Negative surgical margins for prostate cancer patients undergoing robotic-assisted laparoscopic radical prostatectomy result in lower biochemical recurrence rates for low and intermediate risk groups.
Keywords: Prostate cancer, PSA, Recurrence, Robotic surgery
Abstract
Background and Objectives:
To determine prostate cancer biochemical recurrence rates with respect to surgical margin (SM) status for patients undergoing robotic-assisted laparoscopic radical prostatectomy (RALP).
Methods:
IRB-approved radical prostatectomy database was queried. Patients were stratified as low, intermediate, and high risk according to D’Amico's risk classification. Postoperative prostate-specific antigen (PSA) values were obtained every 3 mo for the first year, then biannually and annually thereafter. Biochemical recurrence was defined as ≥0.2ng/mL. Patients receiving adjuvant or salvage treatment were included. Positive surgical margin was defined as presence of cancer cells at inked resection margin in the final specimen. Margin presence (negative/positive), margin multiplicity (single/multiple), and margin length (≤3mm focal and >3mm extensive) were noted. Kaplan-Meier curves of biochemical recurrence-free survival (BRFS) as a function of SM were generated. Forward stepwise multivariate Cox regression was performed, with preoperative PSA, Gleason score, pathologic stage, prostate gland weight, and SM as covariates.
Results:
At our institution, 1437 patients underwent RALP (2003-2009). Of these, 1159 had sufficient data and were included in our analysis. Mean follow-up was 16 mo. Kaplan-Meier curves demonstrated significant increase in BRFS in low-risk and intermediate-risk groups with negative SM. Overall BRFS at 5 y was 72%. Gleason score, pathologic stage, and SM status were significant prognostic factors in multivariate analysis.
Conclusions:
Negative surgical margins resulted in lower biochemical recurrence rates for low-risk and intermediate-risk groups. Multifocal and longer positive margins were associated with higher biochemical recurrence rates compared with unifocal and shorter positive margins. Documenting biochemical recurrence rates for RALP is important, because this treatment for localized prostate cancer is validated.
INTRODUCTION
Robotic-assisted laparoscopic radical prostatectomy (RALP) has gained popularity around the world. This year the number of robotic cases again exceeds that of traditional open prostatectomy cases in the United States, with 85% performed robotically.1 We performed a review of our prostate cancer database to demonstrate trends in biochemical recurrence (BCR) in a large cohort of patients undergoing RALP, as a function of preoperative prostate-specific antigen (PSA) levels, Gleason score, TNM stage, and surgical margins (SM). Our primary objective was to determine prostate cancer BCR rates with respect to SM status for patients undergoing RALP. We also compared our BCR rates to other open and laparoscopic radical prostatectomy series reported in the literature.
MATERIALS AND METHODS
A retrospective review of a prospectively maintained, Internal Review Board (IRB)-approved radical prostatectomy database was performed. Patients undergoing RALP between December 1, 2003 and January 1, 2009 were eligible for analysis.
All RALP procedures were performed via a transperitoneal approach by 1 of 3 surgeons using a modified Vattikuti Institute technique.2 DaVinci, DaVinci S, and DaVinci S HD Surgical Systems (Intuitive Surgical, Sunnyvale, CA, USA) were used to perform the procedures. Learning curves when present for all surgeons were included.
The demographic information, clinical staging, and intraoperative details were prospectively collected and entered into the database. Using preoperative data, patients were stratified as low, intermediate, and high risk according to D’Amico's risk classification.3 Specifically, the low-risk group was defined as having serum PSA <10ng/mL, Gleason score <7, and clinical stage <cT2b. The intermediate-risk group was defined as having serum PSA between 10ng/mL and 20ng/mL or Gleason score equal to 7. The high-risk group was defined as having serum PSA >20ng/mL or Gleason score 8 to 10.
Surgical margin status was determined by pathologic evaluation of the specimen at a single institution. AJCC 2002 staging guidelines were consistently used by the pathologists.4 All specimens were whole-mounted and step-sectioned at 3-mm intervals with apex and base being additionally cross-sectioned. Positive surgical margin was defined as the presence of cancer cells at the inked resection margin in the final specimen. Intraoperative biopsy results or additional tissue excisions were not used to determine margin positivity. Margin presence (negative/positive), margin multiplicity (single/multiple), and margin length (≤3mm focal and >3mm extensive) were noted.
Postoperative PSA values were routinely obtained at 1, 3, 6, 9, 12, 18, and 24 mo and annually thereafter. PSA recurrence was defined as ≥0.2ng/mL. Patients receiving adjuvant treatment were included in our analyses and were considered as having recurred at the time of adjuvant treatment.
We excluded 278 patients due to lack of postoperative PSA or lack of follow-up at our institution. Demographic comparison was performed between excluded and included patients.
Data were extracted from the prostate database and entered into SPSS v 14.0 statistical software (SPSS, Inc., Chicago, IL, USA.) Univariate analysis was performed for the overall cohort, as well as for patients stratified by Gleason score, TNM stage, and D’Amico classification, comparing biochemical recurrence-free survival (BRFS) with respect to SM status. BRFS was defined as time from RALP to BCR. Patients without BCR were censored on the date of their last PSA measurement. Kaplan-Meier plots of BRFS were generated for the above categories. Differences relative to SM status were evaluated using the log-rank test. Multivariate Cox regression analysis was also performed. Pathologic stage (≤pT2N0M0, pT3aN0M0, pT3b/4N0M0, pTxN1Mx), pathologic grade (pathologic Gleason score ≤6, 7, and 8 to 10), SM status (negative/positive), margin multiplicity (single/multiple), and margin length (≤3mm focal and >3mm extensive) were entered as categorical variables with the first category as the reference group. Preoperative PSA, transformed to its natural logarithm to minimize effects of extreme values, an approach previously described in the literature, was entered as a continuous variable.5 Statistical significance was set at 2-sided P < .05.
To evaluate whether nerve-sparing status is a risk factor for positive margins, a univariate analysis was performed for bilateral, unilateral, and non–nerve-sparing procedures. The impact of the learning curve on margin status was examined by performing a univariate analysis on each quartile of cases.
RESULTS
Between December 1, 2003 and January 2009, 1437 patients underwent RALP procedures performed by 3 surgeons at our institution. Of these, 278 patients were excluded due to lack of follow-up at our institution and lack of postoperative PSA. Sixteen patients received adjuvant therapy and were included in our analysis. Specifically, 9 patients underwent adjuvant radiation therapy, 3 received adjuvant androgen deprivation therapy, and 4 received both. A total of 1159 patients were included in our analysis.
Patient demographics are presented in Table 1. Mean age, body mass index (BMI), and preoperative PSA values were 59.3 y, 28.1kg/m2, and 5.9ng/mL, respectively. Patients had a mean follow-up of 15.9 mo (0 to 60). Predictably, preoperative serum PSA demonstrated a statistically significant difference between the 2 groups, with higher values for those with positive surgical margins (P < .001). The distribution of clinical Gleason score (P = .003), D’Amico risk stratification (P = .001), pathological Gleason (P = .001), and pathologic stage (P = .001) also demonstrated a statistically significant difference. Patients with positive margins had higher stage, grade, and risk. Neither performance of pelvic lymphadenectomy (P = .182) nor presence of positive lymph nodes (P = .139) was statistically different between the 2 groups.
Table 1.
Demographic Data
| Negative Margin | Positive Margin | All Patients | P Value | |
|---|---|---|---|---|
| Number of patients (%) | 843 (72.7%) | 316 (27.3%) | 1159 (100.0%) | |
| Mean age, years (SDa) | 59.3 (6.5) | 59.2 (6.4) | 59.3 (6.5) | .987 |
| Mean BMI, kg/m2 (SDa) | 28.1 (7.2) | 28.0 (3.9) | 28.1 (6.4) | .693 |
| Mean preoperative PSAa, ng/mL (SDa) | 5.4 (2.8) | 7.4 (6.7) | 5.9 (4.4) | <.001 |
| Clinical Gleason score | .003 | |||
| ≤6 | 485 (76.6%) | 148 (23.4%) | 633 (100.0%) | |
| 7 | 295 (68.6%) | 135 (31.4%) | 430 (100.0%) | |
| 8–10 | 61 (64.9%) | 33 (35.1%) | 94 (100.0%) | |
| Clinical stage | .073 | |||
| cT1 | 588 (73.2%) | 215 (26.8%) | 803 (100.0%) | |
| cT2 | 246 (72.8%) | 92 (27.2%) | 338 (100.0%) | |
| cT3 | 7 (46.7%) | 8 (53.3%) | 15 (100.0%) | |
| Risk class (D’Amico) | <.001 | |||
| Low | 439 (77.0%) | 131 (23.0%) | 570 (100.0%) | |
| Intermediate | 329 (71.4%) | 132 (28.6%) | 461 (100.0%) | |
| High | 74 (58.3%) | 53 (41.7%) | 127 (100.0%) | |
| Pathologic Gleason score | <.001 | |||
| ≤6 | 294 (82.4%) | 63 (17.6%) | 357 (100.0%) | |
| 7 | 483 (69.1%) | 216 (30.9%) | 699 (100.0%) | |
| 8–10 | 60 (61.9%) | 37 (38.1%) | 97 (100.0%) | |
| Pathologic stage | <.001 | |||
| pT0–pT2N0M0 | 718 (79.9%) | 183 (20.3%) | 901 (100.0%) | |
| T3aN0M0 | 89 (46.8%) | 101 (53.2%) | 190 (100.0%) | |
| T3b and pT4N0M0 | 25 (50.0%) | 25 (50.0%) | 50 (100.0%) | |
| TxN1Mx (positive nodes) | 8 (53.3%) | 7 (46.7%) | 15 (100.0%) | |
| Mean follow-up, months (SDa) | 15.6 (13.0) | 16.5 (14.3) | 15.9(13.4) | .337 |
| Learning curve | .248 | |||
| First quartile | 201 (69.3%) | 89 (30.7%) | 290 (100%) | |
| Second quartile | 217 (74.8%) | 73 (25.2%) | 290 (100%) | |
| Third quartile | 219 (75.8%) | 70 24.2%) | 289 (100%) | |
| Fourth quartile | 206 (71.0%) | 84 (29.0%) | 290 (100%) | |
| Nerve sparing status | .672 | |||
| Bilateral nerve sparing | 564 (72.7%) | 212 (27.3%) | 776 (100%) | |
| Unilateral nerve sparing | 217 (73.8%) | 77 (26.2%) | 294 (100%) | |
| Non nerve sparing | 60 (69.0%) | 27 (31.0%) | 87 (100%) |
SD=standard deviation; BMI=body mass index; PSA=prostate-specific antigen.
Excluded patients were compared with the analyzed patients and no statistically significant differences were found in the demographic, operative, or pathologic characteristics, with exception of frequency of pelvic lymphadenectomy. Pelvic lymphadenectomy was performed on 48.6% of excluded patients vs. 58.9% in the analyzed population (P = .002).
The frequency of positive margins in the overall cohort was 27.3%, 20.3% for pathologic stage T2, and 52.3% for pathologic stage ≥T3a disease. Specifically, in patients with Gleason score ≤6, pathologic stage T2N0M0 and T3aN0M0 had 14.9% and 65% incidence of positive surgical margin (PSM), respectively. In patients with Gleason score 7, the incidence of PSM for stage T2N0M0, T3aN0M0, T3b/T4N0M0, and TxN1Mx was 24.1%, 53.2%, 51.7%, and 12.5%, respectively. In patients with Gleason score 8 to 10, the incidence of PSM for stage T2N0M0, T3aN0M0, T3b/T4N0M0, and TxN1Mx was 17.9%, 45.2%, 50.0%, and 85.7%, respectively.
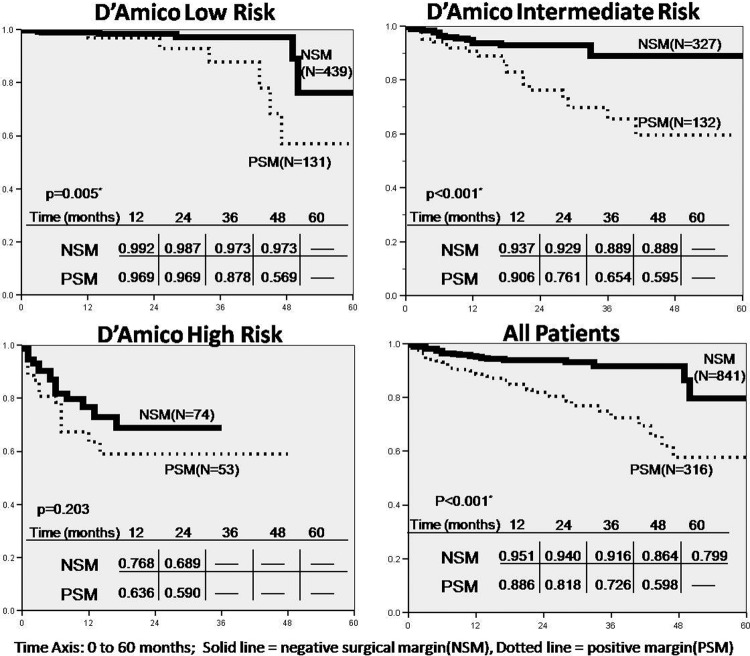
BRFS curves were generated for the overall cohort as well as individual D’Amico risk groups and are depicted in Figure 1. Low-risk and intermediate-risk groups achieved a statistically significant difference in BRSF with respect to SM. In the low-risk group, mean BRFS was 56.7 vs. 51.0 mo (P = .005), and in the intermediate-risk group it was 55.2 vs. 43.5 mo (P < .001) for negative and positive SM, respectively. The high-risk group did not reach statistical significance with respect to SM. The overall cohort mean BRFS was statistically significant, 54.9 vs. 45.9 mo (P < .001) for negative and positive SM, respectively.
Figure 1.
Biochemical recurrence-free survival for D’Amico risk groups and over all by surgical margin status.
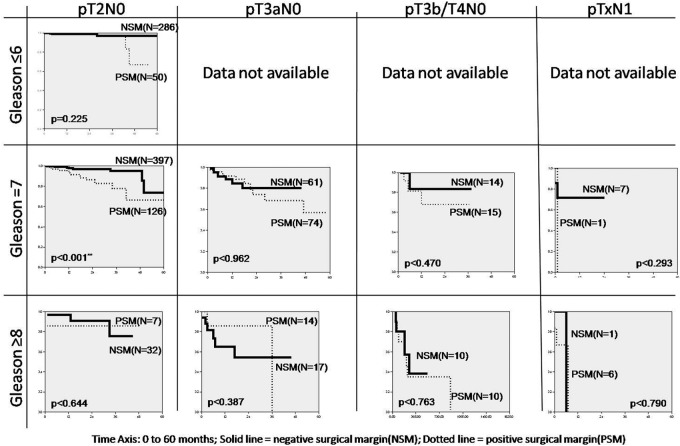
Similar curves were generated for individual pathologic tumor stage and pathologic Gleason score for positive and negative SM and are depicted in Figure 2. Patients with Gleason score of 7 and pathologic stage T2N0M0 had a statistically significant difference in mean BRFS of 55.6 and 48.7 mo (P < .001) for negative and positive margins, respectively. Others did not have a statistically significant difference in BCRF survival with respect to SM.
Figure 2.
Cumulative biochemical recurrence-free survival by Gleason score and pathologic stage.
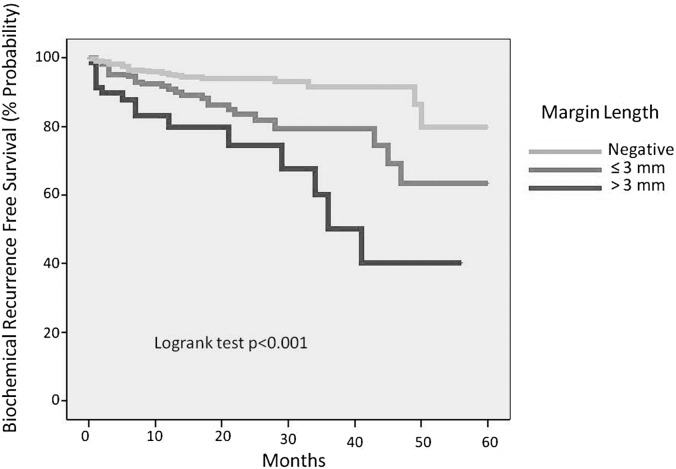
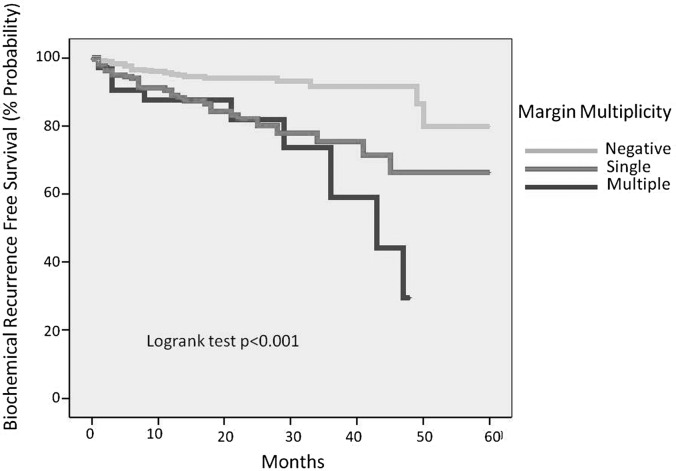
When surgical margins were further categorized by multiplicity and length, multiple margins and extensive margins carried a higher rate of BCR (P < .001) (Figures 3 and 4). The rates of positive surgical margins did not change significantly between quartile of cases (P = .243, Table 1). Nerve-sparing surgical status did not significantly affect the incidence of positive surgical margins (P = .672, Table 1).
Figure 3.
Biochemical recurrence-free survival by margin length.
Figure 4.
Biochemical recurrence-free survival buy margin multiplicity.
The multivariate prediction of time to BRFS showed pathological stage (P < .001), pathologic Gleason grade (P < .001), and SM (P = .035) as significant covariates. Data for preoperative PSA was suggestive but missed statistical significance (P = .053). While margin multiplicity and margin length were significant in univariate analysis, they were not in multivariate analysis. Hazard ratios (HR) and confidence intervals (CI) for each variable in the equation are listed in Table 2.
Table 2.
Multivariate analysis of biochemical recurrence-free survival
| Variable | HRc | CIc (95%) | P Value |
|---|---|---|---|
| Pathologic stage | <.001 | ||
| ≤pT2N0M0 | 1.0 (reference) | ||
| pT3aN0M0 | 2.30 | 1.34–3.97 | .003 |
| pT3b/T4N0M0 | 5.22 | 2.72–9.99 | <.001 |
| pTxN1Mx | 13.48 | 5.61–32.42 | <.001 |
| Pathologic Gleason score | <.001 | ||
| ≤6 | 1.0 (reference) | ||
| 7 | 3.42 | 1.34–8.75 | .01 |
| 8–10 | 10.13 | 3.66–28.02 | <.001 |
| Surgical margin statusa | .035 | ||
| Negative | 1.0 (reference) | ||
| Positive | 1.63 | 1.83–2.56 | .035 |
| Margin multiplicityb | |||
| Single | 1.0 (reference) | ||
| Multiple | .98 | .48–2.01 | .954 |
| Margin lengthb | |||
| <3mm | 1.0 (reference) | ||
| >3mm | 1.45 | .74–2.87 | .281 |
| Preoperative PSAc | 1.44 | 0.995–2.09 | .053 |
Comparing negative vs positive margin for all patients.
Comparing length and multiplicity among those with positive margins.
HR=hazard ratio; CI = confidence interval); PSA=prostate-specific antigen.
DISCUSSION
The importance of positive SM, its impact on BRFS, and as a result, its impact on disease-specific survival has been previously established in univariate and multivariate analyses.6–8 In our series, the frequency of positive SM for the overall cohort was slightly higher than but within the range reported in the literature for both open and robotic series.5,9–13 While some series have shown that margin rates improve with surgical volume, this was not our experience.5,14,15 Our margin rates were fairly constant over the time period examined, most likely because 2 of our 3 surgeons had gone through their learning curves during fellowship and/or prior to joining our institution; the learning curve of only 1 surgeon is present in our data.
With respect to SM status, our analyses demonstrate that the following groups reached statistically significant difference in BRFS: pT2/Gleason 7, D’Amico low-risk and intermediate–risk groups, and the overall cohort. The rest of the groups did not show a statistically significant difference for BCRF survival with respect to SM. One possible explanation is that these groups were underpowered. Alternatively, the lack of statistical difference may be real and represents the minimal impact of SM on BRFS in certain subgroups. It is known that prostate cancer in patients with Gleason score ≤6 tends to not recur, regardless of SM status, as demonstrated by our pT2/Gleason score ≤6 subgroup. This phenomenon is supported by a recent publication by Caire et al.16 where authors concluded that patients with pathologic Gleason score <7 had a significantly delayed BCR as compared with those with Gleason score ≥7. These authors also found that delayed BCR confers a disease-specific survival advantage. Similarly, Karakiewicz et al.13 determined SM to be a significant predictor of BCR only for Gleason score ≥7.
In the multivariate analysis, SM remained a significant predictor of BCR, as did pathologic stage and Gleason score. When positive margins were further categorized by length and multiplicity, multiple positive margins and extensive positive margins carried a higher rate of BCR. Preoperative PSA did not quite reach statistical significance. Pavlovich et al.17 also failed to find significant association between preoperative PSA levels and BCR. Once pathologic stage, Gleason score, and margin status were included in the analysis, the significance of preoperative PSA was lost. Although serum PSA level may play a role in screening and as a tumor marker to detect BCR postoperatively, the prognostic ability of preoperative serum PSA with respect to BCR is not supported by our analysis.
Our cumulative BRFS was 93.3%, 90.6%, 86.2%, 79.7%, and 72.0% at 1, 2, 3, 4, and 5 y after RALP, respectively. The 5-y BRFS of 72% is comparable to other series in the literature. Murphy et al.15 reported 74.0% for robotic-assisted, Rassweiler et al.18 73.1% for laparoscopic, and Karakiewicz et al.13 75.4% for open radical retropubic prostatectomy series.
Sixteen patients in our study received adjuvant therapy and were included in our analysis as failures whether PSA nadired or not. Whether to include or exclude these patients is not well defined in the literature. Swindle et al.6 confirmed the persistent significance of positive surgical margins on BRFS when patients receiving adjuvant therapy were either considered to recur at the time of adjuvant therapy or were excluded. Although our database contains few patients receiving adjuvant therapy, when a similar analysis was performed our outcomes did not change significantly (results not shown).
We acknowledge that the retrospective nature of this study may allow for selection bias. Although patients with positive and negative margins differed in pathologic and clinical characteristics, subgroup analyses by D’Amico risk, TNM stage, and Gleason grade attempted to minimize the above differences.
Patients who had insufficient clinical and pathological data, who were lost to follow-up, or who chose to follow-up elsewhere were not captured by this study, although every effort was made to do so. Analysis of available data for 278 excluded patients compared with the 1159 included patients did not show any significant difference except for the frequency of pelvic lymph node dissection, which was lower in the excluded group (48.6% vs. 58.9%). Performance of lymph node dissection was at the surgeon's discretion, and lower frequency of lymphadenectomy in the excluded patients suggests that this group was at a lower risk for nodal metastasis and recurrence. Thus, if these patients were included, resultant BRFS would likely increase.
This study is also limited by the length of follow-up. Early stage/low-grade prostate cancer is indolent and BCR may not be captured within our follow-up interval, thus possibly minimizing the significance of SM. Because we are a tertiary care center, most of our patients receive follow-up care with their local urologists, and we are exploring other avenues for data capture such as on-line surveys, automated calls, and other such things. Future studies with longer follow-up would allow evaluation of more direct metrics, such as disease-specific survival, metastasis-free survival, and overall survival.
CONCLUSIONS
This study confirms the significance of SM on BRFS in a large cohort of RALP patients. BCR rates for robotic, laparoscopic, and open series appear to be comparable. Our BRFS further validates a robotic-assisted approach for the treatment of prostate cancer. Longer follow-up is needed to better define the impact of margin status on BCR. A multi-institutional approach would further strengthen the role of RALP as comparable to radical retropubic prostatectomy.
Contributor Information
Serge Ginzburg, University of Connecticut Health Center, Farmington, CT, USA..
Thomas Nevers, Hartford Hospital, Hartford, CT, USA..
Ilene Staff, Hartford Hospital, Hartford, CT, USA..
Joseph Tortora, Hartford Hospital, Hartford, CT, USA..
Alison Champagne, Hartford Hospital, Hartford, CT, USA..
Stuart S. Kesler, Hartford Hospital, Hartford, CT, USA..
Vincent P. Laudone, Hartford Hospital, Hartford, CT, USA..
Joseph R. Wagner, Hartford Hospital, Hartford, CT, USA..
References:
- 1. Schwartz BF. Training requirements and credentialing for laparoscopic and robotic surgery–what are our responsibilities? J Urol. 2009; 182 (3): 828–829 [DOI] [PubMed] [Google Scholar]
- 2. Menon M, Tewari A, Peabody J, et al. Vattikuti Institute prostatectomy: technique. J Urol. 2003; 169 (6): 2289–2292 [DOI] [PubMed] [Google Scholar]
- 3. D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280 (11): 969–974 [DOI] [PubMed] [Google Scholar]
- 4. Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 5. Schroeck FR, Sun L, Freedland SJ, et al. Comparison of prostate-specific antigen recurrence-free survival in a contemporary cohort of patients undergoing either radical retropubic or robot-assisted laparoscopic radical prostatectomy. BJU Int. 2008; 102 (1): 28–32 [DOI] [PubMed] [Google Scholar]
- 6. Swindle P, Eastham JA, Ohori M, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2008;179(5 Suppl):S47–S51 [DOI] [PubMed] [Google Scholar]
- 7. Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999; 281 (17): 1591–1597 [DOI] [PubMed] [Google Scholar]
- 8. Pfitzenmaier J, Pahernik S, Tremmel T, Haferkamp A, Buse S, Hohenfellner M. Positive surgical margins after radical prostatectomy: do they have an impact on biochemical or clinical progression? BJU Int. 2008; 102 (10): 1413–1418 [DOI] [PubMed] [Google Scholar]
- 9. Wieder JA, Soloway MS. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998; 160 (2): 299–315 [PubMed] [Google Scholar]
- 10. Smith JA, Jr., Chan RC, Chang SS, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007; 178 (6): 2385–2389 [DOI] [PubMed] [Google Scholar]
- 11. O'Malley PJ, Van Appledorn S, Bouchier-Hayes DM, Crowe H, Costello AJ. Robotic radical prostatectomy in Australia: initial experience. World J Urol. 2006; 24 (2): 165–170 [DOI] [PubMed] [Google Scholar]
- 12. Yossepowitch O, Bjartell A, Eastham JA, et al. Positive surgical margins in radical prostatectomy: outlining the problem and its long-term consequences. Eur Urol. 2009; 55 (1): 87–99 [DOI] [PubMed] [Google Scholar]
- 13. Karakiewicz PI, Eastham JA, Graefen M, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005; 66 (6): 1245–1250 [DOI] [PubMed] [Google Scholar]
- 14. Atug F, Castle EP, Srivastav SK, Burgess SV, Thomas R, Davis R. Positive surgical margins in robotic-assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol. 2006; 49 (5): 866–871 [DOI] [PubMed] [Google Scholar]
- 15. Murphy DG, Kerger M, Crowe H, Peters JS, Costello AJ. Operative details and oncological and functional outcome of robotic-assisted laparoscopic radical prostatectomy: 400 cases with a minimum of 12 months follow-up. Eur Urol. 2009; 55 (6): 1358–1366 [DOI] [PubMed] [Google Scholar]
- 16. Caire AA, Sun L, Ode O, et al. Delayed prostate-specific antigen recurrence after radical prostatectomy: how to identify and what are their clinical outcomes? Urology. 2009; 74 (3): 643–647 [DOI] [PubMed] [Google Scholar]
- 17. Pavlovich CP, Trock BJ, Sulman A, Wagner AA, Mettee LZ, Su LM. 3-year actuarial biochemical recurrence-free survival following laparoscopic radical prostatectomy: experience from a tertiary referral center in the United States. J Urol. 2008; 179 (3): 917–921 [DOI] [PubMed] [Google Scholar]
- 18. Rassweiler J, Schulze M, Teber D, et al. Laparoscopic radical prostatectomy with the Heilbronn technique: oncological results in the first 500 patients. J Urol. 2005; 173 (3): 761–764 [DOI] [PubMed] [Google Scholar]