Abstract
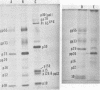
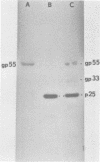
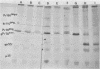
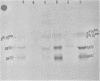
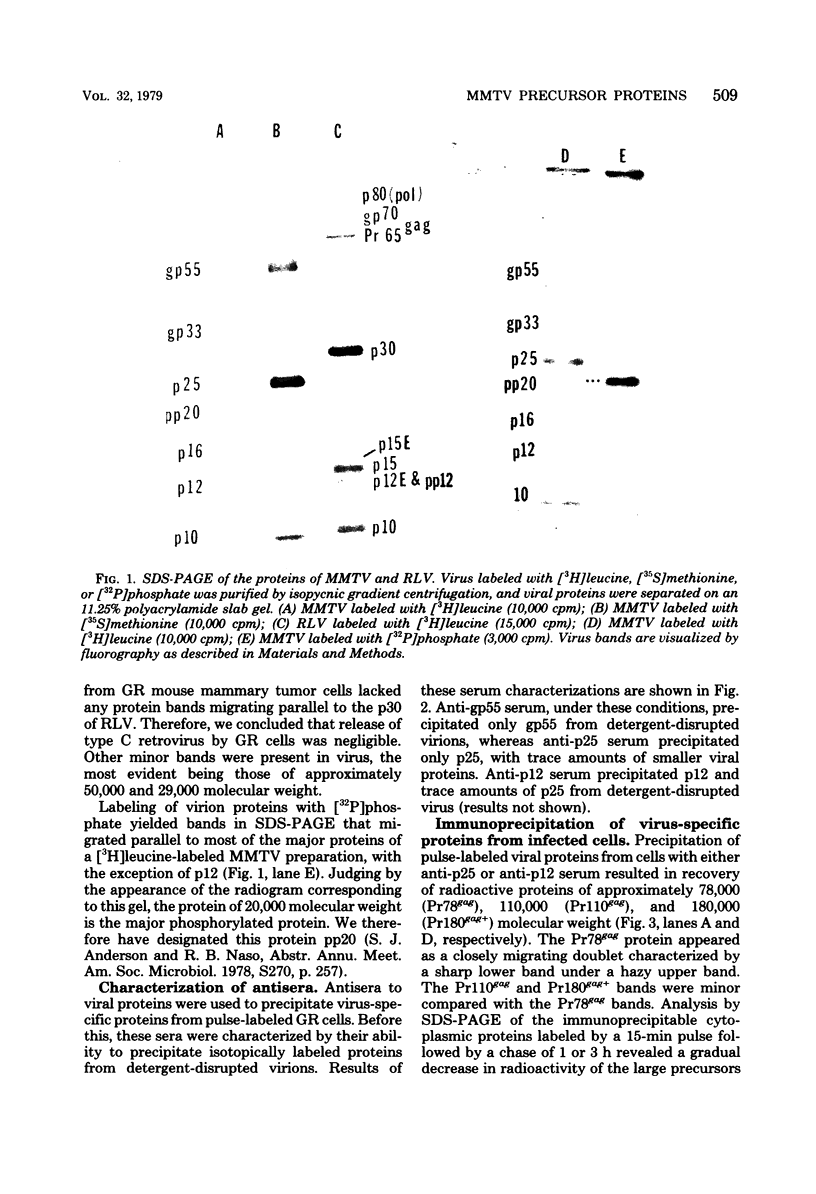
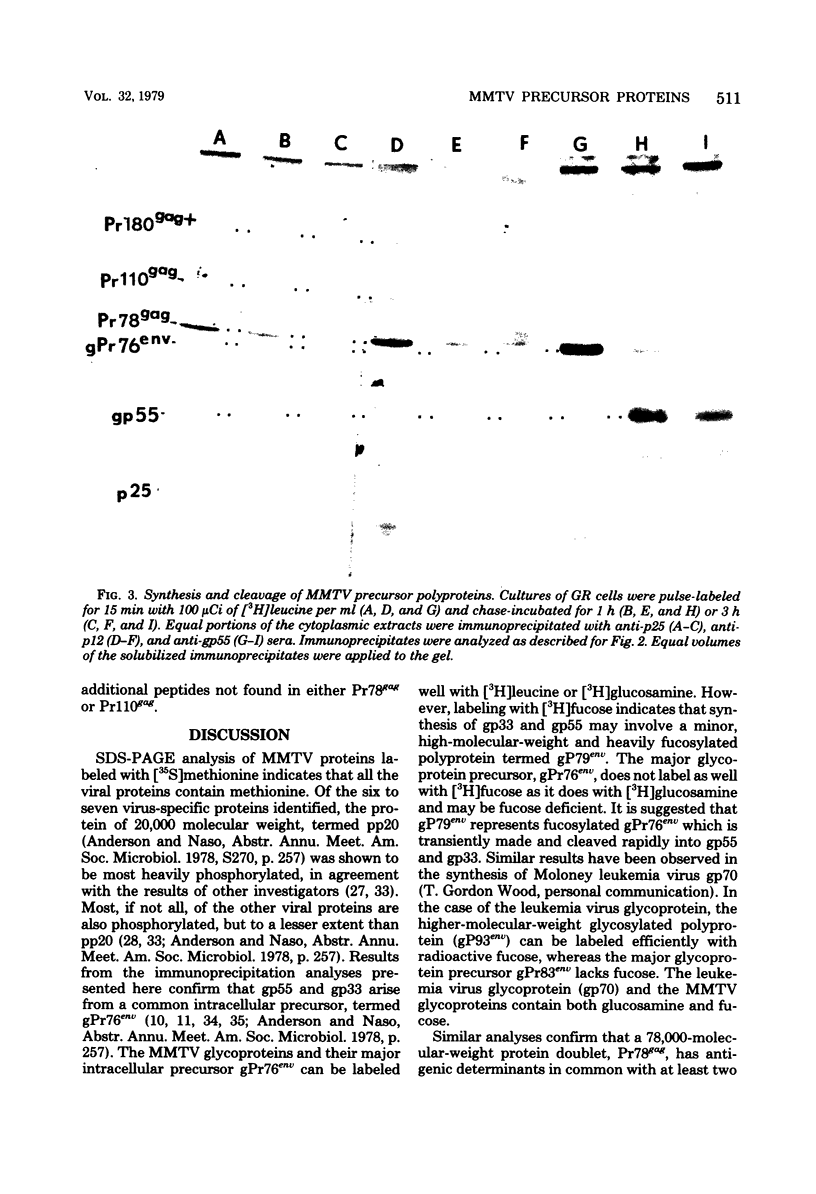
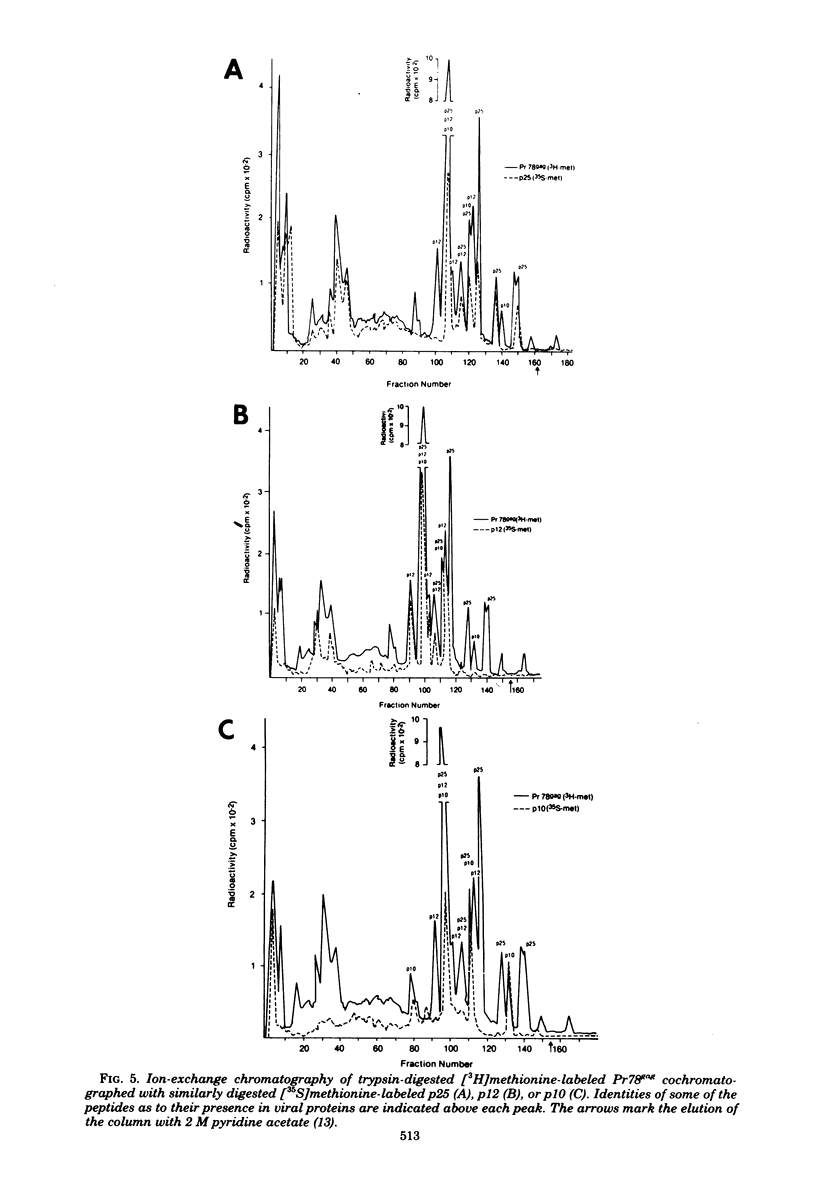
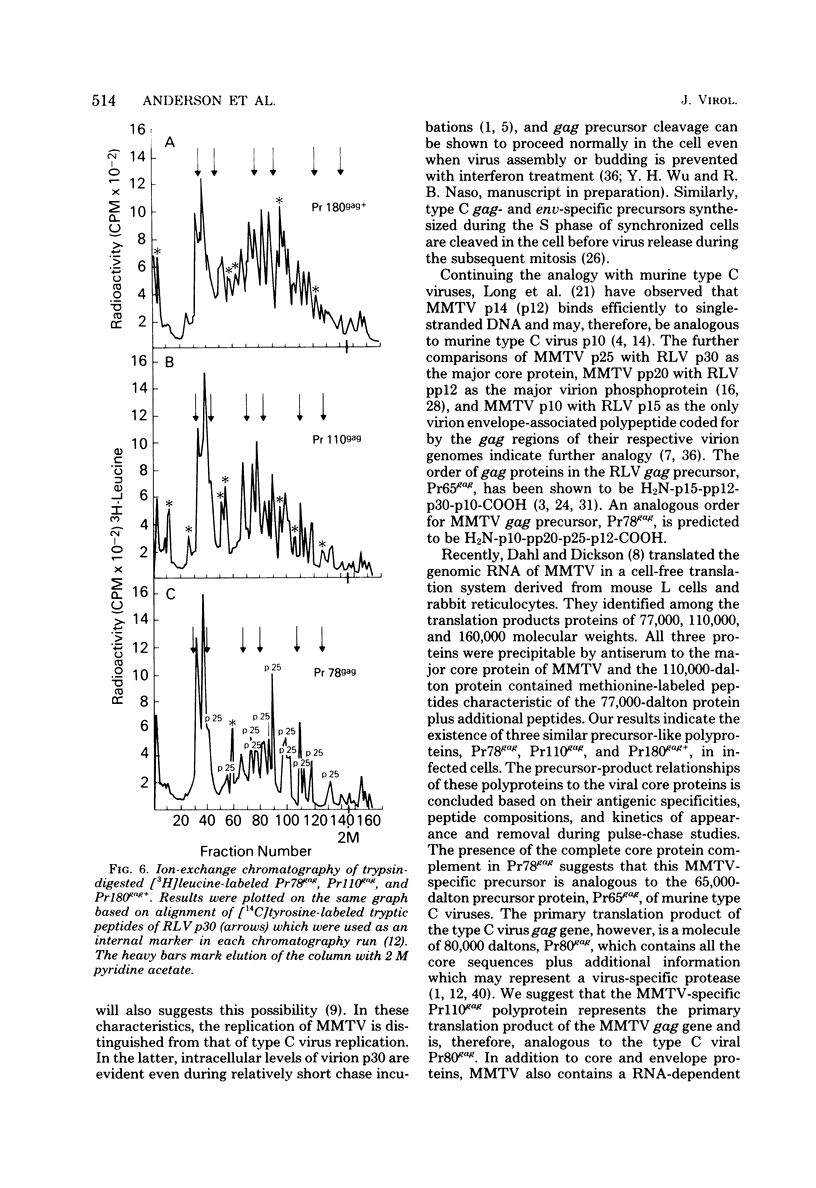
Mouse mammary tumor virus (MMTV) derived from the culture medium of GR cells contained seven proteins, identified as gp55, gp33, p25, pp20, p16, p12, and p10. The major viral phosphoprotein was the 20,000-molecular-weight protein, pp20. Immunoprecipitation of cytoplasmic extracts from pulse-labeled GR cells identified three MMTV gag-specific proteins, termed Pr78gag, Pr110gag, and Pr180gag+. These intracellular polyproteins were precipitable from cytoplasmic extracts by antisera to virions p25 and p12 but not by antisera to gp55. The major intracellular gag-specific precursor polyprotein, Pr78gag, contained antigenic determinants and tryptic peptides characteristic of p25, p12, p10, and presumably pp20. This precursor is presumably derived from nascent chain cleavage or rapid posttranslational cleavage of the larger intracellular precursor-like protein, designated Pr110gag. Pr110gag contained all but one of the leucine-containing tryptic peptides of Pr78gag, plus several additional peptides. In addition to Pr78gag and Pr110gag, monospecific antisera to virion p12 and p25 were also capable of precipitating from pulse-labeled cells a small amount of a 180,000-molecular-weight precursor-like protein, designated Pr180gag+. This large polyprotein contained nearly all of the leucine-containing tryptic peptides of Pr78gag and Pr110gag plus several additional peptides. By analogy to type C viral systems, Pr180gag+ is presumed to represent a gag-pol common precursor which is the major pathway for synthesis of MMTV polymerase. Immunoprecipitation of cytoplasmic extracts from pulse-labeled cells with antisera to gp55 identified two env-specific proteins, designated gPr76env and gP79env. The major env precursor, gPr76env, could be labeled with radioactive glucosamine and was shown to contain antigenic determinants and tryptic peptides characteristic of gp55 and gp33. A minor glycoprotein, gP79env, contained both fucose and glucosamine and was precipitable from cytoplasmic extracts with monospecific serum to gp55. It is suggested that gP79env represents fucosylated gPr76env which is transiently synthesized and cleaved rapidly into gp55 and gp33.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcement L. J., Karshin W. L., Naso R. B., Arlinghaus R. B. "gag" polyprotein precursors of Rauscher murine leukemia virus. Virology. 1977 Sep;81(2):284–297. doi: 10.1016/0042-6822(77)90145-3. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Stephenson J. R., Aaronson S. A. gag Gene of mammalian type-C RNA tumour viruses. Nature. 1976 Aug 12;262(5569):554–559. doi: 10.1038/262554a0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Luftig R., Shaper J. H. Localization of RNA tumor virus polypeptides. I. Isolation of further virus substructures. Virology. 1973 Dec;56(2):549–564. doi: 10.1016/0042-6822(73)90057-3. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E. Proteolytic processing of animal virus proteins. Curr Top Microbiol Immunol. 1977;77:1–41. doi: 10.1007/978-3-642-66740-4_1. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D., Puentes M. J., Young L. J., Smith G. H., Teramoto Y. A., Altrock B. W., Pratt T. S. Serological and biochemical characterization of the mouse mammary tumor virus with localization of p10. Virology. 1978 Mar;85(1):157–167. doi: 10.1016/0042-6822(78)90420-8. [DOI] [PubMed] [Google Scholar]
- Dahl H. H., Dickson C. Cell-free synthesis of mouse mammary tumor virus Pr77 from virion and intracellular mRNA. J Virol. 1979 Mar;29(3):1131–1141. doi: 10.1128/jvi.29.3.1131-1141.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Polyproteins related to the major core protein of mouse mammary tumor virus. J Virol. 1978 Jun;26(3):660–672. doi: 10.1128/jvi.26.3.660-672.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Puma J. P., Nandi S. Identification of a precursor protein to the major glycoproteins of mouse mammary tumor virus. J Virol. 1975 Jan;17(1):275–282. doi: 10.1128/jvi.17.1.275-282.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Puma J. P., Nandi S. Intracellular synthesis of mouse mammary tumor virus polypeptides: indication of a precursor glycoprotein. J Virol. 1975 Aug;16(2):250–258. doi: 10.1128/jvi.16.2.250-258.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar K. J., Moelling K. Biochemical properties of p15-associated protease in an avian RNA tumor virus. J Virol. 1978 Oct;28(1):106–118. doi: 10.1128/jvi.28.1.106-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Fleissner E., Tress E. Isolation of a ribonucleoprotein structure from oncornaviruses. J Virol. 1973 Dec;12(6):1612–1615. doi: 10.1128/jvi.12.6.1612-1615.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamjoom G. A., Ng V. L., Arlinghaus R. B. Inhibition of maturation of Rauscher leukemia virus by amino acid analogs. J Virol. 1978 Jan;25(1):408–412. doi: 10.1128/jvi.25.1.408-412.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karshin W. L., Arcement L. J., Naso R. B., Arlinghaus R. B. Common precursor for Rauscher leukemia virus gp69/71, p15(E), and p12(E). J Virol. 1977 Sep;23(3):787–798. doi: 10.1128/jvi.23.3.787-798.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kopchick J. J., Jamjoom G. A., Watson K. F., Arlinghaus R. B. Biosynthesis of reverse transcriptase from Rauscher murine leukemia virus by synthesis and cleavage of a gag-pol read-through viral precursor polyprotein. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2016–2020. doi: 10.1073/pnas.75.4.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchick J. J., Karshin W. L., Arlinghaus R. B. Tryptic peptide analysis of gag and gag-pol gene products of Rauscher murine leukemia virus. J Virol. 1979 May;30(2):610–623. doi: 10.1128/jvi.30.2.610-623.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Long C. W., Berzinski T. R., Gilden R. V. Immunologic studies of the low molecular weight DNA binding protein of murine oncornaviruses. Int J Cancer. 1977 Jun 15;19(6):843–850. doi: 10.1002/ijc.2910190616. [DOI] [PubMed] [Google Scholar]
- Marcus S. L., Sarkar N. H., Modak M. J. Purification and properties of murine mammary tumor virus DNA polymerase. Virology. 1976 May;71(1):242–254. doi: 10.1016/0042-6822(76)90109-4. [DOI] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Arlinghaus R. B. Tryptic peptide analyses of polypeptides generated by premature termination of cell-free protein synthesis allow a determination of the Rauscher leukemia virus gag gene order. J Virol. 1978 Dec;28(3):929–935. doi: 10.1128/jvi.28.3.929-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlbock O. Note on a new inbred mouse-strain GR-A. Eur J Cancer. 1965 Oct;1(2):123–124. doi: 10.1016/0014-2964(65)90003-4. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Karshin W. L., Jamjoom G. A., Arlinghaus R. B. A fucose-deficient glycoprotein precursor to Rauscher leukemia virus gp69/71. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2326–2330. doi: 10.1073/pnas.73.7.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naso R. B., Brown R. L. Synthesis and cleavage of Rauscher leukemia virus precursor proteins in synchronized cells. Virology. 1977 Oct 1;82(1):247–251. doi: 10.1016/0042-6822(77)90049-6. [DOI] [PubMed] [Google Scholar]
- Nusse R., Asselbergs F. A., Salden M. H., Michalides R. J., Bloemendal H. Translation of mouse mammary tumor virus RNA: precursor polypeptides are phosphorylated during processing. Virology. 1978 Nov;91(1):106–115. doi: 10.1016/0042-6822(78)90359-8. [DOI] [PubMed] [Google Scholar]
- Pal B. K., McAllister R. M., Gardner M. B., Roy-Burman P. Comparative studies on the structural phosphoproteins of mammalian type C viruses. J Virol. 1975 Jul;16(1):123–131. doi: 10.1128/jvi.16.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar-Reddy E., Devare S. G., Vasudev R., Sarma P. S. Simplified radioimmunoassay for viral antigens: use of Staphylococcus aureus as an adsorbent for antigen-antibody complexes. J Natl Cancer Inst. 1977 Jun;58(6):1859–1861. doi: 10.1093/jnci/58.6.1859. [DOI] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. Synthesis and processing of precursor polypeptides to murine mammary tumor virus structural proteins. J Virol. 1978 Jan;25(1):374–383. doi: 10.1128/jvi.25.1.374-383.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. K., Stephenson J. R. Intracistronic mapping of the murine type C viral gag gene by use of conditional lethal replication mutants. Virology. 1977 Sep;81(2):328–340. doi: 10.1016/0042-6822(77)90149-0. [DOI] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Whittington E. S., Racevskis J., Marcus S. L. Phosphoproteins of the murine mammary tumor virus. Virology. 1978 Dec;91(2):407–422. doi: 10.1016/0042-6822(78)90387-2. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Long C. W., Oroszlan S., Arthur L., Fine D. L. Isolation of separate precursor polypeptides for the mouse mammary tumor virus glycoproteins and nonglycoproteins. Virology. 1978 Mar;85(1):168–174. doi: 10.1016/0042-6822(78)90421-x. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Strand M., Billiau A. Synthesis and cleavage processing of oncornavirus proteins during interferon inhibition of virus particle release. Infect Immun. 1977 Jun;16(3):742–747. doi: 10.1128/iai.16.3.742-747.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. H. Evidence for a precursor-product relationship between intracytoplasmic A particles and mouse mammary tumour virus cores. J Gen Virol. 1978 Oct;41(1):193–200. doi: 10.1099/0022-1317-41-1-193. [DOI] [PubMed] [Google Scholar]
- Tanaka H. Precursor-product relationship between nonglycosylated polypeptides of A and B particles of mouse mammary tumor virus. Virology. 1977 Feb;76(2):835–850. doi: 10.1016/0042-6822(77)90263-x. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Properties of a P70 proteolytic factor of murine leukemia viruses. Cell. 1977 Nov;12(3):709–719. doi: 10.1016/0092-8674(77)90271-9. [DOI] [PubMed] [Google Scholar]
- van de Ven W. J., Vermorken A. J., Onnekink C., Bloemers H. P., Bloemendal H. Structural studies on Rauscher murine leukemia virus: isolation and characterization of viral envelopes. J Virol. 1978 Sep;27(3):595–603. doi: 10.1128/jvi.27.3.595-603.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]