Abstract
The objective of this study was to compare the incidence rate of clinical mastitis (IRCM) between cows classified as high, average, or low for antibody-mediated immune responses (AMIR) and cell-mediated immune responses (CMIR). In collaboration with the Canadian Bovine Mastitis Research Network, 458 lactating Holsteins from 41 herds were immunized with a type 1 and a type 2 test antigen to stimulate adaptive immune responses. A delayed-type hypersensitivity test to the type 1 test antigen was used as an indicator of CMIR, and serum antibody of the IgG1 isotype to the type 2 test antigen was used for AMIR determination. By using estimated breeding values for these traits, cows were classified as high, average, or low responders. The IRCM was calculated as the number of cases of mastitis experienced over the total time at risk throughout the 2-year study period. High-AMIR cows had an IRCM of 17.1 cases per 100 cow-years, which was significantly lower than average and low responders, with 27.9 and 30.7 cases per 100 cow-years, respectively. Low-AMIR cows tended to have the most severe mastitis. No differences in the IRCM were noted when cows were classified based on CMIR, likely due to the extracellular nature of mastitis-causing pathogens. The results of this study demonstrate the desirability of breeding dairy cattle for enhanced immune responses to decrease the incidence and severity of mastitis in the Canadian dairy industry.
INTRODUCTION
Mastitis, generally defined as the inflammation of the mammary gland, is a complex disease associated with significant economic loss in the dairy industry (1). Mastitis can be caused by numerous diverse organisms that enter and multiply inside the mammary gland, often as a result of disruption of physical barriers such as the teat and mucosal barriers. Mastitis pathogens are generally categorized as contagious, including Staphylococcus aureus and Streptococcus agalactiae, which are spread from infected quarters to other quarters or cows, or as environmental, including coliforms like Escherichia coli and Klebsiella or streptococci (2, 3). These various mastitis pathogens utilize diverse mechanisms to infect and persist in the host mammary gland and contribute to decreased milk quality and animal welfare.
The immune system controls host defenses to pathogen challenge. Antibody-mediated immune responses (AMIR) and cell-mediated immune responses (CMIR) have been used as indicator traits of adaptive immune responses in dairy cattle and other species (4–7). Both CMIR and AMIR are essential for host protection against intracellular and extracellular microorganisms, respectively, and can be characterized by various functional tests, including measuring the cytokines and antibody isotypes produced in response to challenge. A type 1 response, or CMIR, is typically characterized by gamma interferon and antibody of the IgG2 isotype, whereas a type 2 response, or AMIR, is associated with interleukin-4 and antibody of the IgG1 isotype (8). Although an antagonistic relationship exists between these responses (9, 10), an optimal balance is required for broad-based disease resistance (11). Adaptive immune response traits are heritable (9), and their inclusion in breeding indices has been suggested to improve inherent disease resistance in dairy cattle (12, 13).
A previous study that classified 136 Holstein cows in Ontario based on AMIR found high immune responder (HIR) cows had a lower occurrence of mastitis in 2 out of 3 herds tested, improved response to commercial vaccine, and increased milk and colostrum quality (14). A subsequent study evaluating immune responses of 699 cows in a large commercial dairy in Florida found that, compared to other cows, HIR cows had a lower incidence of multiple diseases, including mastitis, metritis, displaced abomasums, and retained placenta (15), and were less likely to be seropositive for paratuberculosis (16). These previous studies have established many benefits of identifying HIR cows; however, those studies were performed on a limited number of herds within a single region. Therefore, the purpose of this work was to evaluate the association of mastitis and immune responses for Holstein cows from numerous commercial herds on a national scale.
The current study was part of a larger study of the Canadian Bovine Mastitis Research Network (CBMRN), a collaborative research network with the mission to decrease the incidence of mastitis, reduce financial losses, and maintain milk quality on Canadian dairy farms (17). Immune response profiles of cows across Canada were measured to determine associations with the incidence rates of clinical mastitis (IRCM) and the causative pathogens. Significant variations in immune response phenotypes for cows in this study have been reported (18), indicating the potential to classify cows as high, average, or low immune responders. Genetic parameters of AMIR and CMIR were then estimated and found to be heritable, with heritability estimates of of 0.29 and 0.19, respectively, demonstrating the feasibility of breeding for enhanced immune response (9). The objectives of this study were to determine the IRCM for cows from numerous commercial herds on a national scale and to compare the IRCM between cows classified as high, average, or low for AMIR and CMIR based on estimated breeding values (EBV) for these traits.
MATERIALS AND METHODS
Animals.
Immune response profiles of 458 lactating Holsteins, outside the peripartum period, from 41 herds across Canada were evaluated (18). Immune responses were tested between July 2007 and August 2008. Distribution by parity was as follows: 146 in parity 1, 134 in parity 2, 76 in parity 3, and 102 in parity 4 or higher. All experimental procedures were approved by the Animal Care Committee of the University of Guelph under guidelines of the Canadian Council of Animal Care.
Immune response traits.
Cows were immunized with known type 1 and type 2 test antigens outside the peripartum period to stimulate CMIR and AMIR, respectively, as described previously (18). Cutaneous delayed-type hypersensitivity (DTH) to Candida albicans, a type 1 test antigen, was used as an indicator of CMIR (19). Primary antibody production to a type 2 test antigen, hen egg white lysozyme (HEWL), was used as an indicator of AMIR (20). On days 0 and 14, cows received an intramuscular injection of 0.5 mg HEWL (Sigma-Aldrich Canada Ltd., Oakville, ON, Canada), 0.5 mg Candida albicans (Greer Laboratories Inc., Lenior, NC), and 0.5 mg Quil-A adjuvant (Cedarlane Laboratories, Hornby, ON, Canada) dissolved in 1 ml phosphate-buffered saline (PBS; pH 7.4). With a 22-gauge needle, a 1.0-ml injection was divided and administered intramuscularly on both sides of the neck or rump. Serum antibody of the IgG1 isotype to the type 2 test antigen was measured in an enzyme-linked immunosorbent assay as an indicator of AMIR (9).
Breeding value estimation and immune response category classification.
Genetic parameters of CMIR and AMIR in these herds have been estimated and reported previously (9). Complete records for CMIR and AMIR and cow registration numbers were available for 445 cows. The full pedigree records file included a total of 29,336 animals and was provided by the Canadian Dairy Network (Guelph, Ontario, Canada). CMIR and AMIR were analyzed using a series of uni- and bivariate linear animal models, as follows: yijkl = μijkl+ hti + pj + (α × st) + (β × st2) + (γ × dk) + cl + eijkl, where yijkl is the CMIR or AMIR, μijkl is the overall mean, hti is the random effect of herd-technician (41 herds and 9 technicians), pj is the fixed effect of parity (1, 2, 3, and ≥4), st is the stage of lactation measured as days in milk as a linear function (and st2 is the stage of lactation squared), dk is the control site for CMIR or day zero data for AMIR as fixed regressions, α, β, and γ are regression coefficients, cl is a random effect for a cow, and eijkl is the residual error.
In matrix form, the multiple trait animal model is described by the following equation: y = Xb + Z1ht + Za + e, where y is the matrix value for CMIR and AMIR, X, Z1, and Z are the incidence matrices' related to the fixed and random effects, respectively, b is the vector that contains the fixed effects, ht is the vector of random effects of herd-technician, a is the vector of random additive genetic effects for a cow; and e is the vector of random residual effect. The expectations and assumed variance are as follows: E(y) = Xb; E(ht) = E(a) = E(e) = 0, V(ht) = HT; V(a) = G; V(e) = R; cov(a,e′) = 0, and V(y) = ZGZ′ + HT + R, where HT is the direct product of an identity matrix (I) of the order of the number of herd-technicians and the matrix of herd-technician variances (I ⊗ ht0), G is the direct product of the numerator relationship matrix (A) for animals and the matrix of genetic variance and covariance (A ⊗ G0), and R is the direct product of an identity matrix of order of the number of observations and the matrix of error variances and covariances (I ⊗ R0). The genetic analysis was performed with DMU by using an average information-restricted maximum-likelihood (AI-REML) algorithm for estimation of co(variance) components in mixed models (21). Using these parameters, breeding values were estimated. For each trait, cows with an EBV of above +1 or below −1 standard deviation from the mean were considered high immune responders or low immune responders, respectively.
Milk sampling and processing.
Milk samples were taken as described previously for the CBMRN herds (17). Briefly, farmers sampled cows identified as having abnormal milk or clinical signs of mastitis. A milk sample was taken on the day of diagnosis. Milk samples were frozen for storage at −20°C and submitted to 1 of 4 bacteriology laboratories. A standardized protocol based on National Mastitis Council guidelines for bacteriology culture and species identification was followed. Data on the number of colonies and species isolated were made available through the CBMRN database and obtained for use in this study, to allow calculation of the IRCM for cows in the immune response study. A mastitis clinical score was assigned to each case. A mastitis score of 1 was categorized as a quarter having abnormal milk only, a mastitis score of 2 was abnormal milk plus a swollen quarter, and a mastitis score 3 was abnormal milk, swollen quarter, and a sick cow (17).
Isolation of the contagious pathogen Staphylococcus aureus or Streptococcus agalactiae was considered the cause of an intramammary infection if 1 colony (100 CFU/ml) was isolated. If ≥200 CFU/ml of the environmental pathogens Escherichia coli, streptococci other than Streptococcus agalactiae, Enterococcus spp., coagulase-positive staphylococci other than Staphylococcus aureus, Klebsiella spp., Arcanobacterium pyogenes, Serratia spp., Pseudomonas spp., or Pasteurella spp., or ≥1,000 CFU/ml of Corynebacterium bovis, coagulase-negative staphylococci (CNS), yeasts, fungi, or Bacillus spp. were isolated, the quarter was deemed positive for an intramammary infection (22). Samples were considered contaminated if more than 3 species were isolated, unless Staphylococcus aureus or Streptococcus agalactiae was identified, in which case the sample was enumerated (17).
Statistical analysis.
All cases of mastitis in any quarter were considered, regardless of culture result (18). A new case in the same quarter was considered if 14 days had passed from the previous case. Cows were at risk for the entire 2-year period of CBMRN National Cohort of Dairy Farms sampling. Days at risk for each cow were calculated as the total number of days in milk from the start to end of the 2-year period, based on information available through the CBMRN. Time at risk ended if the cow was removed from the herd or was not milking. The incidence rate was calculated as the number of cases of mastitis per 36,500 days at risk (100 cow-years). The association of mastitis incidence with immune response category was analyzed by Poisson regression using PROC GLIMMIX (SAS version 9.1.3) with the natural logarithm of the number of days at risk as the offset. Herd was fit as a random effect.
RESULTS
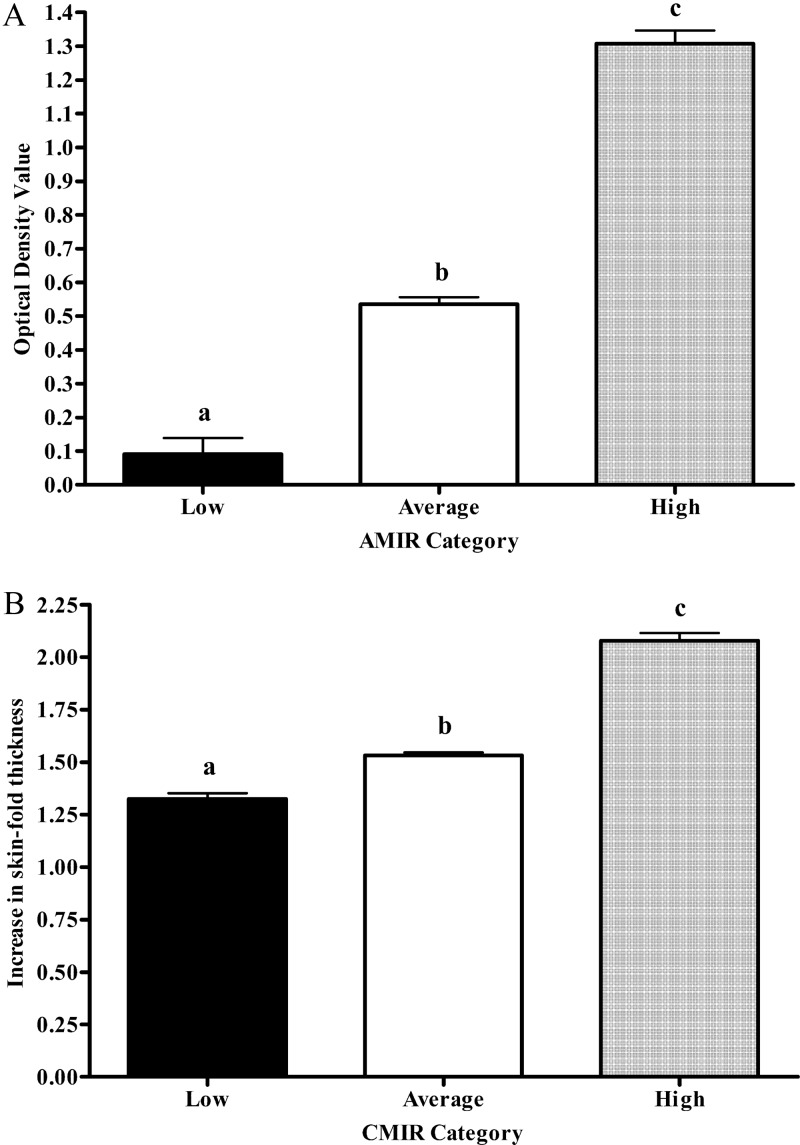
Phenotypic differences in AMIR and CMIR between cows classified as high, average, and low responders based on EBV are presented in Fig. 1. Cows classified as low responders had significantly lower AMIR (Fig. 1A) and CMIR (Fig. 1B) than average or high responder cows. High responder cows had significantly higher responses than average and low responding cows.
Fig 1.
AMIR (A) and CMIR (B) of 458 Holsteins from 41 herds classified as low, average, or high based on estimated breeding values. Different letters indicate significant (P < 0.0001) differences between IR categories.
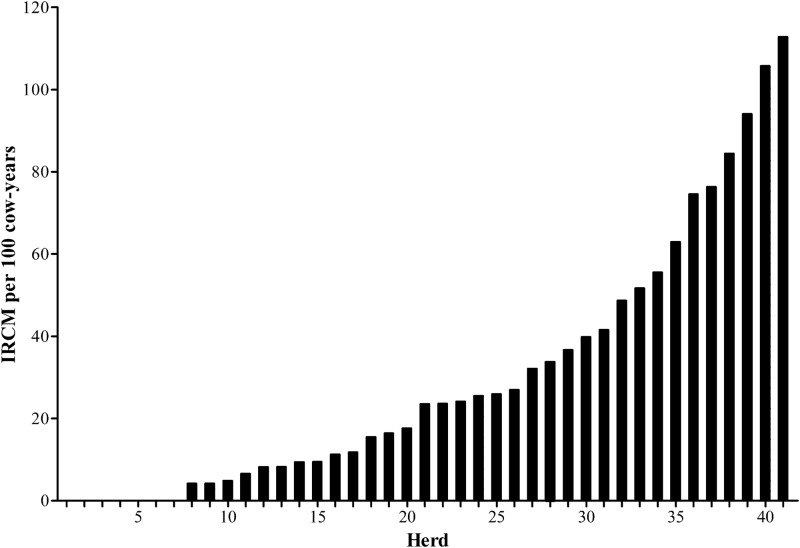
The mean IRCM in this study was 26.3 cases per 100 cow-years. The IRCM per herd ranged from 0.0 to 112.8 (Fig. 2). Escherichia coli was the most frequently isolated pathogen, comprising 29.9% of the isolates, followed by Staphylococcus aureus (22.2%) and Streptococcus spp. (16.2%) (Table 1). Contagious pathogens were responsible for 17.2% of the recorded mastitis cases, and environmental pathogens were responsible for 46.0%. The majority of mastitis cases, 53.3%, had a severity score of 1 (abnormal milk only), followed by severity score of 2 (abnormal milk and swollen udder) for 36.4% and severity of 3 (abnormal milk, swollen udder, and sick cow) constituting 10.3% of the cases.
Fig 2.
IRCM per 100 cow-years for 458 Holstein dairy cows from 41 Canadian herds.
Table 1.
Distribution of mastitis pathogens from 41 herds in Canada
| Pathogen | No. of cases | % of samples | % of isolates |
|---|---|---|---|
| Escherichia coli | 35 | 17.7 | 29.9 |
| Staphylococcus aureus | 26 | 13.1 | 22.2 |
| Streptococcus spp. | 19 | 9.6 | 16.2 |
| Coagulase-negative staphylococcus | 9 | 4.5 | 7.7 |
| Streptococcus uberis | 8 | 4.0 | 6.8 |
| Other Gram negative | 4 | 2.0 | 3.4 |
| Klebsiella spp. | 4 | 2.0 | 3.4 |
| Streptococcus dysgalactiae | 3 | 1.5 | 2.6 |
| Arcanobacterium pyogenes | 3 | 1.5 | 2.6 |
| Yeasts | 2 | 1.0 | 1.7 |
| Bacillus spp. | 2 | 1.0 | 1.7 |
| Prototheca spp. | 1 | 0.5 | 0.9 |
| Enterobacter spp | 1 | 0.5 | 0.9 |
| Culture negative | 39 | 19.7 | |
| Contaminated | 42 | 21.2 |
High-AMIR cows had a significantly lower IRCM than low and average responders (Table 2). High-AMIR cows had 17.1 cases of mastitis per 100 cow-years, compared to average responders with 27.9 cases and low AMIR cows with 30.7 cases. Only the differences for the causative pathogens Escherichia coli, Staphylococcus aureus, and Streptococcus spp. were reported, due to an insufficient number of cases for other pathogens. Differences in the IRCM by pathogen were not significant between the low, average, and high groups. However, the IRCM for both contagious and environmental pathogens were lower for high-AMIR cows than low-AMIR cows. High-AMIR cows had a significantly (P < 0.05) lower IRCM of mastitis, with a severity score of 1, compared to the average responders and also tended (P < 0.1) to be lower in the high-AMIR group than the low responders. Low-AMIR cows tended to have a higher IRCM of severe mastitis than average responders.
Table 2.
ICRM per 100 cow-years by high, average, and low immune response category based on EBV for AMIR for 458 Holstein dairy cows from 41 herds across Canada
| Pathogen or mastitis severity group | IRCM (per 100 cow-yrs) by AMIR categorya |
|||
|---|---|---|---|---|
| Low (n = 86) | Avg (n = 289) | High (n = 83) | All cows | |
| Escherichia coli | 6.15 | 4.53 | 4.26 | 4.77 |
| Staphylococcus aureus | 1.54 | 4.97 | 0.71 | 3.54 |
| Streptococcus spp. | 2.31 | 2.37 | 3.55 | 2.59 |
| Contagiousb | 2.31 | 6.26 | 1.42 | 4.63 |
| Environmentalc | 14.60 | 12.52 | 9.95 | 12.40 |
| Culture negative | 5.38 | 6.04 | 2.84 | 5.31 |
| Overall IRCM | 30.7 (A) | 27.9 (A) | 17.1 (B) | 26.3 |
| Mastitis severity score | ||||
| 1 | 16.14 | 15.76 (A) | 7.10 (B) | 14.17 |
| 2 | 8.45 | 10.58 | 7.81 | 9.67 |
| 3 | 6.15 | 1.94 | 2.13 | 2.72 |
IRCM values in the same row that are followed by different uppercase letters in parentheses show significant differences (P ≤ 0.05).
Contagious pathogens were Staphylococcus aureus, Streptococcus agalactiae, and Streptococcus uberis.
Environmental pathogens were Escherichia coli, Streptococcus spp. (other than S. agalactiae), Serratia spp., Citrobacter spp., Proteus spp., Salmonella spp., Pseudomonas spp., Pasteurella multocida, Corynebacterium spp., yeasts, fungi, Klebsiella spp., Staphylococcus hyicus, Prototheca spp., coagulase-negative Staphylococcus, Arcanobacterium pyogenes, Bacillus spp., non-S. aureus coagulase-positive Staphylococcus, and Enterobacter.
No differences were found in the IRCM between high-CMIR and low-CMIR cows (Table 3). However, average-CMIR cows tended (P < 0.1) to have a higher IRCM than low-CMIR cows, with 28.9 and 18.4 cases per 100 cow-years, respectively. Average-CMIR cows also tended (P < 0.01) to have higher IRCM due to environmental pathogens than did low-CMIR cows. No differences were found in the severities of mastitis between CMIR categories.
Table 3.
ICRM per 100 cow-years by high, average, and low immune response category based on EBV for CMIR for 458 Holstein dairy cows from 41 herds across Canada
| Pathogen or mastitis severity score | IRCM (per 100 cow-yrs) by CMIR categorya |
|||
|---|---|---|---|---|
| Low (n = 68) | Avg (n = 324) | High (n = 66) | All cows | |
| Escherichia coli | 2.76 | 5.56 | 2.90 | 4.77 |
| Staphylococcus aureus | 3.68 | 3.64 | 2.90 | 3.54 |
| Streptococcus spp. | 0.92 | 3.26 | 0.97 | 2.59 |
| Contagiousb | 3.68 | 5.17 | 2.90 | 4.63 |
| Environmentalc | 6.44 | 14.56 | 7.72 | 12.40 |
| Culture negative | 1.84 | 6.13 | 4.83 | 5.31 |
| Overall IRCM | 18.4 | 28.9 | 21.2 | 26.3 |
| Mastitis severity score | ||||
| 1 | 11.96 | 15.14 | 11.58 | 14.17 |
| 2 | 5.52 | 10.73 | 8.69 | 9.67 |
| 3 | 0.92 | 3.45 | 0.97 | 2.72 |
There were no significant differences in these results.
Contagious pathogens were Staphylococcus aureus, Streptococcus agalactiae, and Streptococcus uberis.
Environmental pathogens were Escherichia coli, Streptococcus spp. (other than S. agalactiae), Serratia spp., Citrobacter spp., Proteus spp., Salmonella spp., Pseudomonas spp., Pasteurella multocida, Corynebacterium spp., yeasts, fungi, Klebsiella spp., Staphylococcus hyicus, Prototheca spp., coagulase-negative Staphylococcus, Arcanobacterium pyogenes, Bacillus spp., non-S. aureus coagulase-positive Staphylococcus, and Enterobacter.
DISCUSSION
The incidence rate of clinical mastitis for all cows in this study was 26.3 cases per 100 cow-years, consistent with a previous study across Canada that estimated a national IRCM of 23.0 (22) and with other research (23–25). Escherichia coli was the most frequently isolated pathogen, followed by Staphylococcus aureus, and these findings were similar to previous studies (24, 25). However, a recent study evaluating the IRCM of dairy herds across Canada from 2003 to 2005 found Staphylococcus aureus to represent a greater percentage of isolates than Escherichia coli (22). It has been suggested that the relative and absolute importance of mastitis pathogens has shifted over time (2). Also, substantial geographic variation among mastitis-causing bacteria in Canada has been demonstrated and may contribute to the differences found between these national studies (18, 22). The IRCM per herd ranged from 0.0 to 112.8 cases per 100 cow-years. It should be noted that only mastitis cases for the cows enrolled in the immune response study were included in the analysis. Cases of mastitis were confirmed in all herds; therefore, an IRCM of 0 was not due to a lack of recording.
Cows classified as high AMIR had a significantly lower IRCM than average and low responders. This suggests that cows with superior antibody responses are better immunologically equipped to protect the host from infection with mastitis pathogens. The majority of mastitis pathogens isolated were extracellular in nature, which tend to be controlled by the antibody branch of the adaptive immune system. This finding is consistent with a previous study that determined immune response phenotypes for cows based on AMIR and found high-AMIR cows had less mastitis in 2 out of 3 herds tested (14). Further, more recent research evaluating both AMIR and CMIR in a large commercial dairy found high-AMIR cows had significantly less mastitis, with 16% of cows experiencing at least one case within the lactation of immune response sampling, compared to 22% of low-AMIR cows (15). In those previous studies, mastitis was recorded as a binary trait within the lactation immune response sampling. The results of this work more accurately represent the differences in mastitis by immune response category, since the IRCM accounts for multiple cases per cow throughout lactation, over the 2-year period.
Low-AMIR cows tended to have the most severe mastitis, likely due to a suboptimal ability to respond to challenge. A prompt and appropriate immune response is required for bacterial clearance before systemic signs of severe mastitis present, such as inflammation and fever. Identifying low-AMIR cows for culling or to be managed more intensively is a practical approach to decrease the incidence and severity of mastitis cases within a herd. On the other hand, since AMIR is heritable (9), breeding for enhanced antibody responses may decrease the incidence and severity of clinical mastitis and subsequent economic losses for Canadian dairy farms.
Only the IRCM for Escherichia coli, Staphylococcus aureus, and Streptococcus spp. were reported, due to an insufficient number of cases for other pathogens. Differences between immune response groups by pathogen were not significant, likely due to the low number of cases for each pathogen. Since farmers identified clinical cases based on milk quality, udder inflammation, and sickness behavior, all cases of mastitis were considered in the overall IRCM regardless of bacteriology results.
Cows were also categorized as high, average, or low responders based on CMIR; however, no associations with mastitis were found. This was consistent with previous findings from a large U.S. commercial dairy (15). The cell-mediated branch of the adaptive immune system generally predominates in host defenses to intracellular pathogens, such as Mycobacterium avium paratuberulosis. Previous work found that cows with high CMIR were associated with a lower probability of paratuberculosis seropositivity (16). The majority of mastitis pathogens are extracellular; however, some bacteria, such as Staphylococcus aureus, utilize both intracellular and extracellular mechanisms to infect and persist within the mammary gland. Staphylococcus aureus has the ability to survive intracellularly as small colony variants (SCV), establishing long-term persistence through modulation of the immune system (26, 27). In an experimental intramammary infection, bovine SCV elicited a DTH response as well as induction of antibody with an IgG2 bias, typical of a type 1 immune response (28). Therefore, it is hypothesized that cows with superior or high CMIR are better able to control Staphylococcus aureus in the SCV form; however, this is a less common phenotype. Staphylococcus aureus infection often results in subclinical, chronic infection (26, 29), and since only clinical mastitis cases were recorded in this study, it is possible that differences in mastitis related to SCV that are best controlled by the CMIR were overlooked.
Average-CMIR cows tended (P < 0.1) to have a higher IRCM than low-CMIR cows. This result was similar to a previous study, which found average-CMIR cows had the most mastitis, although the difference between groups was not significant (15). As suggested in the previous study, this tendency may have been due to the standard deviation classification system used, which may have caused the largest number of animals to be categorized in the average immune response group. Genetic correlations between AMIR and CMIR for the cows in the current study have been reported previously and were found to be negative or 0 (9). Therefore, it is possible that some of the cows with high AMIR were average or even low for CMIR, which may have contributed to the lack of association of CMIR with mastitis, which is typically controlled by antibody responses.
Once bacteria have entered the sterile environment of the mammary gland, optimal host defenses are required for prompt bacterial clearance to prevent mastitis. A rapid resolution of infection is critical to prevent damage to the mammary epithelium (30). The bacterial species that have the ability to cause bovine mastitis require various immune responses for host protection, as reviewed recently (3, 31). These pathogen-dependent immune responses can result in acute or chronic symptoms that range in severity (32). For example, Escherichia coli, a Gram-negative bacterium, is associated with the release of the cell wall component lipopolysaccharide (LPS) into the mammary gland, resulting in the pathogenesis of mastitis. E. coli often causes severe clinical mastitis of short duration and that is associated with high bacteria counts (33). On the other hand, Staphylococcus aureus is a Gram-positive bacterium with lipoteichoic acid being a major virulence component, and it is often associated with chronic, subclinical, persistent mastitis (26, 29). Bacteria have a large repertoire of virulence factors that are produced at various concentrations, depending on the stage of infection (34), and these virulence factors in part determine the differences in the magnitude and duration of host immune responses. Given the diversity of mastitis-causing pathogens, it is essential for the host to have a broad range of host defense mechanisms as part of its immunological arsenal. This is the goal of identifying cows by using the HIR test system with both high AMIR and CMIR.
Cows with superior adaptive responses are likely better able to eliminate bacterial challenge and restore immune homeostasis to prevent mastitis or an uncontrolled inflammatory response compared to other cows in the herd. Since appropriate innate immune responses are essential for the initiation of optimal adaptive responses, selecting cows with superior adaptive immune responses will likely also identify cows with superior innate responses, as suggested previously (15). This hypothesis is supported by evidence that showed high immune responder cows had a lower occurrence of not only mastitis but also other diseases, like metritis, displaced abomasums, and retained placenta. These disease are typically controlled, at least in part, by neutrophils of the innate immune system (35, 36). Future studies aimed at evaluating innate responses of high immune response cattle will be beneficial for determining if these cows also have enhanced innate responses, which may explain why they have a lower occurrence of diseases typically controlled by innate host defenses.
Positive genetic correlations (0.19 to 0.49) exist between mastitis and other diseases, like milk fever, ketosis, and retained placenta (37). This was true in a study in a large U.S. commercial dairy that found high immune response cows had not only less mastitis but also less metritis, retained placenta, and displaced abomasums (15). Breeding for enhanced immune responsiveness is therefore expected to decrease overall disease occurrence. Clinical mastitis is also known to have a negative impact on the reproductive performance of dairy cows (38). Associations of immune response with routinely evaluated traits in Canada have been estimated previously for the cows in this study, and significant beneficial EBV correlations were found with reproductive traits such as 56-day nonreturn rate, number of services, first service to conception, and gestation length (9). Although the EBV correlations were low, these findings suggested that breeding for enhanced immune responsiveness may not only decrease disease occurrence but also may be associated with improved reproductive performance.
Current breeding strategies to decrease mastitis include direct selection by using clinical mastitis records or indirect selection methods by using traits genetically correlated with mastitis, such as somatic cell score (SCS) (39). Although these methods in coordination with mastitis control programs have been somewhat successful in controlling mastitis, there are caveats associated with both. Selection against clinical mastitis will likely leave cattle susceptible to infection with other mastitis pathogens, since bacteria require unique immune responses for host protection and mastitis pathogens have been demonstrated to change over time and geographically (2). Further, the heritability of mastitis resistance is low; it was recently demonstrated in Canadian Holsteins to be about 0.02 (40). Breeding for decreased SCS is an alternative, as it is genetically correlated with mastitis and has a higher heritability, of about 0.11 (40, 41). However, SCS tends to useful for monitoring subclinical cases, and although decreasing bulk tank counts have been associated with a decline in subclinical mastitis, clinical mastitis continues to be a problem in many herds (24). Since most of the cells that constitute the SCS are cells of the immune system, an SCS that is too low has been associated with an increased risk of clinical mastitis (42). Breeding for enhanced immune responsiveness, as suggested here, is a solution to provide cows with an overall superior ability to respond to a variety of pathogen types requiring unique responses to provide broad-based disease resistance.
The emergence of genomic selection (43) has provided a promising tool to improve traits such as immune response and health. Since health and immune response phenotypes are relatively intensive to measure, genomics may be a solution, by using data available on the genetics of the animals (44). Future studies to identify genetic profiles associated with high and low immune responses are expected to show the potential for inclusion of immune response traits in genomic breeding indices in order to improve herd health and decrease the incidence of diseases like mastitis.
Conclusions.
Results from this study suggest breeding dairy cows for enhanced adaptive immune responses may decrease the incidence and severity of clinical mastitis on Canadian dairy farms. Cows identified as high antibody-mediated immune responders had significantly lower incidence rates of clinical mastitis than did low- or average-responding cows. Also, low-immune-responding cows tended to have the most severe mastitis. Therefore, culling low-immune-responding cows from the herd is a practical approach to decrease the incidence and severity of mastitis. Identification of immune response phenotypes within herds also allows the producer to group and manage cows differently when culling is not an option. Overall, the results of this study confirm previous work that found dairy cattle with high AMIR had a lower occurrence of mastitis in one or a limited number of herds in a single region. The results presented here from numerous herds on a national scale emphasize the potential to breed dairy cows for enhanced immune response as a sustainable approach to improve inherent disease resistance in the Canadian dairy industry.
ACKNOWLEDGMENTS
This research is financed by grants to B. A. Mallard from the National Sciences and Engineering Research Council of Canada, Alberta Milk, Dairy Farmers of New Brunswick, Nova Scotia, Ontario and Prince Edward Island, Novalait Inc., Dairy Farmers of Canada, DairyGen Council of Canadian Dairy Network, Agriculture and Agri-Food Canada, Public Health Agency of Canada, Technology Prince Edward Island Inc., Université de Montréal, and University of Prince Edward Island through the Canadian Bovine Mastitis Research Network. K. A. Thompson-Crispi is funded by a Dairy Farmers of Ontario doctoral research assistantship.
We have no financial conflicts of interest.
We thank Heba Atalla (University of Guelph) and Simon Dufour (University of Montreal) for their help analyzing the mastitis data.
Footnotes
Published ahead of print 21 November 2012
REFERENCES
- 1. Heikkila AM, Nousiainen JI, Pyorala S. 2012. Costs of clinical mastitis with special reference to premature culling. J. Dairy Sci. 95:139–150 [DOI] [PubMed] [Google Scholar]
- 2. Bradley A. 2002. Bovine mastitis: an evolving disease. Vet. J. 164:116–128 [DOI] [PubMed] [Google Scholar]
- 3. Schukken YH, Gunther J, Fitzpatrick J, Fontaine MC, Goetze L, Holst O, Leigh J, Petzl W, Schuberth HJ, Sipka A, Smith DG, Quesnell R, Watts J, Yancey R, Zerbe H, Gurjar A, Zadoks RN, Seyfert HM. 2011. Host-response patterns of intramammary infections in dairy cows. Vet. Immunol. Immunopathol. 144:270–289 [DOI] [PubMed] [Google Scholar]
- 4. Biozzi G, Mouton D, Heumann AM, Bouthillier Y, Stiffel C, Mevel JC. 1979. Genetic analysis of antibody responsiveness to sheep erythrocytes in crosses between lines of mice selected for high or low antibody synthesis. Immunology 36:427–438 [PMC free article] [PubMed] [Google Scholar]
- 5. Heriazon A, Thompson KA, Wilkie BN, Mathes-Sears W, Quinton M, Mallard BA. 2009. Antibody to ovalbumin and delayed-type hypersensitivity to Candida albicans and mycobacteria in lactating Holstein cows using Quil A or Freund's complete adjuvant. Vet. Immunol. Immunopathol. 127:220–227 [DOI] [PubMed] [Google Scholar]
- 6. Mallard BA, Wilkie BN, Kennedy BW, Quinton M. 1992. Use of estimated breeding values in a selection index to breed Yorkshire pigs for high and low immune and innate resistance factors. Anim. Biotechnol. 3:257–280 [Google Scholar]
- 7. Sarker N, Tsudzuki M, Nishibori M, Yasue H, Yamamoto Y. 2000. Cell-mediated and humoral immunity and phagocytic ability in chicken Lines divergently selected for serum immunoglobulin M and G levels. Poult. Sci. 79:1705–1709 [DOI] [PubMed] [Google Scholar]
- 8. Estes DM, Brown WC. 2002. Type 1 and type 2 responses in regulation of Ig isotype expression in cattle. Vet. Immunol. Immunopathol. 90:1–10 [DOI] [PubMed] [Google Scholar]
- 9. Thompson-Crispi KA, Sewalem A, Miglior F, Mallard B. 2012. Genetic parameters of adaptive immune response traits in Canadian Holsteins. J. Dairy Sci. 95:401–409 [DOI] [PubMed] [Google Scholar]
- 10. Wilson CB, Makar KW, Shnyreva M, Fitzpatrick DR. 2005. DNA methylation and the expanding epigenetics of T cell lineage commitment. Semin. Immunol. 17:105–119 [DOI] [PubMed] [Google Scholar]
- 11. Corbeil LB. 2002. Antibodies as effectors. Vet. Immunol. Immunopathol. 87:169–175 [DOI] [PubMed] [Google Scholar]
- 12. Abdel-Azim GA, Freeman AE, Kehrli ME, Jr, Kelm SC, Burton JL, Kuck AL, Schnell S. 2005. Genetic basis and risk factors for infectious and noninfectious diseases in US Holsteins. I. Estimation of genetic parameters for single diseases and general health. J. Dairy Sci. 88:1199–1207 [DOI] [PubMed] [Google Scholar]
- 13. Mallard BA, Atalla H, Cartwright S, Hine BC, Hussey B, Paibomesai M, Thompson-Crispi KA, Wagter-Lesperance L. 2011. Genetic and epigenetic regulation of the bovine immune system: practical implications of the high immune response technology, p 53–63 Proc. Natl. Mastitis Council 50th Annu. Meet. National Mastitis Council, Verona, WI [Google Scholar]
- 14. Wagter LC, Mallard BA, Wilkie BN, Leslie KE, Boettcher PJ, Dekkers JC. 2000. A quantitative approach to classifying Holstein cows based on antibody responsiveness and its relationship to peripartum mastitis occurrence. J. Dairy Sci. 83:488–498 [DOI] [PubMed] [Google Scholar]
- 15. Thompson-Crispi KA, Hine B, Quinton M, Miglior F, Mallard BA. 2012. Short communication: association of disease incidence and adaptive immune response in Holstein dairy cows. J. Dairy Sci. 95:3888–3893 [DOI] [PubMed] [Google Scholar]
- 16. Pinedo PJ, Donovan A, Rae O, DeLapaz J. 2009. Association between paratuberculosis infection and general immune status in dairy cattle, p 127 International Association for Paratuberculosis, Minneapolis, MN [Google Scholar]
- 17. Reyher KK, Dufour S, Barkema HW, Des CL, Devries TJ, Dohoo IR, Keefe GP, Roy JP, Scholl DT. 2011. The National Cohort of Dairy Farms: a data collection platform for mastitis research in Canada. J. Dairy Sci. 94:1616–1626 [DOI] [PubMed] [Google Scholar]
- 18. Thompson-Crispi KA, Mallard BA. 2012. Type 1 and type 2 immune response profiles of commercial dairy cows in four regions of Canada. Can. J. Vet. Res. 76:120–128 [PMC free article] [PubMed] [Google Scholar]
- 19. Hernandez A, Yager JA, Wilkie BN, Leslie KE, Mallard BA. 2005. Evaluation of bovine cutaneous delayed-type hypersensitivity (DTH) to various test antigens and a mitogen using several adjuvants. Vet. Immunol. Immunopathol. 104:45–58 [DOI] [PubMed] [Google Scholar]
- 20. Heriazon A, Yager JA, Sears W, Mallard BA. 2009. Induction of delayed-type hypersensitivity and interferon-gamma to Candida albicans and anti-hen-egg white lysozyme antibody as phenotypic markers of enhanced bovine immune response. Vet. Immunol. Immunopathol. 129:93–100 [DOI] [PubMed] [Google Scholar]
- 21. Madsen P, Jensen J. 2008. DMU: a user's guide. A package for analysing multivariate mixed models. DJF, Foulum, Denmark [Google Scholar]
- 22. Olde Riekerink RG, Barkema HW, Kelton DF, Scholl DT. 2008. Incidence rate of clinical mastitis on Canadian dairy farms. J. Dairy Sci. 91:1366–1377 [DOI] [PubMed] [Google Scholar]
- 23. Barkema HW, Schukken YH, Lam TJ, Beiboer ML, Wilmink H, Benedictus G, Brand A. 1998. Incidence of clinical mastitis in dairy herds grouped in three categories by bulk milk somatic cell counts. J. Dairy Sci. 81:411–419 [DOI] [PubMed] [Google Scholar]
- 24. Sargeant JM, Scott HM, Leslie KE, Ireland MJ, Bashiri A. 1998. Clinical mastitis in dairy cattle in Ontario: frequency of occurrence and bacteriological isolates. Can. Vet. J. 39:33–38 [PMC free article] [PubMed] [Google Scholar]
- 25. Schukken YH, Grommers FJ, van de Greer D, Brand A. 1989. Incidence of clinical mastitis on farms with low somatic cell counts in bulk milk. Vet. Rec. 125:60–63 [DOI] [PubMed] [Google Scholar]
- 26. Atalla H, Gyles C, Jacob CL, Moisan H, Malouin F, Mallard B. 2008. Characterization of a Staphylococcus aureus small colony variant (SCV) associated with persistent bovine mastitis. Foodborne Pathog. Dis. 5:785–799 [DOI] [PubMed] [Google Scholar]
- 27. Atalla H, Gyles C, Mallard B. 2010. Persistence of a Staphylococcus aureus small colony variants (S. aureus SCV) within bovine mammary epithelial cells. Vet. Microbiol. 143:319–328 [DOI] [PubMed] [Google Scholar]
- 28. Atalla H, Wilkie B, Gyles C, Leslie K, Mutharia L, Mallard B. 2010. Antibody and cell-mediated immune responses to Staphylococcus aureus small colony variants and their parental strains associated with bovine mastitis. Dev. Comp. Immunol. 34:1283–1290 [DOI] [PubMed] [Google Scholar]
- 29. Sutra L, Poutrel B. 1994. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J. Med. Microbiol. 40:79–89 [DOI] [PubMed] [Google Scholar]
- 30. Zhao X, Lacasse P. 2008. Mammary tissue damage during bovine mastitis: causes and control. J. Anim. Sci. 86:57–65 [DOI] [PubMed] [Google Scholar]
- 31. De Vliegher S, Fox LK, Piepers S, McDougall S, Barkema HW. 2012. Invited review. Mastitis in dairy heifers: nature of the disease, potential impact, prevention, and control. J. Dairy Sci. 95:1025–1040 [DOI] [PubMed] [Google Scholar]
- 32. Aitken SL, Corl CM, Sordillo LM. 2011. Immunopathology of mastitis: insights into disease recognition and resolution. J. Mammary Gland Biol. Neoplasia 16:291–304 [DOI] [PubMed] [Google Scholar]
- 33. Smith KL, Hogan JS. 1993. Environmental mastitis. Vet. Clin. North Am. Food Anim. Pract. 9:489–498 [DOI] [PubMed] [Google Scholar]
- 34. Bharathan M, Mullarky IK. 2011. Targeting mucosal immunity in the battle to develop a mastitis vaccine. J. Mammary Gland Biol. Neoplasia 16:409–419 [DOI] [PubMed] [Google Scholar]
- 35. Benedictus L, Jorritsma R, Knijn HM, Vos PL, Koets AP. 2011. Chemotactic activity of cotyledons for mononuclear leukocytes related to occurrence of retained placenta in dexamethasone induced parturition in cattle. Theriogenology 76:802–809 [DOI] [PubMed] [Google Scholar]
- 36. Galvao KN, Felippe MJ, Brittin SB, Sper R, Fraga M, Galvao JS, Caixeta L, Guard CL, Ricci A, Gilbert RO. 2012. Evaluation of cytokine expression by blood monocytes of lactating Holstein cows with or without postpartum uterine disease. Theriogenology 77:356–372 [DOI] [PubMed] [Google Scholar]
- 37. Koeck A, Miglior F, Kelton DF, Schenkel FS. 2012. Health recording in Canadian Holsteins: data and genetic parameters. J. Dairy Sci. 95:4099–4108 [DOI] [PubMed] [Google Scholar]
- 38. Ahmadzadeh A, Frago F, Shafii B, Dalton JC, Price WJ, McGuire MA. 2009. Effect of clinical mastitis and other diseases on reproductive performance of Holstein cows. Anim. Reprod. Sci. 112:273–282 [DOI] [PubMed] [Google Scholar]
- 39. Heringstad B, Klemetsdal G, Ruane J. 2000. Selection for mastitis resistance in dairy cattle: a review with focus on the situation in the Nordic countries. Liv. Prod. Sci. 64:95–106 [Google Scholar]
- 40. Koeck A, Miglior F, Kelton DF, Schenkel FS. 2012. Alternative somatic cell count traits to improve mastitis resistance in Canadian Holsteins. J. Dairy Sci. 95:432–439 [DOI] [PubMed] [Google Scholar]
- 41. Bloemhof S, de Jong G, de Haas Y. 2009. Genetic parameters for clinical mastitis in the first three lactations of Dutch Holstein cattle. Vet. Microbiol. 134:165–171 [DOI] [PubMed] [Google Scholar]
- 42. Suriyasathaporn W, Schukken YH, Nielen M, Brand A. 2000. Low somatic cell count: a risk factor for subsequent clinical mastitis in a dairy herd. J. Dairy Sci. 83:1248–1255 [DOI] [PubMed] [Google Scholar]
- 43. Meuwissen TH, Hayes BJ, Goddard ME. 2001. Prediction of total genetic value using genome-wide dense marker maps. Genetics 157:1819–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boichard D, Brochard M. 2012. New phenotypes for new breeding goals in dairy cattle. Animal 6:544–550 [DOI] [PubMed] [Google Scholar]