This study suggests that laparoscopic cholecystectomy can be safely performed in Child Pugh class A and B cirrhotic patients with symptomatic gallstones with acceptable morbidity and conversion rate.
Keywords: Cirrhosis, Laparoscopic cholecystectomy, Subtotal cholecystectomy
Abstract
Background and Objectives:
Due to the concern of risk of intra- and postoperative complications and associated morbidity, cirrhosis of the liver is often considered a contraindication for laparoscopic cholecystectomy (LC). This article intends to review the literature and underline the various approaches to dealing with this technically challenging procedure.
Methods:
A Medline search of major articles in the English literature on LC in cirrhotic patients over a 16-y period from 1994 to 2011 was reviewed and the findings analyzed. A total of 1310 cases were identified.
Results:
Most the patients who underwent LC were in Child-Pugh class A, followed by Child-Pugh classes B and C, respectively. The overall conversion rate was 4.58%, and morbidity was 17% and mortality 0.45%. Among the patients who died, most were in Child-Pugh class C, with a small number in classes B and A. The cause of death included, postoperative bleeding, liver failure, sepsis, duodenal perforation, and myocardial infarction. A meta-analysis of 400 patients in the literature, comparing outcomes of patients undergoing LC with and without cirrhosis, revealed higher conversion rate, longer operative time, higher bleeding complications, and overall increased morbidity in patients with cirrhosis. Safe LC was facilitated by measures that included the use of ultrasonic shears and other hemostatic measures and using subtotal cholecystectomy in patients with difficult hilum and gallbladder bed.
Conclusions:
Laparoscopic cholecystectomy can be safely performed in cirrhotic patients, within Child-Pugh classes A and B, with acceptable morbidity and conversion rate.
INTRODUCTION
Cholelithiasis in patients with cirrhosis occurs twice as often as in those in the general population, with a reported incidence of 9.5% to 13.7%, versus 5.2% in patients without cirrhosis.1–4 A review of approximately 4895 autopsy records in the literature showed that the frequency of cholelithiasis was 29.4% in patients with cirrhosis, as compared with 12.8% in patients without cirrhosis.3 In the past, when such patients required cholecystectomy, it was invariably performed by an open approach. However, open cholecystectomy in cirrhotic patients has been reported to be associated with greater operative blood loss, longer duration of surgery, and prolonged hospital stay, as compared with those performed laparoscopically.5,6 The morbidity and mortality rates, for open cholecystectomy in patients with cirrhosis were found to be as high as 5% to 23%, and 7% to 20%, respectively.5,6 Such poor results were mainly due to either excessive blood loss with subsequent postoperative liver failure, sepsis, or both.6–34
Although LC became very popular, cirrhosis was initially considered a relative contraindication.1,2 The first report of LC in a cirrhotic patient appeared in the literature in 1993.35 Since then, abundant evidence exists in the literature to show that the LC technique has improved and refined, such that it is now safe for patients with symptomatic gallbladder disease and Child-Pugh classes A or B cirrhosis to undergo.6–32 The safety of performing LC in Child-Pugh class C is however controversial.6,12 This article attempts to review the present status of LC in patients with cirrhosis, by reviewing the literature over 16 y.
MATERIALS AND METHODS
A review of the literature over 16 y (1994 to 2011) was carried out by searching the Medline database using terms laparoscopic cholecystectomy and cirrhosis. Only studies published in English that described well-documented cases of LC in cirrhotic patients were considered.
RESULTS
Review of the literature on cirrhotic patients undergoing LC during this period revealed 1310 cases (Table 1). Majority of the patients (78.75%) who underwent LC were in Child-Pugh class A category, followed by 19.5% and 1.62%, in Child-Pugh classes B and C, respectively. The overall conversion rate was 4.58%, morbidity was 17%, and mortality was 0.45% (Table 1). In a metaanalysis of 25 published reports with over 400 patients, Puggioni et al.6 noted that when patients with cirrhosis were compared with patients without cirrhosis, they had higher conversion rates (7.06% versus 3.64%, P = .024), longer operative time (98.2 min versus 70 min, P = .005), more bleeding complications (26.4% versus 3.1%, P ≤ .001) and increased overall morbidity (20.86% versus 7.99%, P ≥ .001). Acute cholecystitis was evident in 47% of patients with cirrhosis versus 14.7% of patients without cirrhosis (P < .001). When LC was compared with open cholecystectomy in patients with cirrhosis, LC was associated with less operative blood loss (113mL versus 425.2mL, P = .015), shorter operative time of 123.3 min versus 150.2 min, P < .042 and reduced length of hospital stay (6 d versus 12.2 d, P < .001).6
Table 1.
Literature Review of Patients Undergoing Laparoscopic Cholecystectomy in Cirrhotics (1994 to 2011)
| Reference (year) | Patients (n) | Child–Pugh A | Child–Pugh B | Child–Pugh C | Conversion (%) | Morbidity (%) | Hospital stay (days) | Mortality (%) |
|---|---|---|---|---|---|---|---|---|
| Bessa et al10 (2011) | 40 | 27 | 13 | 0 | 3 (7.5%) | 13 (32.5%) | 2 (2 to 5) | 0 |
| Hamed et al14 (2010) | 15 | 10 | 5 | 0 | 0 | 33 | 2.1 ±2.3 | 0 |
| Delis et al19 (2010) | 220 | 194 | 26 | 0 | 12 (5.45%) | 20 (11.6%) | 4 (2 to 9) | 0 |
| Pavlidis et al13 (2009) | 38 | 29 | 9 | 0 | 6 (15.7%) | 3 (7.8%) | 4.40 ±3.5 | 0 |
| Shaik et al18 (2009) | 20 | 12 | 8 | 0 | 2 (10%) | 15 | 2.8 ±0.1 | 0 |
| Mancero et al15 (2008) | 30 | 23 | 7 | 0 | 0 | 2 (13.4%) Child-Pugh A 4 (57.1%) Child-Pugh B | 1.7 (Child-Pugh A 4.1 (Child-Pugh B) | 0 |
| Leandros et al8 (2008) | 34 | 23 | 11 | 0 | 0 | 5 (14.4%) | 3 (1 to 9) | 1 |
| Palanivellu et al7 (2006) | 265 | NA | NA | NA | 2 (0.75%) | 40 (15%) | 4 | 0 |
| Ji et al21 (2005) | 38 | 19 | 15 | 4 | 0 | 5 (13.2%) | 4.6 ±2.4 | 0 |
| Schiff et al11 (2005) | 27 | 24 | 3 | 3 (11.1%) | nil | 2 | 0 | |
| Curro12 (2005) | 42 | 22 | 16 | 4 | 0 | 15 (35%) | 7.3 | 2 (4.76%) |
| Cucinotta17 (2003) | 22 | 12 | 10 | 0 | 3 (11%) | 8 (36)% | 4 (2 to 5) | 0 |
| Yeh et al16 (2002) | 226 | 193 | 33 | 0 | 10 (4.4%) | 15 (6.6%) | 4.7 | 2 (0.88%) |
| Tuech et al22 (2002) | 26 | 22 | 4 | 0 | 0 | 7 (27%) | 5 | 0 |
| Urban et al32 (2001) | 19 | 19 | 0 | 0 | 0 | 0 | 3.5 | 0 |
| Clark et al33 (2001) | 25 | 14 | 9 | 2 | 0 | 13 (52%) | 4 | 1(4%) |
| Fernandes et al31 (2000) | 48 | 38 | 10 | 0 | 4 (8.3%) | NA | 6.5 | 0 |
| Morino et al23 (2000) | 33 | 27 | 4 | 2 | 2 (6%) | 0 | 2.8 | 0 |
| Poggio et al27 (2000) | 26 | 22 | 4 | 0 | 0 | 5 (19%) | 2.8 | 0 |
| Sleeman et al30 (1998) | 25 | 25 | 0 | 0 | NA | 8 (32%) | NA | 0 |
| Angrisani et al28 (1997) | 31 | 20 | 11 | 0 | 10 (3%) | 8 (25%) | 3 | 0 |
| Jan and Chen34 (1997) | 21 | 18 | 3 | 0 | 2 (9.5%) | 2 (9.5%) | 4.1 | 0 |
| Gugenheim et al29 (1996) | 9 | 9 | 0 | 0 | 0 | 2 (22%) | 3.0 | 0 |
| Lacy et al26 (1995) | 11 | 7 | 3 | 1 | 1 (9%) | 0 | 1.8 | 0 |
| D’Albuquerque et al (1995) | 12 | 8 | 4 | 0 | 0 | 4 (33%) | 2.5 | 0 |
| Yerdel et al20 (1994) | 7 | 6 | 0 | 1 | 0 | 0 | 6.7 | 0 |
| Total | 1310 | 823*78.7% | 205*19.6% | 17*1.62% | 60 (4.58%) | 223 (17%) | 3 to 6.9 (Avg- 2.8) | 6 (0.45%) |
Postoperative Complications
The most frequent complications include fever, subcutaneous emphysema, ascites leakage, postoperative liver failure, encephalopathy, worsening of ascites, port-site infection, port-site bleeding, intraoperative hemorrhage, bilious drainage into the drain, and stone formation in the gallbladder remnant, left in type II laparoscopic subtotal cholecystectomy.6,7,10,16,20 In one of the major series of 265 patients, complications included bilious drainage from the intraperitoneal drain (52.8%), intraoperative bleeding 12% (9.8% from gallbladder bed and 2.3% from cholecystohepatic triangle), blood transfusion requirement in 1.5%, postoperative worsening of ascites in 10.6%, postoperative deterioration of liver function in 15%, and recurrent stones in the gallbladder remnant following subtotal cholecystectomy in 1.1%.7 In another large series of 226 patients, complications were noted in 20% of patients and included intraoperative hemorrhage (5.2%), abdominal collection (3.5%), wound infection (1.1%), and pulmonary infection (1.75%).16 Child-Pugh class C patients were associated with a higher rate of liver failure and sepsis.12 These complications are generally found to be significantly higher in cirrhotic than in noncirrhotic patients (P = .008).6 Moreover, the complications are significantly more frequent in the presence of ascites (P = .001) and in Child-Pugh B patients (P = .007), compared with noncirrhotic patients.6 Few patients with Child-Pugh class C have undergone LC, and the reported morbidity in them has been as high as 75% (Table 1).12
Mortality Rate
Although the mortality rate for the great majority of Child-Pugh class A and B cirrhotic patients who undergo LC is low, even in cases of acute cholecystitis, the rate for patients with Child-Pugh C cirrhosis have been reported to be as high as 50% to 83% in individual series.6,12 However, analyzing the 1310 patients who underwent LC in this review, the overall mortality was noted to be 0.45% (Table 1). Among the patients who died, 17.1%, 0.97%, and 0.12% were in Child-Pugh classes C, B, and A, respectively (Table 1). The cause of death included postoperative bleeding, liver failure, sepsis, duodenal perforation, and myocardial infarction.8,12,16,24
DISCUSSION
Cholelithiasis in patients with cirrhosis occurs twice as often as in those in the general population.1–4 LC, although it was contraindicated in cirrhotic patients, has gradually replaced open cholecystectomy as the standard of care of gallstone disease. Improvements in operating skills and equipment have gradually permitted its application in several previously contraindicated circumstances including cirrhotics.5,6 Patients with liver cirrhosis have generally been considered poor candidates for LC, especially those with end-stage liver disease and portal hypertension, the latter being initially regarded as a contraindication to LC. The hardness of the fibrotic liver and the increased vasculature secondary to portal hypertension with a high risk for bleeding are the major operating difficulties encountered during the procedure.6–12 Over the years, the accumulating experience in LC has resulted in an increasing number of authors reporting that LC can be safely performed in cirrhotic patients.6–34
Risk Factors
Cholelithiasis occurs frequently in patients with liver cirrhosis with an odds ratio (OR) of 1.62 (incidence 9.5%) in alcoholic and OR=2.07 (incidence 13.7%) in nonalcoholic cirrhosis, as compared with patients without liver disease.5 This has been attributed to several factors, such as hemolysis, hypersplenism, reduction in biliary acidity, functional alterations in the gallbladder, and metabolic liver failure, resulting in an increase in unconjugated bilirubin secretion.2
Patients with cirrhosis undergoing surgery are at increased risk for morbidity and mortality.6,7,16,19 Several reasons exist why surgeons have considered patients with cirrhosis to be poor candidates for surgery or have tended to refer them to tertiary care centers. Historically, their reluctance was due to concern about the development of end-stage liver disease associated with anesthesia and laparotomy. However, less hepatotoxic regimens are currently used.8,36 The overall risk factors include the type of surgery (emergent or elective), Child-Pugh class C, presence of ascites, encephalopathy, infection, anemia, malnutrition, jaundice, portal hypertension, hypoalbuminemia, prothrombin time (PT) that does not correct with vitamin K, and hypoxemia.6,7,16,19 These problems are further exacerbated by the fact that patients with cirrhoiss are often operated on late in the course of their disease, so the gallbladder tissue is already stiff, woody, and friable.19 A recently published study from the Mayo Clinic found that the model of end-stage liver disease (MELD) score, age, and American Society of Anaesthesiologists class were independent predictors of mortality after major surgery in cirrhotic patients.19 MELD score calculated by using the formula: MELD=9.57xloge (creatinine mg/dL) + 3.78xloge (total bilirubin mg/dL) +11.20xloge international normalized ratio(INR) +6.43 was found to be associated with a higher rate of postoperative complications and conversion rate when the value was above 13.19 In patients with a score above 13, conservative or minimally invasive management (antibiotics, percutaneous drainage) is initially recommended.19
General Risks
Coagulopathy and Increased Risk of Bleeding
The increased risk of bleeding in patients with cirrhoiss is related to increased prothrombin time, thrombocytopenia, and portal hypertension.7,8,10,16 Patients with less than 50,000/mm3 platelets will have to be prepared with platelet infusion, and those with abnormal prothrombin time (PT) would require fresh frozen plasma.7,8 The role of activated recombinant factor V11 (rFV11a) in controlling bleeding has been investigated in several clinical settings.37 A controlled trial found that rFV11a was efficacious in correcting PT and achieving hemostasis in most of the patients with advanced liver disease (Child-Pugh classes B and C) who underwent laparoscopic liver biopsy.36 Some recommend the use of rFV11a in patients with LC at increased risk of bleeding due to difficult cases of coagulopathy disorder.8,37
Preoperative Optimization of Patients Prior to Surgery
Since most of the cirrhotic patients have compromised multiple organ function, special care should be taken to assess the function, prior to surgery, particularly with respect to hepatic, cardiac, and renal function. Individual preoperative preparation should be conducted mainly based on the patient's Child-Pugh classification. Generally, no special preparation is needed for patients with class A.8,21 However special individual measures are required to improve the hepatic function of class B and C cases.6,8,21,36 For patients with Child-Pugh class C cirrhosis, attempts should be made to improve the patients liver function to near class B before surgery.7,36 Measures to improve the hepatic function include hepatic function protection, control of ascites, nutritional support, correction of coagulopathy, and reduction of portal vein pressure.36 Correction of coagulopathy with platelets or fresh frozen plasma before surgery is advised and availability of these products intraoperatively is essential.8,19,36
Laparoscopic cholecystectomy in cirrhotic patients should be performed by experienced laparoscopic surgeons, and equipment to achieve hemostasis like Harmonic shears should be readily available.
Intraoperative Management
Anesthesia Management
Prudent anesthetic management can also help in reducing the perioperative risks in cirrhotic patients undergoing LC. Measures like the use of isoflurane/desflurone/ sevflurone, which undergo less hepatic metabolism, maintaining pCo2 between 35mm Hg and 40mm Hg and the use of nondepolarizing muscle relaxant doxacurium and fentanyl would go a long way in reducing the risk of deterioration of liver function.6–8,36
Insertion of Ports
One of the potential complications while establishing pneumoperitoneum is damage to the recanalized umbilical vein in portal hypertension. When cirrhotic status is known before surgery, this complication can be avoided by creating pneumoperitoneum using a Veress needle placed in the midline subumbilically (as opposed to within the umbilicus in noncirrhotic patients) to avoid undetected enlarged collateral vessels in patients.7,10,19 In patients in whom varices had been documented by preoperative investigations, an open method of peritoneal access is adopted. Others have placed trocars to the right or left of median line under direct vision to avoid this complication.21 In the event of sectioning of the recanalized umbilical vein during insertion of trocars, a transmural ligation technique of the injured parietal vessels has been used.8,11,21
Pneumoperitoneum
During LC, carbon dioxide pneumoperitoneum can cause ischemia-reperfusion injury to the internal organs like the liver and kidney.8,21 This may aggravate the damage to hepatic function that has been positively correlated with the pressure of pneumoperitoneum. To lower this risk, some routinely establish pneumoperitoneum with lower flow of carbon dioxide, maintaining the pressure at about 1.33kPa and gradually relieving the pneumoperitoneum after LC.8,9,21 These measures are believed to reduce further damage to hepatic function. Some recommend the use gasless pneumoperitoneum to avoid ischemia reperfusion injury to internal organs.8,21
Difficulty in Performing Laparoscopic Cholecystectomy
The major difficulties encountered during LC in cirrhotic patients can be predominately classified into 5 areas7,10:
1. Adhesions with increased neovascularity
2. Difficult retraction of the liver
3. Inadequate exposure of the cholecystohepatic triangle
4. A high-risk gallbladder bed
5. A high risk of hilum
Adhesions and Neovascularity
In patients with cirrhosis and portal hypertension, the risk of encountering dilated tortuous veins around the gallbladder is increased.7,10 In addition, omental adhesions to the gallbladder or the liver surface may be unduly vascular (Figure 1). This is handled by the use of ultrasonic shears used for division of all these adhesions. The periumbilical collaterals might be a source of bleeding; so in cases where the cirrhotic condition of the patient is identified preoperatively, an infraumbilical camera trocar is preferred to avoid collaterals present at the umbilicus and above it. For the same reasons the umbilical port is made away from the falciparum ligament, avoiding it completely.7,10
Figure 1.
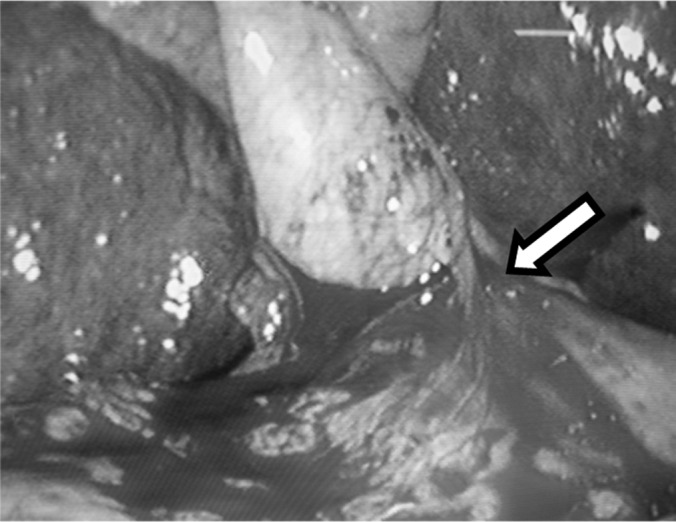
Laparoscopic cholecystectomy in cirrhotic patient. The vascularized omentum (arrow) can be noted, which should be dealt preferably with ultrasonic equipment.
Difficult Retraction of the Liver
During cholecystectomy, retraction of the liver cranially is essential to facilitate gallbladder dissection. A cirrhotic liver is difficult to retract cranially, because it is hard and fibrotic. One additional 5-mm port just to the right of the epigastric port allows passage of a retractor to lift the right lobe.7,10 In case the quadrate lobe is large and obscures the field of vision, an additional blunt retractor is passed through the left lumbar port at the level of the umbilicus to allow its retraction, for effective exposure of the cholecystohepatic triangle.7,10
Inadequate Exposure of the Cholecystohepatic Triangle
If despite the previously mentioned maneuver, the liver cannot be retracted, then applying the retractor on the body of the gallbladder, just beyond the infundibulum rather than at the fundus may help to retract the infundibulum and expose the cholecystohepatic triangle. Finally, if all these fail, the fundus-first method is adopted.7,10
High-Risk Gallbladder Bed
In a cirrhotic liver with portal hypertension, separation of gallbladder from the liver bed is difficult and dangerous. Tortuous, dilated vessels may occur in the gallbladder bed that are easily injured and bleed profusely. Bleeding from the bed is a major problem and is difficult to control. In such patients, laparoscopic subtotal cholecystectomy may be performed by leaving the posterior wall intact with the liver.7,10,14,25 This variant of subtotal cholecystectomy has been designated laparoscopic subtotal cholecystectomy (LSC) I.7,10 Because with this procedure, deliberate iatrogenic gallbladder perforation may lead to stone spillage, abdominal lavage and retrieval of stones will lead to time-consuming consequences.10 The remnant mucosa is removed either by mucosectomy in patients with acute cholecystitis or by electrofulguration in those with chronic cholecystitis.7,10
High-Risk Hilum
In cirrhotic patients, the presence of neovascularity in the hilar region or a cavernamatous transformation of the portal vein renders hilar dissection dangerous.7,10,14,25 This is aggravated by certain risk factors, such as deeply placed hilum, inflammatory phlegmon, pericholecystic fibrosis, or aberrant anatomy. These situations may mask the presence of aberrant vascular channels in the region of the cholecystohepatic triangle making any dissection in this region fraught with risk. So in the presence of any of these risk factors, when one is not certain of abnormal vessels in the hilum, a variant of subtotal cholecystectomy may be performed, called subtotal cholecystectomy II.7,10 The infundibulum is divided circumferentially, as close to the junction of the gallbladder and cystic duct, and as safely, as possible. The mucosa in the proximal remnant is removed by mucosectomy in patients with acute cholecystitis and electrofulguration in those with chronic cholecystitis.7,10 The flap is sutured with continuous suture of polyglactin 3-0. However the drawback of this procedure is the possibility of retained stone in the stump, reported to occur in 1% of these patients requiring relaparotomy for retrieval of stone from the stump.7 The use of Harmonic ACE shears (Ethicon Endo-Surgery, Cincinnati, OH) is reported to reduce this risk, by being able to carry out near total cholecystectomy rather than subtotal cholecystectomy.10 Harmonic ACE is also reported to have a distinct advantage of achieving a complete and safe closure of the gallbladder stump, reducing the postoperative incidence of bile leakage.10 In addition, the spillage of stone into peritoneal cavity due to intentional iatrogenic gallbladder perforation can be avoided and the dissection of gallbladder from the hilar structure can be facilitated.10 In patients having high-risk hilum and high-risk gallbladder bed, a combination of LSC I and LSC II is carried out. This variant has been designated laparoscopic subtotal cholecystectomy III.7,10 In a study involving 265 cirrhotic patients, 23.4% underwent LSC I, 38.5% underwent LSC II, and 15.95% underwent LSC III subtotal cholecystectomy, which amounted to subtotal cholecystectomy being carried out in about three-fourths (77.7%) of the patients.7 Among these, 53.6% required additional ports, and the fundus first technique was used in 8.3% of the patients.7
Compensatory Hypertrophy
Compensatory hypertrophy of the normal liver tissue and nodularity from cirrhotic changes will make exposure of the cholecystohepatic triangle difficult, which is compounded by friability of the liver tissue, precluding excessive traction on the gallbladder. This difficulty is overcome by placing extra ports for retraction of the liver or duodenum and by retracting the body of the gallbladder rather than the fundus (56.3%).7 In the event of failure of other maneuvers, then the fundus-first technique is resorted to in about 8.3%.7
Drawbacks of Subtotal Cholecystectomy
The major drawback is the leakage of bile from the closed stump (when harmonic ACE is not used), which is reported to occur in 38.1% undergoing LSC I, 94.1% of patients undergoing LSC II, and 10.2% undergoing total cholecystectomy.7 In patients in whom the biliary drainage persists beyond 7 d and in significant amounts, predisposing factors like retained stone in common bile duct (CBD) has to be ruled out and promptly treated if found with endoscopic retrograde cholangiography (ERC) sphincterotomy/basketting.7 Intentional opening of the gallbladder in subtotal cholecystectomy will lead to spillage of gallstones and infected bile into the peritoneum. This will add to the operative time spent in retrieving the stones.10 Failure to do so may predispose to complications related to lost stones in the peritoneum, including sepsis. However, the use of Harmonic shears is reported to reduce these complications markedly.10
Equipment Used to Facilitate Dissection and Hemostasis
Ultrasonic shears like the Harmonic ACE (Ethicon Endo-Surgery, Cincinnati, OH) are reported to greatly facilitate bloodless dissection, especially in the face of neovascularization and vascularized adhesions and for closure and division of cystic duct and artery.10,38 Others have reported the use of argon beam coagulation and thrombin spray as useful for hemostatic dissection.10,11,20,22,39 Additional modalities used to control oozing include hemostatic agents, such as oxidized cellulose (Gelfoam [Pfizer, New York, NY]; surgical [Johnson & Johnson, New Brunswick, NJ]), in conjunction with mechanical compression from introduced surgical sponges in these patients.11 However, it is important to note that most of these results are from case series rather than randomized controlled trials. Finally, a tremendous amount of patience is necessary, because conversion may not always help to control the bleeding due to coagulopathy.12 Blunt dissection is avoided to minimize bleeding once the cystic duct is identified and divided and all tissues are clipped/ligated and divided and coagulated using instruments like ultrasonic shears. If the blood is spurting, a figure of eight stitch may at times be necessary if bleeding is not controlled by other means.10,11,22 Before completion of the procedure, all access ports should be checked laparoscopically, after sequentially removing the ports, and if bleeding from a port is noted, it should be promptly managed with transfixing sutures.
Conversion
In recent reports, the rate of conversion to an open procedure is noted in 0% to 15.7%.9,10,13–15 A low threshold for conversion to open cholecystectomy should be maintained. Conversion should not be considered as a failure to achieve a difficult task, but a reflection of sound judgment, because it is meant to prevent more serious complications. These complications include significant bleeding or biliary tract injury, leading to deterioration of liver function and sepsis. Absolute indications for conversion are bleeding not readily controlled laparoscopically and inability to define the anatomy adequately. Uncertainty of safety and efficiency warrants an immediate conversion to an open procedure.10,13–15
A subhepatic drain is placed in patients, because postoperative oozing is likely in the presence of associated coagulopathy.7,15,17 It will facilitate monitoring the postoperative bleeding. However drainage of the liver bed, in the postsurgery stage is, however, controversial.1,15,17 This is mainly because of the concern about cirrhotic patients developing ascites and secondary infection. The manipulation of the gallbladder during operation and possibly decreased function of Kupffer cells and inefficient clearing of enteric micro-organisms in the postoperative period, may be the contributing factors leading to secondary infection of ascites and peritonitis. Extraneous infection of ascitic fluid following a drain insertion is partly circumvented by using a closed drainage system. Drains are usually removed in 24 to 48 h.7,14 In the postoperative period, the patients are started orally after 6 h, unless a complication is suspected. Liver function tests are carried out after 48 h. Patients are discharged as soon as they have tolerated oral food and after the drain is removed. Patients are referred to a medical gastroenterologist for management of cirrhosis and future follow-up.7
Advantages of Laparoscopic Cholecystectomy
Laparoscopic cholecystectomy in cirrhotic patients offers several advantages over open cholecystectomy and include the following6,7,9,10,12,14,16,19,21:
1. Wound-related complications, such as wound infection, dehiscence, and postoperative hernia, are significantly reduced due to the minimally invasive nature of LC. This is particularly true in patients with associated ascites (Figure 2).
2. Inadvertent bacterial seeding and contamination of the ascites is also significantly reduced, because of the less likelihood of contamination of ascitic fluid in the laparoscopic approach compared to the open procedure.
3. The magnification inherent in laparoscopic surgery makes identification of the presence of dilated vascular channels easier, allowing adoption of modified subtotal cholecystectomy.
4. Cirrhotic patients who are likely to be infected with hepatitis B and C pose great risk of needle stick injury to the operating team, which is markedly reduced in the laparoscopic approach because of the reduced possibility of contact with patients’ blood.
5. Patients with cirrhosis have a major problem of coagulopathy. In addition to the risk of bleeding during dissection of the gallbladder, these patients have the potential risk of bleeding in the wound following the muscle-cutting incision in open cholecystectomy. This could lead to subsequent hematoma and infection, which is avoided with the laparoscopic approach.
6. Some patients with cirrhosis may be candidates for liver transplantation in the future. Laparoscopic cholecystectomy offers the potential for fewer right upper quadrant adhesions postoperatively, which may be beneficial during liver transplantation.
Figure 2.
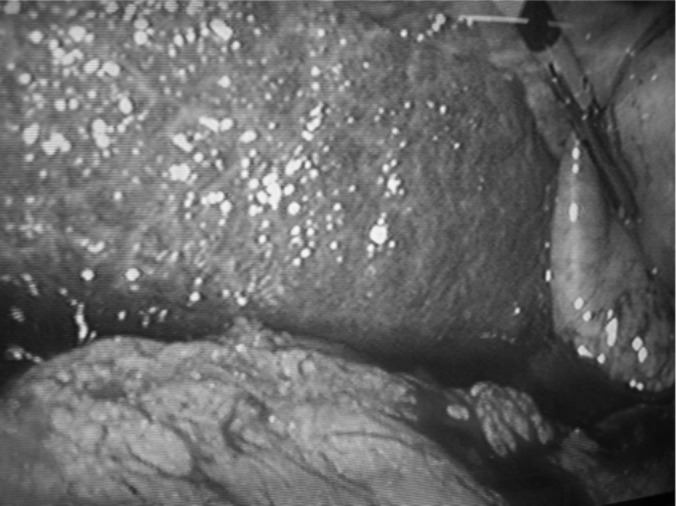
Patient with Child-Pugh class B cirrhosis undergoing laparoscopic cholecystectomy. The cirrhosis of liver and ascites can be noted.
CONCLUSION
Laparoscopic cholecystectomy in cirrhotic patients is associated with a higher complication rate than in noncirrhotic patients, due to several inherent risk factors. The hardness of the fibrotic liver and the increased vasculature secondary to portal hypertension are of considerable significance. Improvements in operating skills, equipment, and accumulating experience in performing LC in difficult conditions over the years has made LC in cirrhotic patients a safe proposition when used judiciously. The postoperative complications are related primarily to Child-Pugh class, being maximum in patients of Child-Pugh class C undergoing LC. Of late, proper selection of the patients, adequate preoperative optimization, and appropriate instrument use have led to lower morbidity and significantly less mortality.
References:
- 1. Aranha GV, Sontag SJ, Greenlee HB. Cholecystectomy in cirrhotic patients. a formidable operation. Am J Surg. 1982; 143 (1): 55–60 [DOI] [PubMed] [Google Scholar]
- 2. Bloch RS, Allaben RD, Walt AJ. Cholecystectomy in patients with cirrhosis. Arch Surg. 1985; 120: 669–672 [DOI] [PubMed] [Google Scholar]
- 3. Bouchier IA. Post mortem study of the frequency of gallstones in patients with cirrhosis of the liver. Gut. 1969; 10 (9): 705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicholas P, Rinaudo PA, Conn HO. Increased incidence of cholelithiasis in Laennec's cirrhosis. Gastroenterology. 1972; 63: 112–121 [PubMed] [Google Scholar]
- 5. Buchner A, Sonnenberg A. Factors influencing the prevalence of gallstones in liver disease: the beneficial and harmful influences of alcohol. Am J Gastroenterol. 2002; 97 (4): 905–909 [DOI] [PubMed] [Google Scholar]
- 6. Puggioni A, Wong LL. A metaanalysis of laparoscopic cholecystectomy in patients with cirrhosis. J Am Coll Surg. 2003; 197 (6): 921–926 [DOI] [PubMed] [Google Scholar]
- 7. Palanivelu C, Rajan PS, Jani K, et al. Laparoscopic cholecystectomy in cirrhotic patients: the role of subtotal cholecystectomy and its variants. J Am Coll Surg. 2006; 203 (2): 145–151 [DOI] [PubMed] [Google Scholar]
- 8. Leandros E, Albanopoulos K, Tsigris C, et al. Laparoscopic cholecystectomy in cirrhotic patients with symptomatic gallstone disease. ANZ J Surg. 2008; 78: 363–365 [DOI] [PubMed] [Google Scholar]
- 9. Ji W, Li LT, Wang ZM, Quan ZF, Chen XR, Li JS. A randomized controlled trial of laparoscopic versus open cholecystectomy in patients with cirrhotic portal hypertension. World J Gastroenterol. 2005; 11 (16): 2513–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bessa SS, Abdel-Razek AH, Sharaan MA, Bassiouni AE, El-Khishen MA, El- Kayal el SA. Laparoscopic cholecystectomy in cirrhosis: A prospective randomized study comparing the conventional diathermy and the harmonic scalpel for gall bladder dissection. J Laproendosc Adv Surg Tech A. 2011; 21 (1): 1–5 [DOI] [PubMed] [Google Scholar]
- 11. Schiff J, Misra M, Rendon G, Rothschild J, Schwaitzberg S. Laparoscopic cholecystectomy in cirrhotic patients. Surg Endosc. 2005; 19 (9): 1278–1281 [DOI] [PubMed] [Google Scholar]
- 12. Curro G, Lapichino G, Melita G, Lorenzini C, Cucinotta E. Laparoscopic cholecystectomy in Child-Pugh class C cirrhotic patients. JSLS. 2005; 9 (3): 311–315 [PMC free article] [PubMed] [Google Scholar]
- 13. Pavlidis TE, Symeonidis NG, Psarras K, et al. Laparoscopic cholecystectomy in patients with cirrhosis of the liver and symptomatic cholelithiasis. JSLS. 2009; 13: 342–345 [PMC free article] [PubMed] [Google Scholar]
- 14. Hamad MA, Thabet M, Badawy A, et al. Laparoscopic versus open cholecystectomy in patients with liver cirrhosis: a prospective randomized study. J Laproendosc Adv Surg Tech A. 2010; 20 (5): 405–409 [DOI] [PubMed] [Google Scholar]
- 15. Mancero JM, D'Albuquerque LA, Gonzalez AM, Larrea FI, de Oliveira eSilva A. Laparoscopic cholecystectomy in cirrhotic patients with symptomatic cholelithiasis: A case control study. World J Surg. 2008; 32 (2): 267–270 [DOI] [PubMed] [Google Scholar]
- 16. Yeh CN, Chen MF, Jan YY. Laparoscopic cholecystectomy in 226 cirrhotic patients. Experience of a single center in Taiwan. Surg Endosc. 2002; 16: 1583–1587 [DOI] [PubMed] [Google Scholar]
- 17. Cucinotta E, Lazzara S, Melita G. Laparoscopic cholecystectomy in cirrhotic patients. Surg Endosc. 2003; 17: 1958–1960 [DOI] [PubMed] [Google Scholar]
- 18. Shaikh AR, Muneer A. Laparoscopic cholecystectomy in cirrhotic patients. JSLS. 2009; 13: 592–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delis S, Bakoyiannis A, Madariaga J, Bramis J, Tassopoulos N, Dervenis C. Laparoscopic cholecystectomy in cirrhotic patients: the value of MELD score and Child-Pugh classification in predicting outcome. Surg Endosc. 2010; 24 (2): 407–412 [DOI] [PubMed] [Google Scholar]
- 20. Yerdel MA, Koksoy C, Aras N, Orito K. Laparoscopic versus open cholecystectomy in cirrhotic patients: a prospective study. Surg Laparosc Endosc. 1997; 7 (6): 483–486 [PubMed] [Google Scholar]
- 21. Ji W, Li L-T, Chen X-R, Li J-S. Application of laparoscopic cholecystectomy in patients with cirrhotic portal hypertension. Hepatobiliary Pancreat Dis Int. 2004; 3 (2): 270–274 [PubMed] [Google Scholar]
- 22. Tuech JJ, Pessaux P, Regenet N, Rouge C, Bergamaschi R, Arnaud JP. Laparoscopic cholecystectomy in cirrhotic patients. Surg Laparosc Endosc Percutan Tech. 2002; 12 (4): 227–231 [DOI] [PubMed] [Google Scholar]
- 23. Morino M, Cavuoti G, Miglietta C, Giraudo G, Simone P. Laparoscopic cholecystectomy in cirrhosis: contra-indication or privileged indication? Surg Laparosc Endosc Percutan Tech. 2000; 10 (6): 360–363 [PubMed] [Google Scholar]
- 24. Clark JR, Wills VL, Hunt DR. Cirrhosis and laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2001; 11 (3): 165–169 [PubMed] [Google Scholar]
- 25. Cucinotta E, Lazzara S, Melita G. Laparoscopic cholecystectomy in cirrhotic patients. Surg Endosc. 2003; 17: 1958–1960 [DOI] [PubMed] [Google Scholar]
- 26. Lacy AM, Balaguer C, Andradi E, et al. Laparoscopic cholecystectomy in cirrhotic patients: a prospective study. Surg Laparosc Endosc. 1997; 7: 483–486 [PubMed] [Google Scholar]
- 27. Poggio JL, Rowland CM, Gores GJ, Nagorney DM, Donohue JH. A comparison of laparoscopic and open cholecystectomy in patients with compensated cirrhosis and symptomatic gallstone disease. Surgery. 2000; 127 (4): 405–411 [DOI] [PubMed] [Google Scholar]
- 28. Angrisani L, Lorenzo M, Corcione F, Vincenti R. Gallstones in cirrhotics revisited by a laparoscopy view. J Laproendosc Adv Surg Tech A. 1997; 7: 213–220 [DOI] [PubMed] [Google Scholar]
- 29. Gugenheim J, Casaccia M, Jr., Mazza D, et al. Laparoscopic cholecystectomy in cirrhotic patients. HPB Surg. 1996; 10: 79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sleeman D, Namias N, Levi D, et al. Laparoscopic cholecystectomy in cirrhotic patients. J Am Coll Surg. 1998; 187 (4): 400–403 [DOI] [PubMed] [Google Scholar]
- 31. Fernandes NF, Schwesinger WH, Hilsenbeck SG, Gross GW, Bay MK, Sirinek KR. Laparoscopic cholecystectomy and cirrhosis: a case control study of outcomes. Liver Transpl. 2000; 6: 340–344 [DOI] [PubMed] [Google Scholar]
- 32. Urban L, Eason G, ReMine S, et al. Laparoscopic cholecystectomy in patients with early cirrhosis. Curr Surg. 2001; 58: 312–315 [DOI] [PubMed] [Google Scholar]
- 33. Clark JR, Wills VL, Hunt DR. Cirrhosis and laparoscopic cholecystectomy. Surg Laparosc Endosc Percut Tech. 2001; 11 (3): 165–169 [PubMed] [Google Scholar]
- 34. Jan YY, Chen MF. Laparoscopic cholecystectomy in cirrhotic patients. Hepatogastroenterology. 1997; 44 (18): 1584–1587 [PubMed] [Google Scholar]
- 35. De Paula AL, Hashiba K, Bafutto M, et al. Colecistectomia laparoscopica em cirroticos: Relato preliminary. Goiania Cir Videolaparosc Braz. 1993; 69–72 [Google Scholar]
- 36. Friedman LC. The risk of surgery in patients with liver disease. Hepatology. 1999; 29 (6): 1617–1623 [DOI] [PubMed] [Google Scholar]
- 37. Jeffers L, Chalasani N, Balart L, Pyrsopoulos N, Erhardtsen E. Safety and efficacy of recombinant factor V11a in patients with liver disease undergoing laparoscopic liver biopsy. Gastroenterology. 2002; 123: 118–126 [DOI] [PubMed] [Google Scholar]
- 38. Power C, Maguire D, McAnena OJ, Calleary J. Use of the ultrasonic dissecting scalpel in laparoscopic cholecystectomy. Surg Endosc. 2000; 14 (11): 1070–1073 [DOI] [PubMed] [Google Scholar]
- 39. Glavic Z, Begic L, Rozman R. A new device for the detection and recognition of blood vessels in laparoscopic surgery. Surg Endosc. 2002; 16 (8): 1197–1200 [DOI] [PubMed] [Google Scholar]


