy and Infectious Diseases Tetramer Core Facility. The tetramers were used at a 1/200 dilution. Cells were analyzed as described above, comparing tetramer+ CD8+ CD62Llow cells generated by vaccine immunization.
Quantitative PCR (qPCR) analysis of proinflammatory cytokines and costimulatory markers.
Bone marrow-derived dendritic cells (BMDCs) were isolated as described below for all in vitro mechanistic studies. Briefly, femurs from 6-week-old mice were mechanically cleared of all muscle tissue and subjected to three sequential 5-min washes in 70% ethanol, followed by immersion in PBS. Subsequently, bone marrow was extracted, and the cells were collected in Dulbecco's modified Eagle's medium 10 (DMEM-10) and cultured in 100-cm dishes with 20 ng/ml of granulocyte-macrophage colony-stimulating factor (GM-CSF) and 50 ng/ml of mouse IL-4. The medium was replaced every 3 days, and BMDCs were collected on day 7 for immediate stimulation or freezing. Subsequently, 2.5 × 106 BMDCs in 1 ml of DMEM-10 were stimulated with vehicle control or with a series of experimental conditions for 2 h at 37°C. For a positive control, 15 ng of LPS or 50 μg of poly(I-C) was added to either C57BL/6-derived BMDCs or tlr4−/− BMDCs, respectively. The stimulatory potential of dtLLO was assayed by adding BL21(DE3) IpxM-purified dtLLO protein (50, 25, or 12.5 μg) to BMDCs. After treatment, RNA was extracted from the BMDCs by use of an RNeasy RNA extraction kit (Qiagen, Valencia, CA) and converted to cDNA by use of a high-capacity reverse transcriptase kit (Applied Biosystems, Carlsbad, CA). Primer sets specific for mouse CD40, MHC II, IL-12 (p35), and tumor necrosis factor alpha (TNF-α) were purchased from SABiosciences and employed to determine stimulation by dtLLO and LPS.
Polymyxin B pretreatment, heat inactivation, and proteinase K treatment.
The contribution of endotoxin to each stimulation condition was controlled by several strategies. A specific inhibitor of LPS pyrogenicity, polymyxin B (20 μg), was incubated with either dtLLO or LPS for 30 min at room temperature prior to addition of the stimulant to cells. To determine whether natively folded dtLLO was responsible for the observed stimulation, samples containing either dtLLO or LPS were heat inactivated by boiling for 30 min. Subsequently, samples were allowed to equilibrate to room temperature before addition to BMDCs. Additionally, pretreatment with proteinase K was employed as a strategy to eliminate any protein-associated stimulation by treatment with dtLLO and LPS. Briefly, samples were pretreated with 20 μg of proteinase K and incubated at 56°C for 15 min, followed by 5 min at 95°C to inactivate the enzyme.
Statistical analysis.
One-tailed Student's t test or the nonparametric Wilcoxon signed-rank test was performed to compare E7 vaccination to either dtLLO-plus-E7 or dtLLO-E7 vaccination for all data, using GraphPad Prism, version 4.0a, for Macintosh (GraphPad, San Diego, CA). Significant P values for all comparisons are depicted in figures as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001. Nonsignificant P values are depicted by “n.s.”
RESULTS
Construction and purification of dtLLO, E7, and dtLLO-E7 protein vaccines.
In order to properly assay the mechanism of LLO as an adjuvant, we created dtLLO. Detoxification was accomplished by introducing point mutations for three selected amino acids important for binding of LLO to cholesterol and for eventual membrane pore formation (21). The three targeted amino acids (underlined) present in the cholesterol binding domain of LLO (483ECTGLAWEWWR493) were modified in the sequence 483EATGLAWEAAR493 by point mutations introduced into the DNA sequence by PCR. The mutated hly gene was cloned into the pET29b expression vector, and the presence of point mutations was verified by sequence analysis. The pET29b expression system was utilized for the production and purification of all proteins, including HPV-16 E7, dtLLO, and the fusion protein dtLLO-E7. Each protein (E7, dtLLO, and dtLLO-E7) was successfully expressed and purified, as indicated by SDS-PAGE analysis followed by Coomassie blue staining (Fig. 1A). The absence of the LPS endotoxin in each preparation was verified by Western blotting using anti-LPS antibody. As indicated, LPS contamination was undetectable in the purified E7, dtLLO, and dtLLO-E7 protein preparations (Fig. 1B).
Fig 1.

Construction, purification, and characterization of a detoxified form of listeriolysin O (dtLLO). (A) dtLLO protein, HPV-16 E7 protein, and a genetic fusion protein of dtLLO and E7 were extracted from BL21(DE3) transformants after IPTG (isopropyl-β-d-thiogalactopyranoside) induction and then purified using a Ni2+ column. Each purified protein was then subjected to 4 to 12% SDS-PAGE analysis, and the gel was stained with Coomassie blue. Each protein is visualized in its respective labeled lane. (B) Western blot analysis to detect LPS contamination after endotoxin removal in protein preparations from BL21(DE3) transformants. Briefly, endotoxin was removed from each purified protein preparation by use of Norgen Proteospin endotoxin removal columns, and subsequently, proteins or LPS (20 ng; positive control) was subjected to 4 to 12% SDS-PAGE analysis. After transfer to a polyvinylidene difluoride (PVDF) membrane, Western blot analysis with anti-LPS antibody was performed to demonstrate removal of endotoxin from purified protein preparations. (C) Detoxification of LLO by mutation of the cholesterol binding domain results in reduced hemolytic activity. Hemolytic activity of dtLLO was determined using an SRBC lysis assay. Briefly, LLO WT and dtLLO protein preparations were initially activated by incubation at an acidic pH (1× PBS-cysteine buffer, pH 5.4) and subsequently were incubated with sheep red blood cells. The number of hemolytic units was determined by measuring released hemoglobin (optical density at 570 nm) with each treatment.
To verify that dtLLO is nonhemolytic, a hemolysis assay was performed using sheep red blood cells as indicator cells (4). The purified dtLLO protein was found to exhibit a very low hemolytic activity at acidic pH (Fig. 1C) and no detectable activity at neutral pH (data not shown). In contrast, wild-type LLO was highly hemolytic at pH 5.5 (Fig. 1C) and retained 100% activity at neutral pH (data not shown). Confirmation of the detoxified nature of our dtLLO preparations allowed further studies to determine its efficacy as an adjuvant in tumor immunotherapy.
dtLLO augments antitumor efficacy of E7-based protein vaccines.
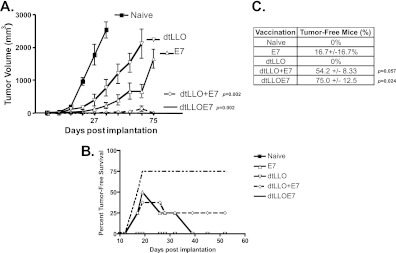
The efficacy of dtLLO as an adjuvant was investigated using TC-1 cells, a mouse tumor model for HPV-16-associated cancer that expresses the HPV-16 E6 and E7 oncoproteins. To determine if dtLLO is an effective adjuvant in a protein vaccination strategy, either fused to or mixed with E7, a tumor regression study was performed using the TC-1 tumor model. Briefly, mice were implanted with TC-1 tumor cells s.c. in the hind flank and vaccinated when tumors were palpable, on day 3, with purified preparations of dtLLO and E7, either alone, mixed, or genetically fused. The vaccinated mice were boosted on day 10 postimplantation, and tumor volumes were measured throughout (Fig. 2A). Following immunization, tumor burdens were significantly reduced in the groups treated with dtLLO-E7 and dtLLO plus E7, and more than 50% of the mice were free of tumors at the completion of the experiment, compared to ∼16% in the E7-treated group (Fig. 2B and C). On the other hand, immunization with dtLLO did not result in tumor-free mice, but a significant delay in the progression of tumor growth was observed with each vaccination (Fig. 2A). As expected, naïve mice developed tumors and were sacrificed by day 32 of the study. This study was repeated three times, and the average number of tumor-free mice at the end of each study is shown in Fig. 2C. These results suggest that dtLLO exhibits adjuvant effects when it is present as a fusion protein or mixed along with tumor-associated antigens (TAA) in cancer immunotherapy.
Fig 2.
dtLLO augments antitumor efficacy of E7 protein vaccination. (A) The ability of dtLLO alone to act as an adjuvant in tumor immunotherapy was determined using the TC-1 tumor model. Mice (8/group) were implanted subcutaneously with 105 TC-1 tumor cells and subsequently vaccinated with protein vaccines (dtLLO, E7, dtLLO-E7, and dtLLO plus E7). Tumor loads are depicted as the tumor volume for each treatment group throughout the course of the experiment (P = 0.002 for the dtLLO and dtLLO-plus-E7 groups compared to the E7 group, using the nonparametric Wilcoxon signed-rank test). (B) Representative tumor load study depicting the percentage of TC-1 tumor-free mice in each treatment group throughout the course of the experiment. (C) Mean percentage of tumor-free mice around day 54 for each treatment group at the end of three separate tumor load studies.
dtLLO augments E7-specific CD8 T cell responses in spleens and tumors.
Previous work has demonstrated that Listeria-based vaccines expressing and secreting LLO-based fusion proteins are able to facilitate regression of tumors due to the generation of efficient tumor-specific CD8+ T cell responses. Thus, we hypothesized that the regression of established tumors induced by vaccination with dtLLO-based vaccines would correlate with increased stimulation of E7-specific CD8+ T cell responses. To determine the number of E7-specific IFN-γ-expressing CD8+ CD62Llow cells in the spleen, intracellular cytokine staining was performed with or without an E7-specific H-2b-restricted CTL peptide, RAHYNIVTF, to induce IFN-γ secretion. A >5-fold induction (Fig. 3A) of E7-specific IFN-γ+ CD8+ CD62Llow cells was detected after vaccination with dtLLO-E7 or dtLLO plus E7 compared to the naïve or dtLLO-vaccinated group, with a 3- to 7-fold increase observed compared to the group vaccinated with E7 alone. These data suggest that the presence of dtLLO augments E7-specific immune responses.
Fig 3.
dtLLO augments tumor-specific adaptive immune responses. (A) Augmentation of E7-specific T cell responses was measured in the spleen by intracellular IFN-γ staining. Splenocytes were harvested from mice in each treatment group from the tumor load study depicted in Fig. 2 and stimulated with IL-2 alone or IL-2 along with an E7-specific CTL epitope peptide (RAHYNIVTF). Splenocytes were then processed for flow cytometric analysis of intracellular production of IFN-γ. Each dot plot depicts activated E7-specific CD8+ T cells from a different treatment group as the percentage of total activated CD8+ T cells in the spleen. (B) Increased infiltration of E7-specific CD8+ TILs into TC-1 tumors after dtLLO protein vaccination. Tumor-infiltrating lymphocytes were isolated from TC-1 tumors at the end of the experiment, pooled within the treatment group, and stained with a tetramer that recognizes E7-specific CTLs. (Bottom row) Tumor-infiltrating E7-specific CTLs are depicted as the percentage of total tumor-infiltrating activated CD8+ CD62Llow CD11b− E7 tetramer+ cells for each treatment group. (Top row) E7-specific CTLs in the spleens of mice from each treatment group.
Additionally, E7-specific tetramer staining was performed on the spleens and tumors of mice treated with different vaccines (Fig. 3B). An increase in the percentage of CD8+ CD62Llow E7-tet+ cells was detected in TILs of mice treated with dtLLO-E7 or dtLLO plus E7 compared to the percentage for mice immunized with E7 or dtLLO alone (Fig. 3B). This result suggests that dtLLO not only enhances the induction of E7-specific T cells but also increases the number of E7-specific TILs in the tumor microenvironment.
dtLLO promotes the expression of proinflammatory cytokines in dendritic cells.
Additionally, we investigated if dtLLO promotes the synthesis of proinflammatory cytokines in mouse BMDCs, similar to a PAMP. An upregulation in the transcription of genes encoding proinflammatory cytokines such as TNF-α (∼90-fold) and IL-12 (∼22-fold) in BMDCs was detected after treatment with dtLLO (Fig. 4A and C). To reduce the contamination of endotoxin in the purified protein preparations of different protein vaccines, such as the E7, dtLLO, and dtLLO-E7 vaccines, we utilized an E. coli BL21 strain that lacks a myristoylated LPS due to a mutation in the enzyme IpxM. The IpxM mutant BL21 strain produces an LPS that has reduced pyrogenicity as measured by activation and maturation of myeloid dendritic cells (DCs) (24). In addition, the induction of cytokine mRNA by dtLLO was refractory to pretreatment with polymyxin B, an inhibitor of LPS pyrogenicity. The increase in proinflammatory cytokine transcription induced by dtLLO was not due to the presence of contaminating LPS. Furthermore, the significant reductions in cytokine secretion by BMDCs after inactivation of dtLLO with heat or proteinase K suggest that a proteinaceous component is responsible for this activity (Fig. 4B and C). As a control PAMP, LPS-induced cytokine stimulation was reduced ∼3-fold in the presence of polymyxin B, but heat inactivation and proteinase K treatment had minimal impacts (Fig. 4A to C).
Fig 4.
dtLLO treatment of BMDCs stimulates induction of mRNAs for proinflammatory cytokines. (A) dtLLO treatment stimulates production of TNF-α mRNA. dtLLO was purified from BL21(DE3) IpxM, followed by endotoxin removal. BMDCs were stimulated for 16 h with LPS, LPS pretreated with a specific inhibitor of LPS signaling (polymyxin B [PMXB]), dtLLO, dtLLO pretreated with PMXB, or PMXB alone. After stimulation, cells were processed for RNA isolation, cDNA conversion, and qPCR analysis with TNF-α primers. TNF-α mRNA production after stimulation is depicted in relation to TNF-α mRNA levels in the untreated BMDC controls. (B and C) TNF-α expression by BMDCs with dtLLO stimulation is dependent on native, intact dtLLO. To determine if the dtLLO protein was responsible for the signaling observed in panel A, the dtLLO protein was either heat denatured by boiling for 10 min (dtLLO-Heat) or treated with proteinase K for 60 min (dtLLO-PK). BMDCs were subsequently stimulated with untreated dtLLO, dtLLO-Heat, dtLLO-PK, LPS alone, LPS-Heat, or LPS-PK, followed by TNF-α (B) and IL-12 (C) qPCR analyses. (D and E) Signaling by dtLLO is independent of TLR4. BMDCs were isolated from tlr4−/− mice and stimulated with dtLLO, dtLLO-Heat, or dtLLO-PK, in addition to poly(I-C) as a positive control and LPS as a negative control. After stimulation for 2 h, cells were processed for qPCR analysis of TNF-α (D) and IL-12 (E) mRNA expression. **, P < 0.01; ***, P < 0.001.
Previously published reports have classified anthrolysin O and other cytolysins expressed by Gram-positive bacteria as TLR4 agonists (26). Thus, the role of the pattern recognition receptor TLR4 was addressed using tlr4−/− BMDCs. We observed that treatment with either dtLLO or poly(I-C) resulted in the synthesis of both IL-12 and TNF-α mRNAs in tlr4−/− BMDCs (Fig. 4D and E). As expected, treatment with the TLR4 agonist LPS did not induce cytokine stimulation in tlr4−/− BMDCs (Fig. 4D and E). This suggests the possibility that dtLLO can interact with another TLR(s) or PRR to stimulate cytokine synthesis. It is worth noting that the magnitudes of both IL-12 and TNF-α mRNA expression in tlr4−/− BMDCs stimulated with dtLLO were substantially lower than those observed in wild-type BMDCs. Importantly, however, these experiments were not run in parallel, so the differences must be interpreted with caution. Nevertheless, while our results strongly suggest that dtLLO is capable of activating cytokine genes through a TLR4-independent pathway, the possibility exists that TLR4 is also involved.
Upregulation of CD40 and MHC II costimulatory molecules by dtLLO.
Since PAMPs are known to facilitate the maturation of DCs, we also analyzed the ability of dtLLO to cause an upregulation in the expression of costimulatory molecules such as CD40 and MHC II, which are markers of this maturation. In BMDCs from wild-type mice, treatment with dtLLO resulted in an ∼50-fold increased synthesis of CD40 mRNA and an ∼15-fold increased synthesis of MHC II mRNA (Fig. 5A and B), comparable to the levels induced by the LPS positive control. The dtLLO-mediated increases in the synthesis of CD40 and MHC II in BMDCs were significantly reduced after heating and proteinase K treatment, again implicating activity by a protein-like component for this effect.
Fig 5.
dtLLO treatment facilitates expression of mRNAs for markers associated with BMDC maturation. (A and B) dtLLO treatment induces expression of costimulatory molecules. BMDCs were stimulated with dtLLO, dtLLO-Heat, dtLLO-PK, LPS, LPS-Heat, or LPS-PK, followed by CD40 (A) and MHC II (B) qPCR analyses. (C and D) Costimulatory molecule expression by dtLLO is independent of TLR4. BMDCs were isolated from tlr4−/− mice and stimulated with dtLLO, dtLLO-Heat, or dtLLO-PK, in addition to poly(I-C) as a positive control and LPS as a negative control. After stimulation for 2 h, cells were processed for qPCR analysis of CD40 (C) and MHC II (D) mRNA expression. **, P < 0.01; ***, P < 0.001.
Furthermore, we confirmed that dtLLO also upregulated costimulatory markers in BMDCs from tlr4−/− mice, resulting in ∼11-fold and ∼4-fold increases in CD40 and MHC II expression, respectively (Fig. 5C and D). The enhancement in CD40 as well as MHC II expression in tlr4−/− BMDCs was completely abolished after degradation of dtLLO by heating or proteinase K treatment. The capacity of dtLLO to induce CD40 and MHC II mRNA expression in tlr4−/− BMDCs suggests that dtLLO is capable of inducing DC maturation through a TLR4-independent pathway. Again, however, the magnitudes of CD40 and MHC II mRNA expression in tlr4−/− BMDCs stimulated with dtLLO were substantially lower than those observed in wild-type BMDCs. This raises the possibility that TLR4-independent and TLR4-dependent pathways may both be involved in dtLLO-induced dendritic cell maturation.
DISCUSSION
The evidence reported in this investigation provides clear and direct support for the hypothesis that a detoxified, nonhemolytic form of LLO is a novel adjuvant and can act in a PAMP-like manner to facilitate TAA-specific cancer immunotherapy. This study showed that dtLLO used as a fusion and administered as a mixture with the TAA E7 provided similar reductions in tumor volume (Fig. 2). Even the administration of adjuvant dtLLO alone in a TC-1 tumor regression study caused a significant delay in tumor progression, as depicted in Fig. 2A. The therapeutic antitumor effects of dtLLO as an adjuvant can likely be attributed to the augmentation of E7-specific CD8 T cell responses, as well as an increased infiltration of E7-specific T cells into the tumor microenvironment. Since augmentation of E7-specific responses was observed when E7 was fused to dtLLO or administered as a mixture, this suggests that the ability of LLO to act as a PAMP is likely responsible for facilitating these immune responses.
Different PAMPs in bacteria, such as CpG DNA, LPS, and flagellin, are recognized by specific PRRs, such as TLRs, in various cell types to activate an immunostimulatory cascade leading to the release of proinflammatory cytokines such as TNF-α and IL-12. The expression of these cytokines in BMDCs was stimulated by dtLLO, indicating a possible interaction with a PRR(s) to promote the secretion of these cytokines. Additionally, dtLLO stimulation induced an upregulation on the surfaces of DCs of costimulatory signals such as MHC II and CD40, which are markers of maturation of these DCs. DCs play a key role in the initiation and instruction of adaptive immunity. Thus, our evidence suggests that dtLLO acts in a PAMP-like manner to stimulate production of the proinflammatory cytokines IL-12 and TNF-α, as well as to facilitate DC maturation. This is consistent with the previous finding that L. monocytogenes-based vaccines expressing the LLO fusion protein Lm-LLO-E7 cause rapid and effective phenotypic and functional maturation of myeloid DCs (27). The enhancement of DC maturation by LLO likely induces higher levels of in vitro T cell proliferation and in vivo antitumor immunity.
Previously, all Gram-positive bacterial CDCs, such as LLO, anthrolysin O, perfringolysin O, and streptolysin O, were reported to act in a TLR4-dependent manner (26). However, our results suggest that dtLLO can induce the expression of proinflammatory cytokines and markers associated with DC maturation in the absence of TLR4. This observation supports a hypothesis in which dtLLO PAMP activity is not exclusive to TLR4 and signals through another, as yet unidentified PRR. Note that the levels of cytokine mRNA induced by dtLLO as well as poly(I-C) (TLR3 agonist) were significantly lower in tlr4−/− BMDCs than those induced by dtLLO or LPS in wild-type BMDCs, suggesting that these cells were possibly less conducive to stimulation of proinflammatory cytokine mRNA production. Recently, a key virulence factor of Streptococcus pneumoniae and another CDC, pneumolysin, were shown to promote DC maturation and cytokine secretion in a TLR4-independent manner (28). The pore-forming Shigella toxin porin has been shown to signal the TLR2-TLR6 complex (29). It has also been suggested that seeligeriolysin O, a member of the cholesterol-dependent cytolysins of Listeria seeligeri, induces activation of peritoneal macrophages to secrete IL-12 by a mechanism involving both TLR2 and TLR4 (30).
As a Gram-positive pathogen, Listeria lacks LPS, and it therefore does not use this molecule to signal the innate immune system through TLR4. Macrophages of Tlr4−/− mice express listeriocidal activity similar to that of wild-type macrophages, suggesting that TLR4 signaling is possibly not essential for clearance of Listeria (31). Other known PAMPs displayed by Listeria include the TLR ligands peptidoglycan (TLR2), lipoteichoic acid (TLR2), unmethylated CpG sequences (TLR9), and flagellin (TLR5), all of which signal the cell through myeloid differentiation factor 88 (MyD88) (32). Identified bacterial protein PAMPs are relatively rare. To our knowledge, flagellin, which binds to TLR5, is the only identified L. monocytogenes protein PAMP (33). TLR5 and TLR7 are surprisingly absent on spleen and bone marrow-derived murine DCs (34, 35) and are thus unlikely to be the targets of LLO. The only well-defined ligand for TLR9 is bacterial CpG DNA. However, there is some evidence that it may bind non-nucleic acid ligands (36). TLR9 is found not at the cell surface but in endosomal compartments, including phagosomes (37). Since LLO expression by L. monocytogenes is maximal in the phagosome, it may interact with receptors or other proteins in this compartment. Thus, future studies on the involvement of other PRRs that engage LLO as a ligand are warranted.
In conclusion, the evidence presented in this study supports the hypothesis that dtLLO is a novel adjuvant with PAMP-like activity that augments both innate and adaptive immune responses. The ability of dtLLO to act as an adjuvant, as a fusion partner, or mixed with other antigens makes it versatile and attractive for application in the treatment of various malignant diseases.
ACKNOWLEDGMENTS
This work was supported by grant CA69632 from the National Institutes of Health.
We acknowledge Jamila Abraham and Mathew Seavey for their assistance in the purification of dtLLO protein for the hemolysis assay.
Yvonne Paterson discloses that she has a financial interest in Advaxis Inc., a publicly traded immunotherapy company that has licensed or has an option to license all patents from the University of Pennsylvania that concern the use of L. monocytogenes or listerial products as immunotherapies. Anu Wallecha is currently an employee of Advaxis Inc. Vafa Shahabi is a shareholder of Advaxis Inc.
Footnotes
Published ahead of print 7 November 2012
REFERENCES
- 1. Tweten RK. 2005. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 73:6199–6209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cossart P, Vicente MF, Mengaud J, Baquero F, Perez-Diaz JC, Berche P. 1989. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57:3629–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marquis H, Bouwer HG, Hinrichs DJ, Portnoy DA. 1993. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect. Immun. 61:3756–3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Portnoy DA, Jacks PS, Hinrichs DJ. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rose F, Zeller SA, Chakraborty T, Domann E, Machleidt T, Kronke M, Seeger W, Grimminger F, Sibelius U. 2001. Human endothelial cell activation and mediator release in response to Listeria monocytogenes virulence factors. Infect. Immun. 69:897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dramsi S, Cossart P. 2003. Listeriolysin O-mediated calcium influx potentiates entry of Listeria monocytogenes into the human Hep-2 epithelial cell line. Infect. Immun. 71:3614–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamon MA, Batsche E, Regnault B, Tham TN, Seveau S, Muchardt C, Cossart P. 2007. Histone modifications induced by a family of bacterial toxins. Proc. Natl. Acad. Sci. U. S. A. 104:13467–13472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hussain SF, Paterson Y. 2004. CD4+CD25+ regulatory T cells that secrete TGFbeta and IL-10 are preferentially induced by a vaccine vector. J. Immunother. 27:339–346 [DOI] [PubMed] [Google Scholar]
- 9. Nitcheu-Tefit J, Dai MS, Critchley-Thorne RJ, Ramirez-Jimenez F, Xu M, Conchon S, Ferry N, Stauss HJ, Vassaux G. 2007. Listeriolysin O expressed in a bacterial vaccine suppresses CD4+CD25high regulatory T cell function in vivo. J. Immunol. 179:1532–1541 [DOI] [PubMed] [Google Scholar]
- 10. Carrero JA, Calderon B, Unanue ER. 2004. Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J. Immunol. 172:4866–4874 [DOI] [PubMed] [Google Scholar]
- 11. Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. 2004. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118:285–296 [DOI] [PubMed] [Google Scholar]
- 12. Lamikanra A, Pan ZK, Isaacs SN, Wu TC, Paterson Y. 2001. Regression of established human papillomavirus type 16 (HPV-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8(+) T-cell responses that home to the tumor site. J. Virol. 75:9654–9664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng X, Treml J, Paterson Y. 2007. Adjuvant properties of listeriolysin O protein in a DNA vaccination strategy. Cancer Immunol. Immunother. 56:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh R, Dominiecki ME, Jaffee EM, Paterson Y. 2005. Fusion to listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J. Immunol. 175:3663–3673 [DOI] [PubMed] [Google Scholar]
- 15. Darji A, Chakraborty T, Wehland J, Weiss S. 1995. Listeriolysin generates a route for the presentation of exogenous antigens by major histocompatibility complex class I. Eur. J. Immunol. 25:2967–2971 [DOI] [PubMed] [Google Scholar]
- 16. Neeson P, Pan ZK, Paterson Y. 2008. Listeriolysin O is an improved protein carrier for lymphoma immunoglobulin idiotype and provides systemic protection against 38C13 lymphoma. Cancer Immunol. Immunother. 57:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. 2001. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J. Immunol. 167:6471–6479 [DOI] [PubMed] [Google Scholar]
- 18. Schnupf P, Portnoy DA, Decatur AL. 2006. Phosphorylation, ubiquitination and degradation of listeriolysin O in mammalian cells: role of the PEST-like sequence. Cell. Microbiol. 8:353–364 [DOI] [PubMed] [Google Scholar]
- 19. Sewell DA, Douven D, Pan ZK, Rodriguez A, Paterson Y. 2004. Regression of HPV-positive tumors treated with a new Listeria monocytogenes vaccine. Arch. Otolaryngol. Head Neck Surg. 130:92–97 [DOI] [PubMed] [Google Scholar]
- 20. Sewell DA, Shahabi V, Gunn GR, 3rd, Pan ZK, Dominiecki ME, Paterson Y. 2004. Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7. Cancer Res. 64:8821–8825 [DOI] [PubMed] [Google Scholar]
- 21. Michel E, Reich KA, Favier R, Berche P, Cossart P. 1990. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol. Microbiol. 4:2167–2178 [DOI] [PubMed] [Google Scholar]
- 22. Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. 1996. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 56:21–26 [PubMed] [Google Scholar]
- 23. Wood LM, Pan ZK, Shahabi V, Paterson Y. 2010. Listeria-derived ActA is an effective adjuvant for primary and metastatic tumor immunotherapy. Cancer Immunol. Immunother. 59:1049–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cognet I, de Coignac AB, Magistrelli G, Jeannin P, Aubry JP, Maisnier-Patin K, Caron G, Chevalier S, Humbert F, Nguyen T, Beck A, Velin D, Delneste Y, Malissard M, Gauchat JF. 2003. Expression of recombinant proteins in a lipid A mutant of Escherichia coli BL21 with a strongly reduced capacity to induce dendritic cell activation and maturation. J. Immunol. Methods 272:199–210 [DOI] [PubMed] [Google Scholar]
- 25. Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. 2002. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 156:1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park JM, Ng VH, Maeda S, Rest RF, Karin M. 2004. Anthrolysin O and other gram-positive cytolysins are Toll-like receptor 4 agonists. J. Exp. Med. 200:1647–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peng X, Hussain SF, Paterson Y. 2004. The ability of two Listeria monocytogenes vaccines targeting human papillomavirus-16 E7 to induce an antitumor response correlates with myeloid dendritic cell function. J. Immunol. 172:6030–6038 [DOI] [PubMed] [Google Scholar]
- 28. McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, Moran B, Fitzgerald KA, Tschopp J, Petrilli V, Andrew PW, Kadioglu A, Lavelle EC. 2010. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 6:e1001191 doi:10.1371/journal.ppat.1001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ray A, Biswas T. 2005. Porin of Shigella dysenteriae enhances Toll-like receptors 2 and 6 of mouse peritoneal B-2 cells and induces the expression of immunoglobulin M, immunoglobulin G2a and immunoglobulin A. Immunology 114:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ito Y, Kawamura I, Kohda C, Tsuchiya K, Nomura T, Mitsuyama M. 2005. Seeligeriolysin O, a protein toxin of Listeria seeligeri, stimulates macrophage cytokine production via Toll-like receptors in a profile different from that induced by other bacterial ligands. Int. Immunol. 17:1597–1606 [DOI] [PubMed] [Google Scholar]
- 31. Edelson BT, Unanue ER. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 169:3869–3875 [DOI] [PubMed] [Google Scholar]
- 32. Barton GM, Medzhitov R. 2003. Toll-like receptor signaling pathways. Science 300:1524–1525 [DOI] [PubMed] [Google Scholar]
- 33. Way SS, Thompson LJ, Lopes JE, Hajjar AM, Kollmann TR, Freitag NE, Wilson CB. 2004. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell. Microbiol. 6:235–242 [DOI] [PubMed] [Google Scholar]
- 34. Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. 2003. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 33:827–833 [DOI] [PubMed] [Google Scholar]
- 35. Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. 2003. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 170:5165–5175 [DOI] [PubMed] [Google Scholar]
- 36. Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, Yamamoto M, Takeuchi O, Itagaki S, Kumar N, Horii T, Akira S. 2005. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J. Exp. Med. 201:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Underhill DM, Gantner B. 2004. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 6:1368–1373 [DOI] [PubMed] [Google Scholar]