Abstract
To initiate and establish infection in mammals, the opportunistic fungal pathogen Cryptococcus neoformans must survive and thrive upon subjection to host temperature. Primary maintenance of cell integrity is controlled through the protein kinase C1 (PKC1) signaling pathway, which is regulated by a Rho1 GTPase in Saccharomyces cerevisiae. We identified three C. neoformans Rho GTPases, Rho1, Rho10, and Rho11, and have begun to elucidate their role in growth and activation of the PKC1 pathway in response to thermal stress. Western blot analysis revealed that heat shock of wild-type cells resulted in phosphorylation of Mpk1 mitogen-activated protein kinase (MAPK). Constitutive activation of Rho1 caused phosphorylation of Mpk1 independent of temperature, indicating its role in pathway regulation. A strain with a deletion of RHO10 also displayed this constitutive Mpk1 phosphorylation phenotype, while one with a deletion of RHO11 yielded phosphorylation similar to that of wild type. Surprisingly, like a rho10Δ strain, a strain with a deletion of both RHO10 and RHO11 displayed temperature sensitivity but mimicked wild-type phosphorylation, which suggests that Rho10 and Rho11 have coordinately regulated functions. Heat shock-induced Mpk1 phosphorylation also required the PKC1 pathway kinases Bck1 and Mkk2. However, Pkc1, thought to be the major regulatory kinase of the cell integrity pathway, was dispensable for this response. Together, our results argue that Rho proteins likely interact via downstream components of the PKC1 pathway or by alternative pathways to activate the cell integrity pathway in C. neoformans.
INTRODUCTION
Cell wall integrity is crucial for fungal growth, survival, and pathogenesis. Responses to some environmental stresses are mediated by the highly conserved protein kinase C1 (PKC1) cell integrity pathway and are thought to be transduced at least in part by conserved Rho GTPases. The Rho family of GTPases, a subfamily of the Ras superfamily, has a fundamental role in regulation of polarized growth and the actin cytoskeleton, microtubule assembly, membrane transport factors, and transcription factor activity (reviewed by Etienne-Manneville and Hall [1]). In Saccharomyces cerevisiae, Rho GTPases consist of six well-studied proteins, Rho1 to Rho5 and Cdc42 (reviewed by Levin [2]). These proteins are all localized to the plasma membrane and serve distinct yet related functions in the cell. Rho1p is a master regulator of cell wall signaling involved in both Pkc1p activation and β-1,3-glucan synthesis. It may have a role in both receiving signals from outside the cell and regulating outputs involved in cell wall biogenesis, actin organization, and polarized secretion (reviewed in Cabib et al. [3]). Rho2p is somewhat functionally redundant with Rho1p (4–6), and Rho3p and Rho4p may share functions involving bud formation and actin polarization in S. cerevisiae (7–9). Rho5p has a role in osmotic stress response and peroxide-induced apoptotic-like cell death (10, 11). Cdc42p is important for bud site assembly and polarized cell growth and a late exocytosis step (12–14). Rho1p and Cdc42p are essential in S. cerevisiae. C-terminal prenylation of the Rho proteins, which serves to increase hydrophobicity and membrane association, is necessary for proper localization and function of all Rho proteins in this organism.
Cryptococcus neoformans is a pathogenic yeast that causes pulmonary infections and meningoencephalitis in immunocompromised persons, primarily those infected with HIV (for a review, see reference 15). Individuals with AIDS are particularly vulnerable to opportunistic fungal infections and cryptococcosis. It has been estimated that approximately 12% of patients with AIDS in the United States and 40 to 50% of HIV-infected people in portions of Africa will contract cryptococcosis, and, if left untreated, the disease is fatal (16). C. neoformans is ubiquitous and found worldwide in the soil. Fungal cells, including C. neoformans, are continuously exposed to a wide variety of environmental stresses in both their natural habitats as well as in their resident hosts. One principal stress that fungal cells encounter is change in temperature, and adaptation to physiologic temperature is a prerequisite for gaining successful access to a mammalian host. Fungi subsequently sense and react to changes in their environment, including temperature, through a complex network of signal transduction pathways, thus adapting to their surroundings and ensuring repair to cellular damage. Therefore, understanding this network and elucidating components of signaling pathways may provide insights for future antifungal therapies.
Previous work in our laboratory has begun to reveal an understanding of the primary PKC1 cell integrity pathway and how fungal cells respond to various stresses that they may encounter (17, 18). Rho GTPases are evolutionarily conserved in all eukaryotes as well as some plants. They presumably function upstream of known PKC1 pathway components and are known to play a critical role in signaling in other organisms. In order to characterize upstream signaling components in C. neoformans, we identified three protein homologues to S. cerevisiae Rho1p and two homologues to Cdc42p. We termed these Rho1, Rho10, Rho11, Cdc42, and Cdc420, respectively, based on highest protein BLAST scores. Ballou et al. previously characterized Cdc42 and Cdc420 C. neoformans proteins, demonstrating that both are nonessential, that Cdc42 is important for thermotolerance, morphogenesis, cytokinesis, and mating, and that this protein is also induced during growth at host temperature (19). Here, we focus on elucidating functions for the Rho proteins. It is likely that one or more of these proteins interact with Pkc1, the major kinase involved in cell integrity and mitogen-activated protein kinase (MAPK) signaling (17). Responses to environmental stresses are mediated by MAPK phosphorylation cascades in eukaryotic cells. In work presented here, we distinguish functions for each of the three Rho proteins by utilizing point mutants and deletion strains subjected to thermal stress and monitoring phosphorylation of the downstream kinase in the cell integrity pathway, Mpk1, as an output for activation of the PKC1 cell integrity pathway. We demonstrate that RHO1 is essential for C. neoformans viability and describe two different point mutants in this protein that constitutively phosphorylate Mpk1 regardless of thermal stress (20; also this study). Additionally, we show that deletion of RHO10 confers temperature sensitivity and causes constitutive Mpk1 phosphorylation but that deletion of RHO11 has no discernible effects and results in phosphorylation of Mpk1 at a level similar to that of the wild type (WT). Furthermore, a mutant strain lacking both RHO10 and RHO11 demonstrates temperature sensitivity similar to a rho10Δ strain yet, like the rho11Δ strain, phosphorylates Mpk1 in a manner indistinguishable from that of the wild type. An unexpected result from this study was that although genes downstream of PKC1 in the cell integrity pathway are necessary for PKC1 pathway activation in response to thermal stress, PKC1 itself is dispensable, suggesting alternative pathway involvement. Thus, we have begun to reveal separate and distinct functions for the Rho GTPases in C. neoformans yet propose a model where these small proteins likely act in concert with each other to produce a balance necessary for survival of fungal cells and maintenance of cell integrity.
MATERIALS AND METHODS
Fungal strains and media.
KN99α was used in this study as the wild-type strain (21). All mutant strains used in this study were generated in either the KN99 or KN99 Mpk1 Flag-tagged background; KN99 containing the Mpk1-Flag epitope was previously shown to be functionally indistinguishable from KN99 (17). C. neoformans was routinely grown at 30°C on yeast peptone dextrose (YPD) (1% yeast extract, 2% Bacto peptone, and 2% dextrose) medium. Solid medium contained 2% Bacto agar. All strains with a deletion of PKC1 (along with appropriate controls) were grown on YPD medium supplemented with 1 M sorbitol. Selective YPD medium contained 100 μg/ml nourseothricin (Werner BioAgents, Jena-Cospeda, Germany), 200 U/ml hygromycin (HYG) B (Calbiochem, La Jolla, CA), or 200 μg/ml Geneticin (Gibco). For regulating the CTR4 promoter, YPD medium contained either 25 μM cupric sulfate (Sigma, St. Louis, MO) or 200 μM bathocuproinedisulphonic acid (BCS) (Sigma, St. Louis, MO).
To assess melanin production, strains were grown overnight in 2 ml of YPD medium at 30°C with shaking and diluted to an optical density at 650 nm (OD650) of 1.0 with phosphate-buffered saline (PBS). Five microliters each of 10-fold serial dilutions were plated on solid l-DOPA (l-3,4-dihydroxyphenylalanine) medium (13 mM glycine, 15 mM glucose, 29.4 mM KH2PO4, 10 mM MgSO4-7H2O, 3 μM thiamine, 5 μM d-biotin, 2% agar, and 1 mM l-DOPA, pH 5.5) and incubated at 30°C for up to 7 days in the dark. The strains' ability to produce pigment was assessed visually.
To determine sensitivity to nitrosative stress, serial dilutions of cells were plated on YNB medium, pH 4.0 (6.7 g/liter Difco yeast nitrogen base without amino acids, 2% glucose, 2% agar, 25 ml/liter 1 M succinate [adjusted to pH 4.0 with HCl]). To determine oxidative stress sensitivity, serial dilutions of cells were plated on YNB medium, pH 4.0 (see above), and also on YNB medium, pH 7.0 (6.7 g/liter Difco yeast nitrogen base without amino acids, 2% glucose, 2% agar, 50 ml/liter 1 M MOPS [morpholinepropanesulfonic acid, adjusted to pH 7.0 with NaOH]). All strains were maintained at 30°C unless otherwise indicated.
Generation of deletion constructs and transformation of C. neoformans.
Gene-specific deletion constructs of the Rho GTPases were generated using overlap PCR gene technology, described previously (18, 22), and included either the hygromycin resistance cassette (HYGR) (23), nourseothricin resistance cassette (NATR) (24), or a Geneticin resistance cassette (G418R) (see Fig. S1A in the supplemental material). The primers used to disrupt the genes are shown in Table S1 in the supplemental material. The rho10 deletion cassette contained the nourseothricin resistance cassette resulting in a 1,062-bp replacement of the coding sequence between regions of primers 3-Rho10 and 6-Rho10 (shown in uppercase in Table S1). The RHO11 deletion cassette contained the hygromycin resistance cassette, resulting in an 841-bp replacement of the coding sequence between regions of primers 3-Rho11 and 6-Rho11 (shown in uppercase in Table S1). Constructs were introduced into either KN99 or the Mpk1 Flag-tagged strain using biolistic techniques (24, 25). For the generation of strains lacking both RHO10 and RHO11, the rho10 deletion construct was biolistically transformed into two independent rho11Δ Mpk1 Flag-tagged strains. Cells were grown to late log phase in YPD medium, concentrated, and plated onto YPD agar for transformation. The cells were bombarded with 0.6-μm gold particles (Bio-Rad) coated with DNA according to the manufacturer's recommendations and allowed to sit for 4 h on nonselective medium following transformation. Cells were recovered by adding 0.8 ml of PBS to the plate and scraping the cells into suspension. They were then transferred to the appropriate selective medium and incubated at 30°C. Transformants were observed within 3 to 5 days.
Analysis of transformants.
Stable transformants were obtained by passaging cells five times on nonselective solid YPD medium, followed by testing for resistance on the appropriate selective marker. Only the isolates that grew on both selective and nonselective media were considered to be stable transformants. These isolates were then further analyzed by a three-primer PCR screen that verifies the homologous integration at both the 5′ and 3′ ends of the deletion cassette. Another PCR screen using primers outside the deletion construct was used to demonstrate a single copy of the deletion cassette had been inserted at the desired locus (see Fig. S1A in the supplemental material). To rule out ectopic integration elsewhere in the genome, Southern blotting was performed, using the selectable marker as a probe. All rho deletions created for this study were found to have a single deletion construct homologously integrated at the appropriate locus with no other insertions in the genome (data not shown).
C. neoformans genomic DNA preparation.
Genomic DNA was prepared by a modification of the glass bead DNA extraction protocol described by Fujimura and Sakuma (26). Cells were suspended in a microcentrifuge tube in 500 μl of lysis buffer (50 mM Tris, pH 7.5, 20 mM EDTA, 1% SDS), with 400 mg of glass beads (425 to 600 μm; Sigma). Cells were disrupted by 10 min of vortexing, followed by 10 min of incubation at 70°C. After brief vortexing, 200 μl of 5 M potassium acetate (KOAc) and 200 μl of 5 M NaCl were added. The tubes were placed on ice for 5 min and centrifuged at 14,000 rpm for 20 min. The supernatant was mixed with 500 μl of phenol-chloroform and spun for 2 min at 14,000 rpm. The aqueous phase was then mixed with 500 μl of chloroform and spun for 2 min at 14,000 rpm. The DNA was precipitated by the addition of 200 μl of ethanol, washed with 70% ethanol, and allowed to dry. Fifty microliters of a 10 μg/ml solution of RNase A (Roche) was added to each tube, and tubes were incubated at 37°C for 30 min. One-tenth volume (5 μl) of 3 M sodium acetate, pH 5.2, and 3 volumes (150 μl) of ethanol were added to each tube, and the DNA was precipitated by spinning at 14,000 rpm for 10 min. The pellets were resuspended in 50 μl of sterile Tris-EDTA (TE) buffer, pH 7.5.
Southern hybridizations.
Approximately 10 μg of genomic DNA from each strain was digested with various restriction endonucleases according to the manufacturer's recommendations. Restriction fragments were separated on a 1% agarose gel and transferred to nitrocellulose membranes using a Turbo-Blot apparatus (Schleicher & Schuell) and 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) as a transfer buffer. Probes for Southern analysis were prepared by random priming (random priming kit; Roche) using 50 μCi of [α-32P]dCTP (AA0005; Amersham) according to the manufacturer's instructions. The blots were incubated in 10 ml of 2× phosphate buffer (0.5 M Na2HPO4 · 7H2O, 0.34% H3PO4, 2 mM EDTA) mixed 1:1 with 14% SDS for 1 h at 65°C. Next, the probe was added to the solution, and the blots were hybridized at 65°C for 14 to 20 h. The blots were washed twice in 2× SSC–0.1% SDS at room temperature for 10 min and once for 10 min in 0.2× SSC–0.1% SDS that had been prewarmed to 65°C. The blots were then UV cross-linked for 2 min and exposed on film or a phosphor screen for imaging.
Generation of constitutively active rho1 mutant strains.
The C. neoformans constitutively active rho1 mutant strains were constructed by nucleotide substitution mutations in the phosphate or magnesium binding sites to create a deregulated GTPase (20). PCR products were generated using overlap PCR technology (22). The nucleotide substitution construct consists of 1,012 bp of genomic sequence upstream of the nucleotide substitution at amino acid position 15 or 1,272 bp of genomic sequence upstream of the nucleotide substitution at amino acid position 64, the G418 resistance cassette, and either 1,305 bp or 1,045 bp of genomic sequence downstream of the respective substitution site (see Fig. S1B in the supplemental material). The construct was biolistically transformed resulting in the amino acid mutation of either glycine to valine at position 15 or glutamine to leucine at position 64 and the insertion of a G418 resistance marker by homologous recombination. Stable transformants were obtained by passaging cells five times on nonselective solid YPD medium and then on solid YPD medium containing 200 μg/ml Geneticin (Gibco). Only transformants that grew on the selective medium were considered stable and were screened by PCR to check for the insertion nucleotide substitution at the desired location. Lastly, the entire RHO1 gene was sequenced for both point mutants to confirm the nucleotide substitutions and check for any unwanted mutations (data not shown). The primers used are shown in Table S1 in the supplemental material. The resulting strains carry rho1G15V and rho1Q64L mutations.
Generation of reconstituted rho1 point mutants and rho10Δ strains with wild-type genes.
C. neoformans rho1 point mutant strains were reconstituted by cotransformation of two PCR products (27). Product one consisted of KN99 genomic sequence of RHO1 spanning the wild-type gene plus 800 bp upstream and downstream, and product two consisted of the hygromycin resistance cassette. Two micrograms each of product one and two were biolistically cotransformed into both rho1 point mutation strains selecting for hygromycin-resistant isolates that were viable at 30°C (17, 27). Stable transformants were obtained by passaging cells five times on nonselective solid YPD medium, followed by streaking on both selective and nonselective media. Transformants were screened by PCR for the presence of the RHO1 gene. Isolates that showed the presence of the wild-type gene and the absence of the original deletion resistance marker (G418r) used to create the rho1 point mutation strains, together with those that were HYG sensitive (had not retained the cotransformed marker cassette), were chosen for further study and rescue of point mutation phenotypes. A C. neoformans rho10 deletion strain was reconstituted by cotransformation of two PCR products (27). Product one consisted of KN99 genomic sequence of RHO10 spanning the wild-type gene plus 1,000 bp upstream and downstream, and product two consisted of the G418 resistance cassette. Two micrograms each of product one and two were biolistically cotransformed into the rho10Δ strain selecting for G418 resistant isolates that were viable at 39°C (17, 27). Stable transformants were obtained by passaging cells five times on nonselective solid YPD medium, followed by streaking on both selective and nonselective media. Transformants were screened by PCR for the presence of the RHO10 gene as well as for the presence of the resistance marker (G418). Isolates that showed the presence of the wild-type gene and the absence of the original deletion resistance marker (HYGr) used to create the rho10Δ strain, together with those that were G418 sensitive (had not retained the cotransformed marker cassette), were chosen for further study and rescue of deletion phenotypes. Multiple efforts to generate reconstituted rho11Δ and rho10Δ rho11Δ strains (both containing the Mpk1 Flag-tagged epitope) were unsuccessful in the RHO11 locus. However, two independent mutant strains were generated carrying the rho11Δ mutation, and three were generated carrying the rho10Δ rho11Δ mutations.
Replacement of the endogenous RHO1 promoter with a repressible copper transporter promoter.
The repressible copper transporter promoter, PCTR4 was generously provided by Tamara Doering as pCTR4-2 (28) and used to create promoter replacement constructs. The promoter replacement construct was created using overlapping PCR technology, similar to the deletion constructs (17, 22). The RHO1 promoter replacement construct consists of 1,004 bp of genomic sequence upstream of the RHO1 promoter, the nourseothricin resistance cassette, PCTR4, and 1,025 bp of genomic sequence downstream of the RHO1 start codon (see Fig. S1C in the supplemental material). The construct was biolistically transformed and plated on YPD medium containing 200 μM bathocuproinedisulphonic acid (BCS; Sigma, St. Louis, MO), resulting in deletion of 956 bp upstream of the RHO1 gene and the insertion of PCTR4 and the nourseothricin resistance cassette by homologous recombination. The primers used to replace the endogenous promoter with a repressible promoter are shown in Table S1 in the supplemental material. Stable transformants were obtained by passaging cells five times on nonselective solid YPD medium containing BCS and then on solid YPD medium containing 100 μg/ml nourseothricin and BCS. Only the transformants that grew equally well on both the selective and nonselective media were considered stable transformants and were screened by PCR to check for homologous recombination of the promoter replacement construct at the desired locus. Lastly, Southern blot analysis that probed for the nourseothricin resistance marker was done to confirm single integration of the PCTR4 cassette that replaced the endogenous RHO1 promoter (data not shown). All media used in this experiment contained 200 μM BCS for induction of the CTR4 promoter. The primers used are shown in Table S1. The resulting strain was designated PCTR4-RHO1.
Plating assay for determination of the essentiality of RHO1.
Strains were grown in 2 ml of YPD medium containing 200 μM BCS at 30°C with shaking overnight. After 14 to 20 h, the cultures were diluted to an OD650 of 1.0 with sterile PBS. Tenfold serial dilutions were made, and 5 μl of each dilution was plated on YPD medium containing either 25 μM cupric sulfate (CuSO4) or 200 μM BCS. Plates were incubated at 30°C for up to 5 days.
Cell wall inhibitor, temperature, nitrosative, and oxidative stress sensitivity assays.
Strains were grown in 2 ml of YPD medium overnight at 30°C in a shaking incubator. After 14 to 20 h, the cultures were diluted to an OD650 of 1.0 with PBS. Five microliters each of 10-fold serial dilutions were plated on YPD solid medium containing the following cell wall stressors: 0.5, 1.0, and 1.5 mg/ml calcofluor white (CFW) (Fluorescent Brightner 28, F-3543; Sigma); 0.2, 0.5, and 1.0 mg/ml caffeine (C-0750; Sigma); 0.01%, 0.03%, and 0.06% SDS, and 0.5% Congo red (C-6767; Sigma). Plates were incubated at 30°C for 2 to 5 days and photographed. To test for temperature sensitivity, strains were plated on solid YPD medium and incubated at 25°C, 30°C, 37°C, or 39°C for up to 5 days. To test for nitrosative and oxidative stress sensitivities, strains were plated on solid YNB or YPD medium (pH 4.0 and pH 7.0) containing 1 mM NaNO2 (pH 4.0 only), 0.5 mM or 1 mM H2O2, or 1 mM diamide and incubated at 30°C for up to 7 days (17).
Capsule analysis.
Cells grown on YPD plates for 2 days were plated on 13.4 g/liter Dulbecco's modified Eagle's medium (DMEM) (D-5648; Sigma) containing 25 mM MOPS, pH 7.0, and 1.8% agar and incubated in the presence of 5% CO2 for 5 days at 30°C. Capsule-induced strains were resuspended in a 1:4 India ink-H2O solution and photographed on an Olympus BX61 microscope. Capsule diameter was measured and averaged for a minimum of 100 cells per strain using SlideBook, version 5.0. Differences in capsule diameters were tested for statistical significance using a one-way analysis of variance (ANOVA) with a Dunnett's t posthoc test to compare each mutant strain to WT. The level of significance (α) was set at 0.05, and statistical analyses were done using PASW Statistics 18, release version 18.0.3 (SPSS, Inc., Chicago, IL).
Analysis of melanin production.
Strains were grown overnight in 2 ml of YPD medium at 30°C with shaking and diluted to an OD650 of 1.0 with PBS. Five microliters each of 10-fold serial dilution were plated on solid l-DOPA medium (13 mM glycine, 15 mM glucose, 29.4 mM KH2PO4, 10 mM MgSO4-7H2O, 3 μM thiamine, 5 μM d-biotin, 2% agar, and 1 mM l-DOPA, pH 5.5) and incubated at 30°C for up to 7 days in the dark. The ability of cells to produce pigment was assessed visually.
Western blot analysis.
Yeast cells were grown overnight at 25°C to late logarithmic phase in 50 ml of YPD medium or YPD medium supplemented with 1 M sorbitol for the pkc1Δ strain and relevant controls. For heat shock, cells were diluted 1:1 with YPD medium or YPD medium supplemented with 1 M sorbitol prewarmed at 55°C and further grown at 39°C for 1 h unless noted otherwise. Control cells were diluted 1:1 with room temperature YPD or YPD medium supplemented with 1 M sorbitol medium and grown further at 25°C. After indicated times, cells were diluted 1:1 with ice-cold stop mix (0.9% NaCl, 1 mM NaN3, 10 mM EDTA, and 50 mM NaF) (29, 30). The cells were harvested at 652 × g at 4°C for 10 min and then washed once in ice-cold stop buffer. The cell pellet was resuspended in 1 ml of 1× lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, pH 8.2, 5 mM EGTA, 0.2 mM Na3VO4, 50 mM potassium fluoride [KF], 30 mM sodium pyrophosphate, 15 mM p-nitrophenylphosphate, 1× protease inhibitor cocktail [11836170001; Roche], and 10 μl/ml, each, phosphatase inhibitor cocktail I and II [P-2850 and P-5726; Sigma]). Approximately 1 ml of the resuspended cells was beaten for 10 min at 4°C with 0.75 ml of 0.5 mm zirconia/silica beads (11079105z; BioSpec Products, Inc.) on a Disruptor Genie. Lysates were spun down at 20,800 × g at 4°C for 20 min, and the supernatants were collected. Protein concentrations were determined using Quick Start Bradford dye reagent (500-0205; Bio-Rad). For each sample, 50 μg of total protein was loaded on 10% Mini-Protean TGX precast gels (456-1033; Bio-Rad) and transferred to nitrocellulose. For phosphorylated Mpk1 determination, a 1:2,500 dilution of phospho-p44/42 MAPK (Thr202/Tyr204) rabbit polyclonal antibody (4370; Cell Signaling Technology) was used. Total Mpk1 protein independent of phosphorylation was detected using a 1:5,000 dilution of DYKDDDDK (Flag) tag rabbit polyclonal antibody (2368; Cell Signaling Technology). All primary antibodies were diluted in TBST (25 mM Tris-HCl, pH 8.1, 145 mM NaCl, 0.1% Tween 20) plus 5% bovine serum albumin and allowed to bind overnight at 4°C. Secondary antibodies used were goat anti-rabbit immunoglobulin G, peroxidase conjugated (A-6154; Sigma) in a dilution of 1:1,000 in Tris-buffered saline–Tween (TBST). Proteins were detected using an ECL Plus Western blotting detection system (RPN2132; GE Healthcare).
RESULTS
Identification of C. neoformans Rho GTPases.
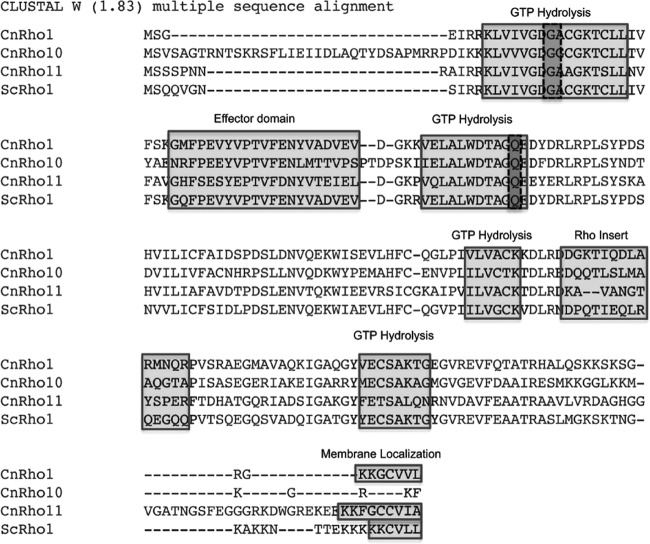
In S. cerevisiae, maintenance of cell integrity is controlled through the protein kinase C1 (PKC1) signaling pathway, which is regulated by the Rho1 GTPase (2). We utilized BLASTp homology with S. cerevisiae Rho1p to identify potential C. neoformans GTPases. This search yielded five homologues of Rho1. Reciprocal BLAST analysis revealed that two of these genes were more homologous to the S. cerevisiae GTPase Cdc42p than to Rho1p. C. neoformans CDC42 and CDC420 have recently been characterized by Ballou et al. (19). The remaining three C. neoformans homologues were more similar to S. cerevisiae Rho1p than to Rho2p, Rho3p, Rho4p, or Rho5p. Therefore, we named the cryptococcal Rho proteins Rho1, Rho10 and Rho11 (Table 1). Further analysis revealed that all three C. neoformans Rho homologues are highly conserved and that Rho1, Rho11, Cdc42, and Cdc420 contained prenylation modification sites at the C terminus, which are important for proper localization and function (Fig. 1) (2, 19; also the present study). Interestingly, Rho10 did not possess a predicted prenylation site at the C terminus, despite its high homology to both S. cerevisiae and C. neoformans Rho1 proteins. Thus, C. neoformans Rho10 may be atypical among the small GTPases (31).
Table 1.
Identification of C. neoformans Rho and Cdc42 small GTPases
Fig 1.
C. neoformans has three Rho GTPase homologues. Alignment of three identified C. neoformans (Cn) Rho1 homologues, Rho1, Rho10, and Rho11, with S. cerevisiae (Sc) Rho1p. Boxed in light gray are conserved domains for GTP hydrolysis, effector (putative site for interaction with target proteins), Rho insert (essential for Rho kinase activation), and putative membrane localization, including the prenylation site CAAX, where A represents an aliphatic amino acid (note that this site is conserved in CnRho1 and Rho11 but not in Rho10). Boxed in dark gray are the conserved glycine at amino acid position 15 and glutamine at position 64 that were mutated to valine and leucine, respectively, in the C. neoformans Rho1 protein to generate constitutively active point mutants.
Two RHO1 homologues, RHO10 and RHO11, are nonessential, and a rho10Δ rho11Δ deletion strain is viable.
In order to elucidate functional roles for the Rho GTPases, individual targeted gene deletions of C. neoformans homologues RHO10, RHO11, CDC42, and CDC420 were generated by homologous recombination in KN99α or the Mpk1-Flag fusion background strain. A strain containing gene deletions in both RHO10 and RHO11 was created by homologous recombination utilizing biolistic transformation of the rho10 deletion construct into the rho11Δ strain containing the Mpk1-Flag fusion protein. Gene deletion cassettes were generated using overlap PCR (22). Identification of specific KN99 gene sequences and design of the primers to generate the gene deletion cassettes were performed using the Gene Deletion Pipeline (18, 32). All mutants generated in this work were positive for homologous recombination with 5′ and 3′ PCR screens, amplified a product of the appropriate size for the entire locus, and had a single insertion site by Southern blotting using a portion of the drug resistance marker as a probe, and all were analyzed by the same methods, as described previously (18, 32). Although only one isolate may be shown for each strain, an additional independent isolate each for the rho10Δ and rho11Δ strains as well as three independent isolates for the rho10Δ rho11Δ strain yielded identical results in all experiments. Table 2 depicts the mutant strains created and used in this study.
Table 2.
Strains used and generated in this study
| Strain | Resistance marker(s) | Mating type | Strain background | Strain designation | Source or reference |
|---|---|---|---|---|---|
| KN99 | None | MATα | JLCN434 | 21 | |
| Mpk1-Flag | G418R | MATα | JLCN434 | JLCN559 | 17 |
| PCTR4-RHO1 | NATR | MATα | JLCN434 | JLCN574 | This study |
| rho1G15V strain | G418R | MATα | JLCN434 | JLCN671 | This study |
| rho1G15V strain | G418R, NATR | MATα | JLCN559 | JLCN711 | This study |
| rho1G15V::RHO1 | NATR | MATα | JLCN559 | JLCN771 | This study |
| rho1Q64L strain | G418R | MATα | JLCN434 | JLCN672 | This study |
| rho1Q64L strain | G418R, NATR | MATα | JLCN559 | JLCN714 | This study |
| rho1Q64L::RHO1 strain | NATR | MATα | JLCN559 | JLCN773 | This study |
| rho10Δ strain | NATR | MATα | JLCN434 | WLCN724 | This study |
| rho10Δ strain | NATR, G418R | MATα | JLCN559 | WLCN831 | This study |
| rho10Δ::RHO10 strain | None | MATα | JLCN434 | WLCN732 | This study |
| rho10Δ::RHO10 strain | G418R | MATα | JLCN559 | WLCN1088 | This study |
| rho11Δ strain | HYGR | MATα | JLCN559 | WLCN817 | This study |
| rho11Δ strain | HYGR | MATα | JLCN559 | WLCN1129 | This study |
| rho10Δ rho11Δ strain | NATR, HYGR | MATα | JLCN559 | WLCN1036 | This study |
| rho10Δ rho11Δ strain | NATR, HYGR | MATα | JLCN559 | WLCN1132 | This study |
| pkc1Δ strain | NATR, G418R | MATα | JLCN559 | KGCN858 | 17 |
| bck1Δ strain | HYGR, G418R | MATα | JLCN559 | KGCN986 | This study |
| mkk2Δ strain | HYGR, G418R | MATα | JLCN559 | KGCN988 | This study |
| mpk1Δ strain | NATR | MATα | JLCN559 | KGCN861 | 17 |
C. neoformans RHO1 is essential.
After numerous failed attempts in generating a targeted gene deletion for RHO1, we attempted to determine if this gene was essential by replacing the endogenous promoter with the repressible copper promoter, PCTR4 (28). The resulting strain, PCTR4-RHO1, was viable in the absence of copper; however, when the gene was repressed using CuSO4, it failed to grow (Fig. 2). This result demonstrated that C. neoformans RHO1 was essential for viability and suggests that it may be functionally similar to the S. cerevisiae RHO1, which is also essential and controls many aspects of growth in baker's yeast.
Fig 2.
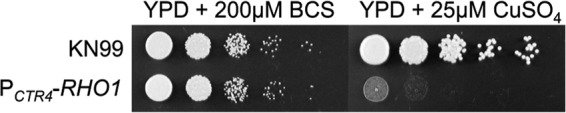
C. neoformans RHO1 is essential. KN99α and PCTR4-RHO1, a strain with the endogenous RHO1 promoter replaced by a copper-repressible promoter, were grown overnight in YPD medium containing 200 μM BCS at 30°C and then diluted to an OD650 of 1.0 in PBS. Five microliters of 10-fold serial dilutions were plated onto YPD plates containing either 200 μM BCS to allow expression of RHO1 from the copper promoter or 25 μM CuSO4 to repress expression of RHO1 from this promoter. The ability of cells to grow was analyzed after 2 and 5 days (shown) at 30°C.
The rho1G15V point mutant and the rho10Δ and rho10Δ rho11Δ deletion mutants exhibit growth defects, and the rho1Q64L strain reveals smaller colonies at elevated temperatures.
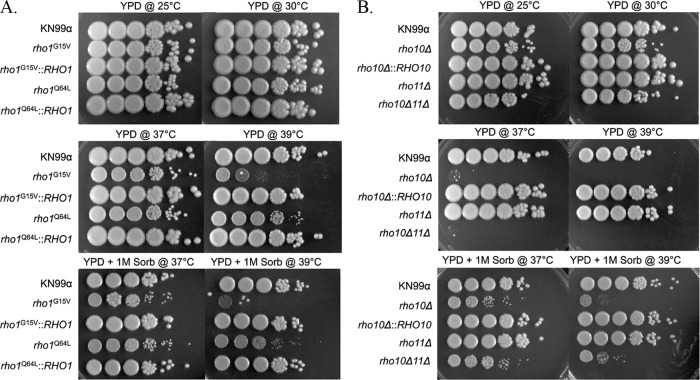
C. neoformans is thought to initially establish infection in the lungs via inhalation of spores or small yeast cells by mammalian hosts. It is logical to believe that elevated temperature is one of the first stresses that C. neoformans encounters upon inhalation by the host. C. neoformans Rho1 point mutants rho1G15V and rho1Q64L, first generated by Chang and Penoyer (20), were previously described as constitutively active based on amino acid conservation and homology to Rho1 proteins from other organisms. We generated these mutants in the KN99 background along with single and double deletion mutants of the remaining RHO1 homologues, RHO10 and RHO11. To determine if any of the rho mutants exhibited growth defects at elevated temperatures, we examined the growth of rho1G15V, rho1Q64L, rho10Δ, rho11Δ, and rho10Δ rho11Δ strains at 37°C and 39°C compared to growth at 25o and 30°C (Fig. 3A and B). Although neither rho1 mutant displayed pronounced growth defects at 37°C, the rho1G15V strain showed markedly impaired growth at 39°C of 4 to 5 orders of magnitude compared to growth at permissive temperatures and to the wild type at 39°C. Additionally, the rho1Q64L mutant exhibited a noticeably smaller colony size at 39°C that was not apparent at any other temperature (Fig. 3A). The high temperature growth defect of each point mutant was remediated by replacing each mutant allele with the wild-type RHO1 gene in the original locus (Fig. 3A). Addition of the osmotic stabilizer sorbitol to the medium was unable to rescue the growth defect of the rho1G15V strain at 39°C (Fig. 3A).
Fig 3.
The rho1G15V and rho1Q64L point mutants as well as both rho10Δ and rho10Δ rho11Δ strains are temperature sensitive, and this sensitivity is remediated by osmotic stabilization only for strains containing a deletion of RHO10. Cultures of strains were grown overnight in YPD medium at 30°C then diluted to an OD650 of 1.0. Five microliters of 10-fold serial dilutions were plated onto YPD plates with or without 1 M sorbitol (Sorb) and grown at indicated temperatures for 5 days. Strain names or genotypes are indicated to the left of each panel.
A strain with a deletion of RHO10 displayed pronounced growth defects at 37°C of 4 to 5 orders of magnitude compared to the wild type and to growth of the rho10Δ strain at permissive temperatures and was unable to grow at 39°C (Fig. 3B). Temperature sensitivity was fully remediated by the complemented rho10Δ::RHO10 strain (Fig. 3B). Similarly to growth of the rho10Δ strain, growth of a strain lacking both RHO10 and RHO11 was severely impaired at 37°C and 39°C (Fig. 3B). Partial rescue of temperature sensitivity by the addition of sorbitol to the medium occurred but was more enhanced for the rho10Δ rho11Δ strain than for one containing only a single rho10 deletion (Fig. 3B). A strain with a deletion of RHO11 exhibited no defects at any temperature tested (Fig. 3B).
The PKC1 cell integrity pathway is activated in response to thermal stress.
The PKC1 cell integrity pathway in C. neoformans is comprised of three downstream MAP kinases: Bck1 (MAPK kinase kinase [MAPKKK]), Mkk2 (MAPKK), and the presumed terminal kinase Mpk1 (MAPK) (18, 33). For all experiments in this study, we chose to examine the kinetics of PKC1 pathway activation by shifting cells rapidly from 24°C to 39°C by dilution in prewarmed medium for 1 h (29). To first determine whether the PKC1 pathway was activated in response to thermal stress, a wild-type strain containing the Mpk1 epitope tagged with Flag was grown at 24°C and then subjected to thermal stress at 39°C. Utilizing an antibody specific for phosphorylated Mpk1, an increase in Mpk1 phosphorylation was observed in protein lysates after heat shock at 39°C at 15, 30, 45, and 60 min compared to cells that had been left at 24°C with no heat shock (Fig. 4A). This indicated that the PKC1 pathway was activated in response to thermal stress. Using an anti-Flag antibody to detect total Mpk1 independent of phosphorylation, we demonstrated that the total amount of Mpk1 protein did not change when cells were heat shocked at 39°C compared to 24°C. The specificity of the anti-Mpk1 phospho-antibody was also validated because there was no detectable phosphorylation of Mpk1 with or without heat shock in a strain with a deletion of MPK1 (Fig. 4B). Thus, we concluded that phosphorylation of Mpk1, the downstream kinase in the PKC1 pathway, was increased as a result of thermal stress, that this response served as a readout and defined PKC1 pathway activation, and that this phosphorylation event was specific to Mpk1.
Fig 4.
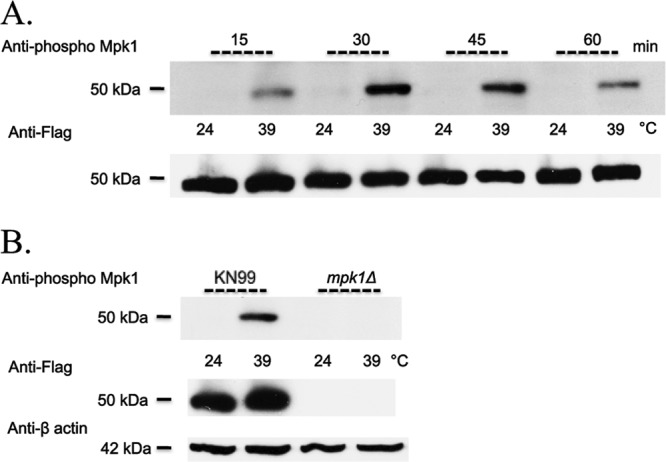
Mpk1 phosphorylation is induced in wild-type cells in response to thermal stress and the anti-Mpk1 phospho-antibody is specific for phosphorylation of Mpk1. (A) KN99α cells harboring the Mpk1 Flag-tagged construct were grown overnight in YPD medium at 24°C and then split the next morning into two aliquots. To one aliquot, an equal volume of YPD medium at room temperature was added, and cells were grown for an additional 15, 30, 45, and 60 min at 24°C. To the second aliquot, an equal volume of YPD medium prewarmed to 55°C was added, and the cells were heat shocked for the same number of minutes at 39°C. Western blots containing 50 μg of total protein from cell lysates were analyzed by probing sequentially with anti-Mpk1 phospho-antibody and then anti-Flag antibody after the membrane was stripped. (B) The Mpk1 Flag-tagged and mpk1Δ strains were treated as described for panel A, but heat shocked for 1 h only. Blots were probed sequentially with anti-Mpk1 phospho-antibody, anti-Flag antibody, and finally anti-β actin antibody for a loading control after the membrane was stripped.
Rho1 rho1G15V and rho1Q64L point mutants are constitutively active as measured by PKC1 pathway activation via Mpk1 phosphorylation.
It has been shown in S. cerevisiae that Rho1p interacts both genetically and biochemically with Pkc1p, and it is likely that at least one of the C. neoformans Rho proteins, presumably Rho1, interacts directly with Pkc1 and is involved in cell integrity pathway activation (34, 35). To test if there is a relationship between Rho1 and the PKC1 pathway in C. neoformans, we decided to generate constitutively active alleles of Rho1. In S. cerevisiae, two specific point mutants of RHO1 were each shown to be constitutively active, and both prevent intrinsic and GTPase-activating protein (GAP)-induced GTP hydrolysis, locking the strains in the activated form (36). The analogous substitutions in C. neoformans RHO1 were predicted to be constitutively active based on amino acid conservation and homology to Rho1 proteins from other organisms and growth phenotypes when these mutants were heterologously expressed in S. cerevisiae (20). We generated C. neoformans strains carrying these alleles with amino acid substitutions of Val for Gly at amino acid 15 or Leu for Gln at amino acid 64 in the Mpk1 Flag-tagged strain.
In an attempt to demonstrate definitive constitutive activation, we tested the Rho1 point mutants for their ability to phosphorylate Mpk1 at 24°C in the absence of stress. Both the rho1G15V and rho1Q64L strains were able to phosphorylate Mpk1 at 24°C after cells were grown overnight and then split into aliquots and diluted with fresh YPD medium at room temperature for an additional hour. Using Mpk1 phosphorylation as a readout for PKC1 pathway activation, these results demonstrated definitively that the rho1G15V and rho1Q64L mutants were constitutively active and suggested that Rho1 was involved directly in the activation of the cell integrity pathway (Fig. 5, lanes 3 and 7). Furthermore, replacement of the rho1 point mutant with the wild-type RHO1 allele restored the Mpk1 phosphorylation of the wild-type strain, indicating that constitutive activation of Mpk1 was a direct result of mutation of either glycine or glutamine at amino acid position 15 or 64, respectively (Fig. 5, lanes 5 and 9).
Fig 5.
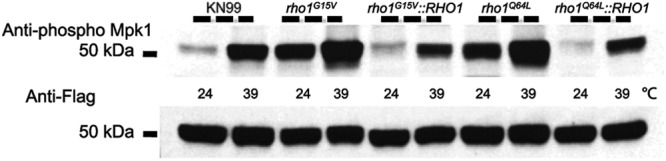
Mutants that harbor either rho1G15V or rho1Q64L in place of endogenous Rho1 are constitutively active independent of heat shock, and Mpk1 phosphorylation is increased with thermal stress compared to that observed at permissive temperatures. KN99α, rho1G15V, rho1G15V::RHO1, rho1Q64L, and rho1Q64L::RHO1 strains were grown overnight in YPD medium at 24°C and then split the next morning into two aliquots. To one aliquot, an equal volume of YPD medium at room temperature was added, and cells were grown an additional hour at 24°C. To the second aliquot, an equal volume of YPD medium prewarmed to 55°C was added, and the cells were grown for one additional hour at 39°C. Western blots containing 50 μg of total protein from cell lysates were analyzed by probing sequentially with anti-Mpk1 phospho-antibody and then anti-Flag antibody after the membrane was stripped. All strains are in the Mpk1 Flag-tagged background.
The rho1G15V and rho1Q64L constitutively active Rho1 point mutants result in increased phosphorylation of Mpk1 at high temperatures.
Although we demonstrated that the rho1G15V and rho1Q64L strains with point mutations of Rho1 phosphorylated Mpk1 at 24°C and thus that both of these mutants constitutively activated the PKC1 pathway in the absence of stress (Fig. 5, lanes 3 and 7), it was also important to determine the effect of heat shock on these mutants. After cells were shifted to 39°C for 1 h, there was an increase in Mpk1 phosphorylation compared to that observed with lysates of cells that remained at 24°C (Fig. 5, lanes 4 and 8). Thus, the introduction of thermal stress to both the rho1G15V and rho1Q64L constitutively active rho1 mutant strains had a synergistic effect on the phosphorylation status of Mpk1 although no variation of total levels of Mpk1 in either of these strains was observed (Fig. 5).
A strain with a RHO10 deletion is constitutively active for Mpk1 phosphorylation.
To determine the involvement of RHO10 in the activation of the PKC1 cell integrity pathway in response to thermal stress, we first analyzed phosphorylation of Mpk1 in a rho10Δ strain at 24°C. When RHO10 was deleted, Mpk1 was phosphorylated at 24°C, independent of any stress induction (Fig. 6A, lane 3). This was similar to what was observed biochemically with both rho1 constitutively active mutants. The rho10Δ mutant was temperature sensitive at 37°C (Fig. 3), yet Mpk1 phosphorylation was constitutive even at a permissive temperature in this strain, which suggested that the PKC1 pathway was activated. Total amounts of Mpk1 remained unchanged at 24°C for this mutant, and the levels were similar to those seen for wild type (Fig. 6A, bottom panel).
Fig 6.
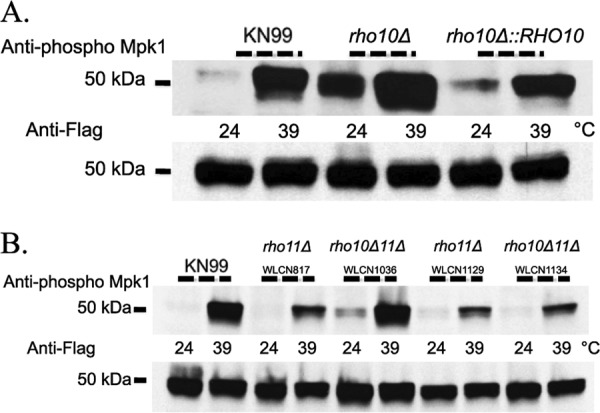
A strain with a deletion of RHO10 demonstrates constitutive Mpk1 phosphorylation independent of heat shock, phosphorylation is increased at high temperature, and both rho11Δ and rho10Δ rho11Δ strains confer wild-type Mpk1 phosphorylation. Strains were grown overnight in YPD medium at 24°C and then split the next morning into two aliquots. To one aliquot, an equal volume of YPD medium at room temperature was added, and cells were grown an additional hour at 24°C. To the second aliquot, an equal volume of YPD medium prewarmed to 55°C was added, and the cells were grown for one additional hour at 39°C. Western blots containing 50 μg of total protein from cell lysates were analyzed by probing sequentially with anti-Mpk1 phospho-antibody and then anti-Flag antibody after the membrane was stripped. All strains are in the Mpk1 Flag-tagged background. Strain names and/or genotypes are given above the blots.
A strain with a deletion of RHO10 had increased phosphorylation of Mpk1 in response to heat shock.
Although we demonstrated that, similar to the constitutively active rho1 point mutant strains, the rho10Δ strain phosphorylated Mpk1 at 24°C and thus constitutively activated the PKC1 pathway in the absence of stress (Fig. 6A, lane 3), it was also imperative to determine the biochemical effect of heat shock on a strain with a deletion of RHO10. After cells were shifted to 39°C for 1 h, there was an increase in Mpk1 phosphorylation compared to that observed in lysates of cells that remained at 24°C (Fig. 6A, lane 4). Thus, subjection of a strain with a deletion of RHO10 to thermal stress had a synergistic effect on Mpk1 phosphorylation, similar to what was observed for both rho1 point mutants. Moreover, similar to what was seen for the rho1G15V and rho1Q64L mutants, no variation in the total amount of Mpk1 was observed for the rho10Δ strain (Fig. 6A, bottom panel).
RHO11 is not required for Mpk1 phosphorylation in response to thermal stress.
To determine the role of the third RHO1 homologue, RHO11, in the activation of the PKC1 pathway in response to thermal stress, we measured the phosphorylation of Mpk1 in two independently generated rho11Δ strains at both 24o and 39°C. In the absence of RHO11, C. neoformans was able to phosphorylate Mpk1 in response to heat shock similarly to wild type; the MAPK was phosphorylated at 39°C, but phosphorylation was essentially absent at a permissive temperature (Fig. 6B, lanes 3, 4, 7, and 8). The total amount of Mpk1 remained essentially unchanged (Fig. 6B, bottom panel).
A strain with a deletion of both RHO10 and RHO11 demonstrates Mpk1 phosphorylation similar to wild type upon subjection to thermal stress.
A strain containing deletions in both RHO10 and RHO11 was barely able to survive at 37°C, similar to a rho10 single deletion strain (Fig. 3B). Therefore, we hypothesized that, like a strain lacking only RHO10, a rho10Δ rho11Δ strain would constitutively activate the PKC1 pathway and phosphorylate Mpk1 even at permissive temperatures. Surprisingly, this was not the case. Rather, Mpk1 phosphorylation of four independent rho10Δ rho11Δ strains mimicked that of the wild type, thus demonstrating the lack of a direct correlation between PKC1 pathway activation as measured by Mpk1 phosphorylation and the cells' ability to survive at high temperatures (Fig. 6B, lanes 5, 6, 9, and 10 and data not shown). The contrasting phosphorylation patterns of strains containing single rho10 or rho11 deletions taken together with these data regarding the double mutant led to the idea that perhaps RHO10 and RHO11 have opposing roles in C. neoformans regarding cell integrity and PKC1 pathway activation.
The rho1G15V and rho1Q64L point mutants and rho10Δ and rho10Δ rho11Δ deletion mutants exhibit cell integrity defects.
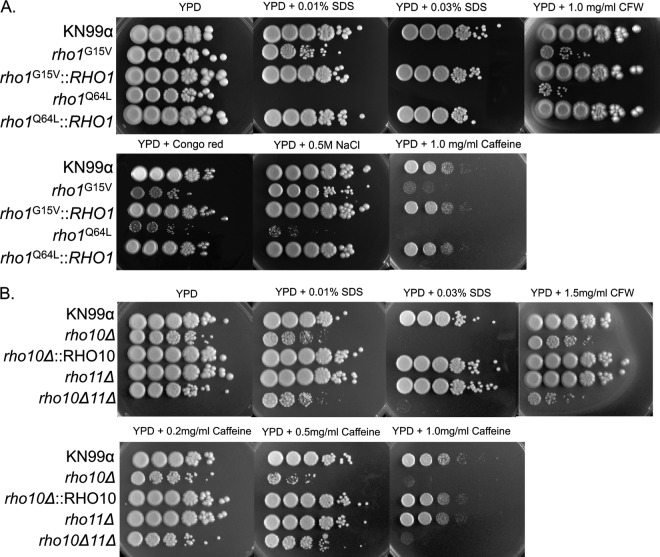
PKC1 cell integrity pathway components and regulators of C. neoformans that have been identified previously have been shown to be critical for resistance to a variety of cell wall stresses (17, 18, 33). Therefore, we examined potential involvement of the Rho GTPases with the PKC1 pathway by determining mutant strain sensitivities to cell wall stressors. Mutant strains were subjected to medium containing sodium dodecyl sulfate (SDS), which disrupts the plasma membrane and lyses cells with cell wall defects, calcofluor white (CFW), which binds to chitin and to a lesser extent glucan and disrupts the cell wall (37–39), sodium chloride (NaCl), which induces osmotic stress, Congo red, which binds to β-1,4-glucans and interferes with cell wall construction (40), and caffeine, which has been used extensively in S. cerevisiae to identify mutants defective for signal transduction (41). The constitutively active rho1G15V mutant exhibited smaller colonies on and was more sensitive to both calcofluor white and Congo red by 2 to 3 orders of magnitude than both the wild type and the rho1G15V mutant on YPD medium alone. This point mutant was approximately 3 orders of magnitude more sensitive to caffeine and dead on 0.03% SDS compared to both growth on YPD medium alone and also to the wild type (Fig. 7A). In contrast, the rho1Q64L mutant was severely impaired (∼4 orders of magnitude more sensitive) when exposed to either calcofluor white or NaCl and also exhibited smaller colonies on both types of media and did not grow on 0.01% SDS and 1 mg/ml caffeine (Fig. 7A). Importantly, all phenotypes for both point mutant strains were rescued by the corresponding complemented strains (Fig. 7A). It is intriguing that the two constitutively active rho1 mutants displayed such marked differences when they were subjected to cell wall-perturbing agents. The cell integrity of the rho1Q64L mutant was more compromised than that of the rho1G15V mutant, correlating with an observed increase in constitutive Mpk1 phosphorylation at 24°C (Fig. 5 and 7A). This indicated that although both mutants constitutively activated the PKC1 pathway biochemically, these mutations have additional effects on cell integrity that differ from each other, thereby pointing toward separate and distinct roles for RHO1 in the cell (Fig. 5, lanes 1, 3 and 7, and 7A).
Fig 7.
RHO1 and RHO10 are necessary for protection against cell wall stress but RHO11 is dispensable for this response. Cultures of strains were grown overnight in YPD medium at 30°C then diluted to an OD650 of 1.0. Five microliters of 10-fold serial dilutions were plated onto YPD plates plus the indicated inhibitor (SDS, sodium dodecyl sulfate; CFW, calcofluor white) and grown at 30°C for 5 days. Strain names or genotypes are indicated to the left of the panels.
A strain with a deletion of RHO10 also exhibited cell integrity phenotypes. This mutant had a slight sensitivity to calcofluor white (and also exhibited smaller colony size) and failed to grow on 0.03% SDS and also on 1 mg/ml caffeine compared to growth on YPD medium alone and also to growth of KN99. The cell integrity defects shown by the rho10Δ mutant were completely restored with the complemented strain carrying rho10Δ::RHO10 (Fig. 7B).
The rho10Δ rho11Δ double mutant, similar to a strain containing only a single deletion of RHO10, was severely impaired when subjected to 0.03% SDS and 1 mg/ml caffeine (Fig. 7B). A strain with a deletion of both RHO10 and RHO11 produced cells that were more sensitive to calcofluor white than a strain with a deletion of only RHO10, but the rho10Δ rho11Δ strain fared notably better than the single mutant rho10Δ strain on 0.5 mg/ml caffeine (Fig. 7B). A strain with a deletion of RHO11 demonstrated no cell integrity phenotypes when subjected to any agent tested (Fig. 7B). Taken together, these data suggest that both RHO1 and RHO10 may play an integral role in the regulation of the PKC1 cell integrity pathway in C. neoformans.
A strain with a deletion of RHO10 confers sensitivity to nitrosative stress.
We previously demonstrated that both oxidative and nitrosative stresses activate the PKC1 cell integrity pathway in wild-type cells, as measured by phosphorylation of Mpk1. We also determined that PKC1 was necessary for defense against both H2O2 and NaNO2 in vitro (17). Given the probability that Cryptococcus would encounter both or either of these stresses rapidly in the lung following inhalation by a host, we sought to determine if any of the RHO genes was necessary for protection. Only a strain with a deletion of RHO10 was sensitive to 1 mM NaNO2 (Fig. 8). Furthermore, we observed a decrease in growth of the rho10Δ rho11Δ strain in the presence of NaNO2 (∼1 order of magnitude compared to growth of this mutant in the absence of nitrosative stress), but the double mutant displayed much less sensitivity than the single rho10Δ mutant. None of the mutants exhibited any sensitivity to the oxidative stressors H2O2 and diamide (data not shown).
Fig 8.
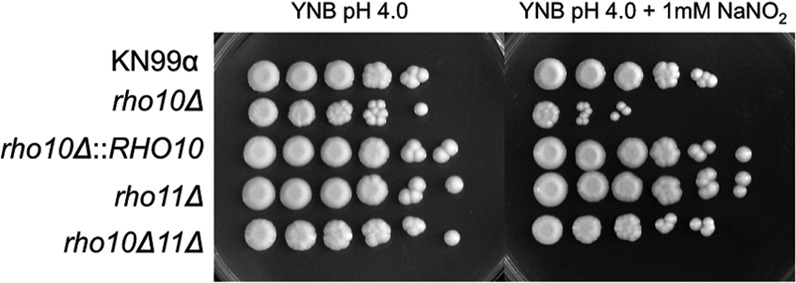
A strain with a deletion of RHO10 but not both RHO10 and RHO11 is sensitive to nitrosative stress. Cultures of strains were grown overnight in YPD medium at 30°C and then diluted to an OD650 of 1.0. Five microliters of 10-fold serial dilutions were plated onto freshly made YNB, pH 4.0, plates containing 1 mM NaNO2 and grown for 5 days at 30°C.
All rho mutants have melanin production levels similar to those of the wild type, but rho1G15V and rho1Q64L mutant strains display decreased capsule.
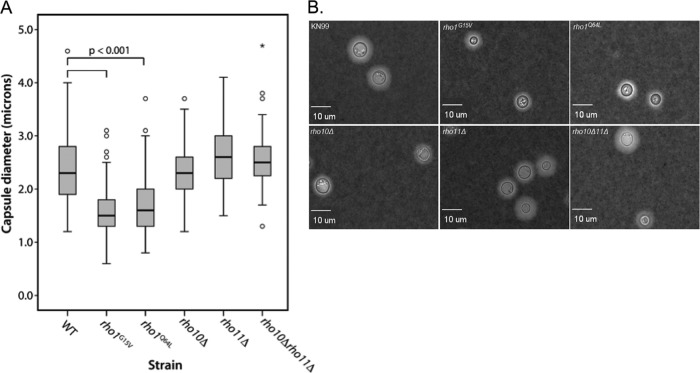
To further characterize our panel of Rho GTPase mutants, we chose to analyze two components known to be critical for virulence, polysaccharide capsule and melanin production. After induction of capsule on solid DMEM in the presence CO2 for 5 days, 100 to 300 cells per strain were analyzed microscopically in the presence of India ink (India ink is excluded from the capsule and therefore easily visualized), and capsule diameter was measured. Both the rho1G15V and rho1Q64L point mutant strains had significantly decreased capsule sizes (Fig. 9). The median capsule diameter of the rho1G15V mutant was 1.6 ± 0.43 μm, and that of the rho1Q64L mutant was 1.7 ± 0.56 μm, whereas wild-type cells had a median capsule diameter of 2.4 ± 0.57 μm (Fig. 9A). However, the cell body diameters of both of these mutants were also somewhat smaller than the cell body diameter of the wild type, and it remains uncertain if induced capsule size was a reflection of this trait (Fig. 9B). All strains exhibited melanin production similar to wild-type levels when plated on medium containing l-DOPA (data not shown).
Fig 9.
The constitutively active rho1G15V and rho1Q64L mutant strains display decreased capsule size compared to wild-type cells. Capsule was induced by growing cells on DMEM plates in the presence of 5% CO2 for 5 days at 30°C. Cells were stained with a 1:4 India ink-H2O solution, and capsule diameter was measured for a minimum of 100 cells per strain. (A) Differences in capsule diameters were tested for statistical significance using a one-way ANOVA with a Dunnett's t posthoc test to compare each mutant or deletion strain to the WT (see Materials and Methods). Open circles represent measurements that were >1.5 to 3.0 times the interquartile range. Asterisks represent measurements that were greater than three times the interquartile range. (B) Microscopic representation of India ink-stained cells for each strain at a magnification of ×400.
PKC1 pathway components Bck1 and Mkk2 are required for Mpk1 phosphorylation in response to thermal stress.
To further explore the role of the PKC1 cell integrity pathway in response to thermal stress, we analyzed deletions in known components of the pathway upon heat shock at 39°C for 1 h. The MAP kinases downstream of Pkc1 in C. neoformans consist of Bck1, Mkk2, and Mpk1, and targeted gene deletions were previously generated in the Mpk1 Flag-tagged background (18, 33). In C. neoformans, Bck1 and Mkk2 were required for Mpk1 phosphorylation in response to either oxidative or nitrosative stress. However, they were dispensable for resistance to these stresses in vitro. This was in contrast to what was seen at host temperature; both the bck1Δ and mkk2Δ strains were temperature sensitive at 37o and 39°C (18). To determine if Bck1 and Mkk2 were required for Mpk1 phosphorylation during heat shock, we analyzed Mpk1 phosphorylation in bck1Δ and mkk2Δ strains. In the absence of BCK1 or MKK2, C. neoformans was unable to phosphorylate Mpk1 in response to heat shock at 39°C, but the total amounts of Mpk1 remained unchanged (Fig. 10A). These data showed definitively that the PKC1 downstream pathway components Bck1 and Mkk2 were required for Mpk1 phosphorylation and thereby activation of the cell integrity pathway in response to thermal stress.
Fig 10.
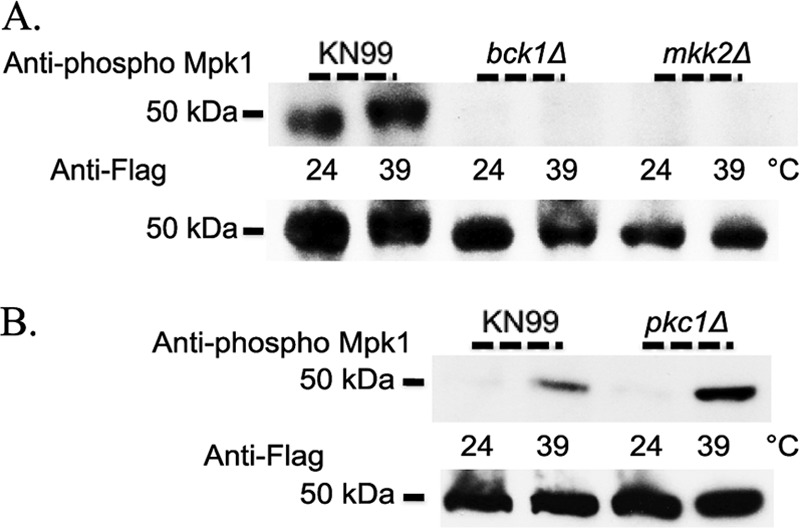
The PKC1 pathway components Bck1 and Mkk2 are necessary but Pkc1 is dispensable for phosphorylation of Mpk1 in response to thermal stress in C. neoformans. (A) KN99α, bck1Δ, and mkk2Δ strains were grown overnight in YPD medium at 24°C and then split the next morning into two aliquots. To one cell aliquot, an equal volume of YPD medium at room temperature was added, and cells were grown an additional hour at 24°C. To the other cell aliquot, an equal volume of YPD medium prewarmed to 55°C was added, and the cells were grown for one additional hour at 39°C. Western blots containing 50 μg of total protein from cell lysates were analyzed by probing sequentially with anti-Mpk1 phospho-antibody and then anti-Flag antibody after the membrane was stripped. All strains are in the Mpk1 Flag-tagged background. Identical results were obtained when experiments with KN99α, bck1Δ, and mkk2Δ strains were done using YPD medium containing 1 M sorbitol. (B) KN99α and pkc1Δ strains in the Mpk1 Flag-tagged background were treated identically to samples described in panel A, but all YPD medium contained 1 M sorbitol due to the conditional lethality of the pkc1 deletion strain.
PKC1 is dispensable for Mpk1 phosphorylation in response to thermal stress.
The major regulator of the PKC1 cell integrity kinase cascade in C. neoformans is believed to be PKC1, and a conditional lethal gene deletion was previously generated in the Mpk1 Flag-tagged strain. PKC1 was previously shown to be essential for Mpk1 phosphorylation in response to both nitrosative and oxidative stresses (17). This gene was also found to be required for response to high temperature; a pkc1Δ strain did not grow at either 37o or 39°C in vitro. Because the PKC1 pathway components BCK1 and MKK2 were shown to be necessary for Mpk1 phosphorylation in response to heat shock, we also chose to examine the response of a strain with a deletion of the vital regulator of cell integrity, PKC1. We hypothesized that, given this gene's importance in maintenance of cell integrity and the myriad of phenotypes observed in a pkc1Δ mutant, this gene would be required for cell integrity pathway activation as measured by Mpk1 phosphorylation. Surprisingly, a strain with a deletion of PKC1 was able to phosphorylate Mpk1 upon induction of thermal stress in a manner that mirrored the wild-type strain. Furthermore, the total amount of Mpk1 remained unchanged (Fig. 10B). These results indicated that PKC1 was dispensable for the Mpk1 phosphorylation response to heat shock. Taken together with results demonstrating that the pathway components BCK1 and MKK2 are necessary for Mpk1 phosphorylation, these data implied that there are inputs downstream and independent of PKC1 that are crucial for signaling and/or regulating the response of C. neoformans to thermal stress.
DISCUSSION
In this study, we analyzed mutant strains of C. neoformans RHO homologues (rho1G15V, rho1Q64L, rho10Δ, rho11Δ, and rho10Δ rho11Δ strains) and their responses to thermal stress via the PKC1 cell integrity pathway. S. cerevisiae has multiple Rho proteins, and Rho1p is the major Rho protein involved in activation of the cell integrity pathway. C. neoformans encodes three proteins which have closer homology to S. cerevisiae Rho1p than to the other S. cerevisiae Rho proteins, hinting that C. neoformans may use multiple Rho proteins to modulate the PKC1 cell integrity pathway. We determined that these three genes have distinct roles in signaling in response to high temperature and showed that RHO1 is an essential gene by utilization of the repressible copper promoter, PCTR4. C. neoformans Rho1 protein is 77% identical to S. cerevisiae Rho1p, and the latter has been shown to interact both biochemically and genetically in its GTP-bound form with the MAP kinase Pkc1p (34, 35). Rho1p is also required in S. cerevisiae for 1,3-β-d-glucan synthase activity (42). Given that RHO1 is essential, it is likely that this gene has similar important roles in C. neoformans. In this study, we have begun to reveal functions for each individual Rho homologue and its distinct role in response to thermal stress.
The C. neoformans Rho1 rho1G15V and rho1Q64L point mutants, which generate amino acid substitutions in phosphate and magnesium binding sites, were first generated by Chang and Penoyer (20) and described as constitutively active based on amino acid conservation and homology to Rho1 proteins from other organisms. In this study, we further characterized these mutants in C. neoformans to demonstrate that both of them constitutively activate the PKC1 pathway. These activating mutations made in highly conserved amino acids prevent intrinsic and GTPase-activating protein (GAP)-induced GTP hydrolysis have been utilized extensively in other systems (36). Using phosphorylation of Mpk1, the presumed terminal kinase in the PKC1 cell integrity pathway, as a measure of activation, we demonstrated that each of the Rho1 point mutant strains constitutively phosphorylates Mpk1 independent of stress (Fig. 5).
Interestingly, rho1G15V and rho1Q64L strains, when subjected to cell wall-inhibiting agents, both exhibit detrimental phenotypes, but the rho1Q64L strain is more severe, and one could speculate that this is a result of persistent increased phosphorylation of Mpk1 that reduces the cells' ability to maintain integrity (Fig. 5 and 7A). Conversely, the rho1G15V strain exhibits greater temperature sensitivity than the rho1Q64L mutant at 39°C (Fig. 3A). The differential effects of these two alleles are supported by previous work in which C. neoformans RHO1 or RHO1 point mutants identical to the two used in this study were overexpressed in C. neoformans using a GAL7 promoter in a strain that still contained wild-type RHO1. Induced expression of the wild-type RHO1 or the rho1G15V allele produced no discernible growth defects, but induced expression of the more severe rho1Q64L allele resulted in detrimental phenotypes. This suggests that the two mutant alleles have inherent differences in their activity. Because the phosphorylation levels of Mpk1 were similar for both mutants, these differences could be a result of Rho1 activation or expression levels of Rho1 that affect other cellular functions. Preliminary data from our laboratory indicate that expression of RHO1 is decreased in the rho1Q64L strain approximately 2-fold compared to the wild type when cells are grown at 24°C and 3-fold when cells are subjected to heat shock at 39°C for 1 h, whereas RHO1 expression is largely unaffected in the rho1G15V strain at either temperature (unpublished data). Taken together, these data suggest that expression of Rho1 as well as regulation of the PKC1 pathway is critical for fitness of fungal cells, and that balance likely contributes to cells' ability to flourish.
It is intriguing that mutants which presumably render Rho1 constitutively active display phenotypes similar to cell wall mutants. One hypothesis is that constitutive phosphorylation of Mpk1 has no deleterious effects but that constitutive activation of Rho1 results in adverse phenotypes that are unrelated to the PKC1 cell integrity pathway. Another possibility is that extended activation of the PKC1 pathway itself is deleterious and that the ability to downregulate is critical for proper maintenance of cell integrity. The importance of downregulation of the PKC1 pathway is suggested by the gradual decrease in Mpk1 phosphorylation following a peak at approximately 30 min after the introduction of thermal stress for wild-type cells (Fig. 4). Either of these hypotheses is supported by previous work in which C. neoformans rho1G15V and rho1Q64L point mutants similar to the two used in this study were overexpressed in S. cerevisiae under the control of the GAL1 promoter. When S. cerevisiae was grown on galactose, both alleles inhibited growth although the Q64L allele had a more severe defect than the G15V allele (20).
Considerable effort went into the generation of independent rho11 deletion strains, and it is worth noting that multiple unsuccessful attempts were made to complement the deletion strain in the RHO11 locus. This could be due at least in part to the close proximity of RHO11 to the centromere of chromosome 7, a region known to contain transposable element-like repetitive sequences, making it difficult for homologous recombination to occur. Another reason for this difficulty could be that the 3′ untranslated region (UTR) of this gene is proximal to the promoter region of the catalytic subunit of polymerase alpha, which is most certainly an essential gene in C. neoformans and unequivocally important for DNA replication and cell survival.
Our data suggest that, in addition to Rho1, both Rho10 and Rho11 together play a vital role in regulating the cell integrity pathway. At 24°C, wild-type C. neoformans does not activate the cell integrity pathway, as measured by Mpk1 phosphorylation (Fig. 4A), but when Rho10 is absent, Mpk1 is abundantly phosphorylated at 24°C and hyperphosphorylated at 39°C, and the wild-type pattern of phosphorylation is restored when RHO11 and RHO10 are both deleted (Fig. 6). Figure 11 depicts a model that is consistent with these observations as well as with the in vitro phenotypes concerning temperature and cell wall stress in these mutant strains. In this proposed model, Rho1 does not activate the pathway at lower temperatures but is the major activator of the pathway at higher temperatures. Rho11 can activate the pathway at all temperatures, but Rho10 interferes with or represses this activation at permissive temperatures. At higher temperatures, Rho10 repression is released, allowing Rho11 to activate the pathway. This is consistent with the fact that deletion of RHO11 has no effect on Mpk1 phosphorylation at a permissive temperature and causes a slight reduction of Mpk1 phosphorylation at 39°C. In further support of the model, deletion of RHO10 has increased phosphorylation at 24°C, and the absence of both RHO10 and RHO11 rescues the hyperphosphorylation seen in the rho10Δ strain. The increased phosphorylation of Mpk1 in the rho10Δ strain may be responsible for the detrimental growth defects, similar to what is seen with the Rho1 point mutant strains. In the absence of RHO10, cells exhibit growth sensitivities to both elevated temperatures and cell wall inhibitors (Fig. 3B and 7B). However, a strain with a deletion of RHO11 yields results similar to those of the wild type, suggesting that Rho1 is sufficient for activation of the cell integrity pathway during thermal stress. Interestingly, the rho11Δ strain, with slightly reduced Mpk1 phosphorylation at 39°C compared to the wild type (Fig. 6B), demonstrates extreme temperature sensitivity at both 37o and 39°C when plated on YPD medium containing hygromycin, the selectable marker used in the generation of the rho11 deletion cassette (data not shown). This suggests that two stresses combined, high temperature and the presence of drug, elicit a phenotype that is not apparent with a single stress of high temperature.
Fig 11.
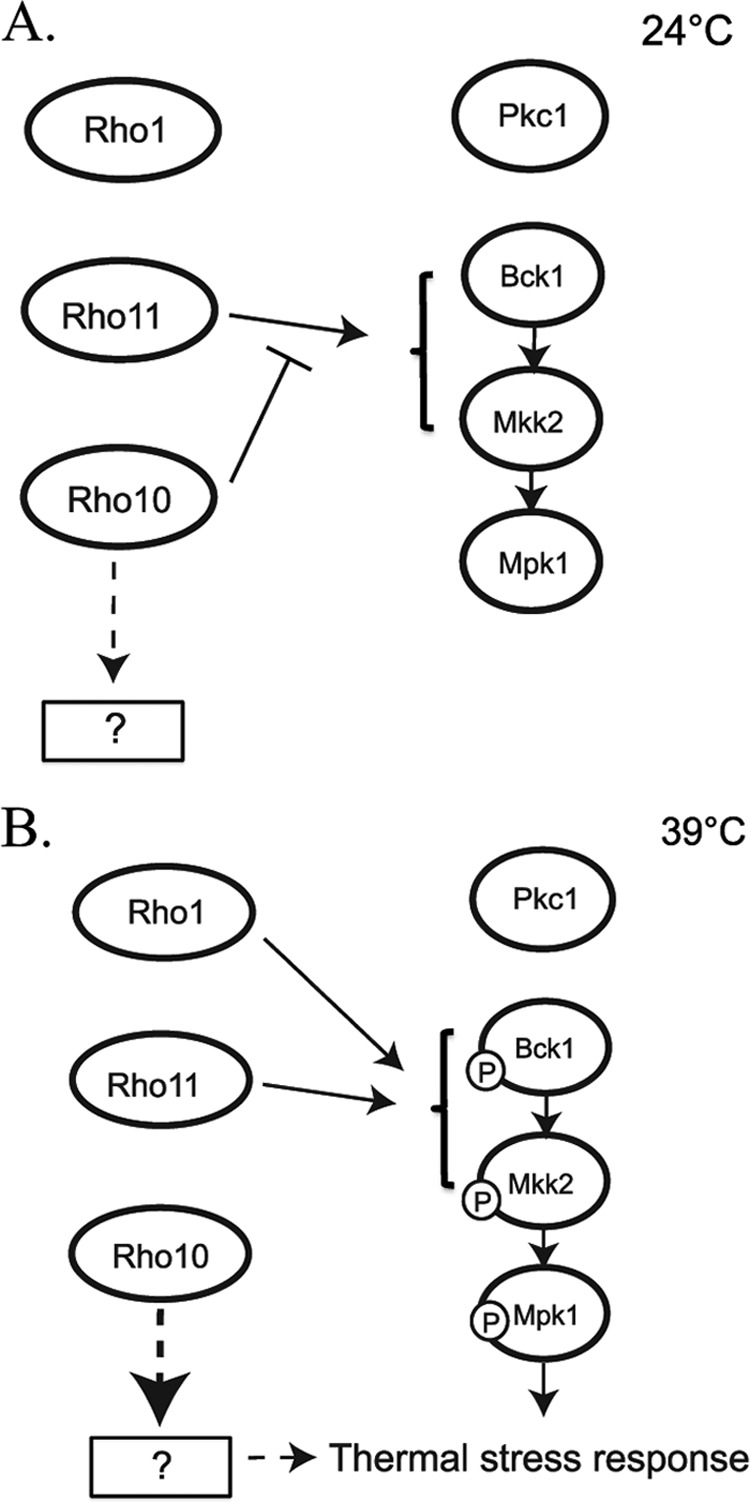
Model for C. neoformans Rho GTPases in protection against thermal stress and their relationship to the PKC1 cell integrity pathway. (A) Under growth conditions at 24°C, Rho1 is not active, and Rho11 is negatively inhibited by Rho10. This leads to inactivation of the cell integrity pathway as measured by Mpk1 phosphorylation. (B) Under thermal stress conditions (39°C for 1 h), Rho1 signals the activation of the cell integrity pathway, and Mpk1 is phosphorylated. Rho10 is inactivated, thereby releasing inhibition of Rho11, resulting in activation of the cell integrity pathway. Rho10 may also positively regulate an unknown protein or process that leads to the activation of the thermal stress response independent of Mpk1 phosphorylation (dashed arrows). For thermal stress response, Pkc1 is dispensable; therefore, Rho proteins likely interact with downstream components of the PKC1 pathway (brackets and solid arrows). Rho1, Rho10, and Rho11 act in concert and require balance to regulate the precise and critical amount of Mpk1 phosphorylation needed for growth and combating thermal stress.
Our model predicts that Rho10 represses Rho11 activity at 24°C, but our data also suggest that Rho10 has other functions as well. If Rho10 were only a repressor of Rho11, then the double mutant rho10Δ rho11Δ strain would have no in vitro phenotypes, but it behaves similarly to a rho10Δ strain in that it grows slightly more slowly than the wild type, even at room temperature. Furthermore, it is sensitive to high temperatures and cell wall-perturbing agents (Fig. 3B and 7B) even though the phosphorylation pattern of Mpk1 is similar to that of the wild type. These data suggest that Rho10 is required for other activities in the cell that contribute to cell wall integrity and high temperature growth. One explanation is that RHO10 may be modulating its effects via as yet undiscovered components alternative to those in the PKC1 pathway, indicating that the PKC1 pathway is necessary, but not sufficient, for protection against thermal stress. Interestingly, Rho10 does not possess a classic C-terminal prenylation site, CAAX (where A represents an aliphatic amino acid), which is important for membrane localization and function in other known Rho proteins. It does, however, possess a C-terminal polybasic region that could be instrumental for membrane localization and also possibly for interaction of this small GTPase with guanine nucleotide exchange factors (43). This may point to a unique and as yet undefined function for this protein in C. neoformans.
Our phenotypic and biochemical data combined suggest that Rho10 and Rho11 may have opposing functions in the cell and act in concert to balance and coordinately regulate certain aspects of cell integrity and PKC1 pathway activation. All three of the activating mutations, rho10Δ, rho1G15V, and rho1Q64L, result in a paradoxical negative effect on cell integrity, and this may be due to a sequestering or imbalance of signaling partners or guanine exchange factors (GEFs) that make them active (GTP-bound) and/or GTPase-activating proteins (GAPS) that render them inactive (GDP bound). Furthermore, deletion of the C. neoformans GAP Lrg1, which would likely cause constitutive activation of at least one of the Rho proteins, also demonstrates severe detrimental phenotypes similar to those seen with rho10Δ, rho1G15V, and rho1Q64L strains, such as sensitivities to Congo red, SDS, and growth at high temperature (18). The phenotypes of an lrg1Δ strain emphasize yet another striking distinction between C. neoformans and S. cerevisiae, as deletion of LRG1 in baker's yeast does not result in severe cell integrity defects. Additionally, dominant-activating mutations in either RHO1 or RHO10 may cause altered localization of these Ras-like signaling components, including GEFS and GAPS, contributing to the severity of phenotypes and decreased maintenance of cell integrity observed with rho1G15V, rho1Q64L, and rho10Δ strains. Strains harboring either the rho1G15V or rho1Q64L point mutation in place of wild-type Rho1, however, do not share the nitrosative stress sensitivity exhibited by a rho10Δ strain, supporting the idea that Rho1 and Rho10 have functions in the cell distinct from each other.
We were interested in determining the response of the PKC1 cell integrity pathway to thermal stress and the RHO gene family's involvement in this pathway. Therefore, we chose to examine the effect of heat shock on strains with deletions of known pathway components (18). A striking finding in this study was that, although Bck1 and Mkk2 are necessary for Mpk1 phosphorylation and thus PKC1 pathway activation upon heat shock, Pkc1 is not required for this response (Fig. 10). This is in direct contrast to what has been observed with pkc1 mutants in S. cerevisiae, where Pkc1 was found to be necessary for pathway activation in response to thermal stress (29). These data strengthen the idea that signaling components in C. neoformans, although highly conserved, have diverged enough to elicit distinct responses to stress that differ dramatically from those in the well-studied baker's yeast. Furthermore, Pkc1 was previously shown to be required for response to nitrosative stress in C. neoformans, so it is clear that the response of Mpk1 phosphorylation and PKC1 pathway activation differ in specificity between thermal and nitrosative stress in this opportunistic pathogen (17). The current studies emphasize that although Pkc1 has multiple functions and pleiotropic effects and exhibits temperature sensitivity, its involvement in Mpk1 phosphorylation in response to heat shock is dispensable (17).
Taken together, these findings demonstrate that the Rho GTPases have distinct roles for cell stress responses, specifically thermal stress, and that this response is not necessarily transmitted by Pkc1 and likely involves alternate pathways or signaling components (Fig. 11). Bahn et al. have previously characterized the C. neoformans MAP kinase Hog1 and shown its importance in response to a wide variety of external stresses (44). Preliminary data from our laboratory indicate that in a strain with a deletion HOG1, Mpk1 phosphorylation is increased regardless of temperature and that phosphorylation increases when cells are subjected to heat shock at 39°C for 1 h compared to growth at 24°C (data not shown). Not surprisingly, this strengthens the notion that cross talk between pathways is likely occurring and that the cells' ability to survive upon encountering thermal stress is dependent on more than one pathway.
Ballou et al. demonstrated elegantly that Cdc42, a closely related Rho1 homologue, was necessary for thermal tolerance in C. neoformans and that this protein was essential for virulence in a mouse model of infection. We showed in this study that additional small Rho GTPases are important for the response to heat shock, but the effect of deletions in Rho proteins on virulence has yet to be determined. We hypothesize that, due to the temperature-sensitive phenotypes of rho10Δ and rho10Δ rho11Δ strains, these mutants will be avirulent or at least demonstrate reduced pathogenesis in a mouse model of infection.
Expanding on data presented in this study, it is plausible that Rho1, Rho10, and Rho11 share GEFs and/or GAPS and that these regulators preferentially bind to one or the other Rho protein in response to specific stresses. We have indentified three C. neoformans GEFS, Rom2, Rom20, and Rom21, and four GAPS, Lrg1, Bag7, Bem3, and Rga1, and it will be interesting to determine which of these proteins are involved in regulation of Rho1, Rho10, and Rho11 (18; also unpublished data). This knowledge should greatly increase our understanding of the regulation and function of the Rho GTPases in C. neoformans.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Maureen Donlin for BLAST analysis of the RHO genes and for expert statistical analyses performed on capsule measurements. We thank Marshall Michener and Maureen Donlin for critical reading of the manuscript, Raj Uphadya and Roger Herr for lively debates, and Kyla Selvig for advice.
This work was supported by NIH NIAID grants R01AI072195 and R01AI050184 to J.K.L.
Footnotes
Published ahead of print 16 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.05305-11.
REFERENCES
- 1. Etienne-Manneville S, Hall A. 2002. Rho GTPases in cell biology. Nature 420:629–635 [DOI] [PubMed] [Google Scholar]
- 2. Levin DE. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69:262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cabib E, Drgonová J, Drgon T. 1998. Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu. Rev. Biochem. 67:307–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helliwell SB, Howald I, Barbet N, Hall MN. 1998. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics 148:99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madaule P, Axel R, Myers AM. 1987. Characterization of two members of the RHO gene family from the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 84:779–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ozaki K, Tanaka K, Imamura H, Hihara T, Kameyama H, Nonaka H, Haiano H, Matsuura Y, Takai Y. 1996. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:2196–2207 [PMC free article] [PubMed] [Google Scholar]
- 7. Imai J, Toh-e A, Matsui Y. 1996. Genetic analysis of the Saccharomyces cerevisiae RHO3 gene, encoding a Rho-type small GTPase, provides evidence for a role in bud formation. Genetics 142:359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kagami M, Toh-e A, Matsui Y. 1997. SRO9, a multicopy suppressor of the bud growth defect in the Saccharomyces cerevisiae rho3-deficient cells, shows strong genetic interactions with tropomyosin genes, suggesting its role in organization of the actin cytoskeleton. Genetics 147:1003–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsui Y, Toh-E A. 1992. Yeast RHO3 and RHO4 ras superfamily genes are necessary for bud growth, and their defect is suppressed by a high dose of bud formation genes CDC42 and BEM1. Mol. Cell. Biol. 12:5690–5699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Annan RB, Wu C, Waller DD, Whiteway M, Thomas DY. 2008. Rho5p is involved in mediating the osmotic stress response in Saccharomyces cerevisiae, and its activity is regulated via Msi1p and Npr1p by phosphorylation and ubiquitination. Eukaryot. Cell 7:1441–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh K, Kang PJ, Park H-O. 2008. The Rho5 GTPase is necessary for oxidant-induced cell death in budding yeast. Proc. Natl. Acad. Sci. U. S. A. 105:1522–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adamo JE, Rossi G, Brennwald P. 1999. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell 10:4121–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson DI. 1999. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63:54–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson DI, Pringle JR. 1990. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J. Cell Biol. 111:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heitman J, Kozel TR, Kwon-Chung J, Perfect JR, Casadevall A. 2010. Cryptococcus: from human pathogen to model yeast. ASM Press, Washington, DC [Google Scholar]
- 16. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530 [DOI] [PubMed] [Google Scholar]
- 17. Gerik KJ, Bhimireddy SR, Ryerse JS, Specht CA, Lodge JK. 2008. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot. Cell 7:1685–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerik KJ, Donlin MJ, Soto CE, Banks AM, Banks IR, Maligie MA, Selitrennikoff CP, Lodge JK. 2005. Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol. Microbiol. 58:393–408 [DOI] [PubMed] [Google Scholar]
- 19. Ballou ER, Nichols CB, Miglia KJ, Kozubowski L, Alspaugh JA. 2010. Two CDC42 paralogues modulate Cryptococcus neoformans thermotolerance and morphogenesis under host physiological conditions. Mol. Microbiol. 75:763–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang YC, Penoyer LA. 2000. Properties of various Rho1 mutant alleles of Cryptococcus neoformans. J. Bacteriol. 182:4987–4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nielsen K, Cox GM, Wang P, Toffaletti DL, Perfect JR, Heitman J. 2003. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and α isolates. Infect. Immun. 71:4831–4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davidson RC, Blankenship JR, Kraus PR, de Jesus Berrios M, Hull CM, D'Souza C, Wang P, Heitman J. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607–2615 [DOI] [PubMed] [Google Scholar]
- 23. McDade HC, Cox GM. 2001. A new dominant selectable marker for use in Cryptococcus neoformans. Med. Mycol. 39:151–154 [DOI] [PubMed] [Google Scholar]
- 24. Hua J, Meyer JD, Lodge JK. 2000. Development of positive selectable markers for the fungal pathogen Cryptococcus neoformans. Clin. Diagn. Lab. Immunol. 7:125–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujimura H, Sakuma Y. 1993. Simplified isolation of chromosomal and plasmid DNA from yeasts. Biotechniques 14:538–540 [PubMed] [Google Scholar]
- 27. Goins CL, Gerik KJ, Lodge JK. 2006. Improvements to gene deletion in the fungal pathogen Cryptococcus neoformans: absence of Ku proteins increases homologous recombination, and co-transformation of independent DNA molecules allows rapid complementation of deletion phenotypes. Fungal Genet. Biol. 43:531–544 [DOI] [PubMed] [Google Scholar]
- 28. Ory JJ, Griffith CL, Doering TL. 2004. An efficiently regulated promoter system for Cryptococcus neoformans utilizing the CTR4 promoter. Yeast 21:919–926 [DOI] [PubMed] [Google Scholar]
- 29. Kamada Y, Jung US, Piotrowski J, Levin DE. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Gene Dev. 9:1559–1571 [DOI] [PubMed] [Google Scholar]
- 30. Surana U, Robitsch H, Price C, Schuster T, Fitch I, Futcher AB, Nasmyth K. 1991. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell 65:145–161 [DOI] [PubMed] [Google Scholar]
- 31. Chenette EJ, Abo A, Der CJ. 2005. Critical and distinct roles of amino- and carboxyl-terminal sequences in regulation of the biological activity of the Chp atypical Rho GTPase. J. Biol. Chem. 280:13784–13792 [DOI] [PubMed] [Google Scholar]
- 32. Missall TA, Lodge JK. 2005. Thioredoxin reductase is essential for viability in the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell 4:487–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kraus PR, Fox DS, Cox GM, Heitman J. 2003. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 48:1377–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamada Y, Qadota H, Python CP, Anraku Y, Ohya Y, Levin DE. 1996. Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271:9193–9196 [DOI] [PubMed] [Google Scholar]
- 35. Nonaka H, Tanaka K, Hirano H, Fujiwara T, Kohno H, Umikawa M, Mino A, Takai Y. 1995. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14:5931–5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bishop AL, Hall A. 2000. Rho GTPases and their effector proteins. Biochem. J. 348:241–255 [PMC free article] [PubMed] [Google Scholar]
- 37. Elorza MV, Rico H, Sentandreu R. 1983. Calcofluor white alters the assembly of chitin fibrils in Saccharomyces cerevisiae and Candida albicans cells. J. Gen. Microbiol. 129:1577–1582 [DOI] [PubMed] [Google Scholar]
- 38. Murgui A, Elorza MV, Sentandreu R. 1985. Effect of papulacandin B and calcofluor white on the incorporation of mannoproteins in the wall of Candida albicans blastospores. Biochim. Biophys. Acta 841:215–222 [DOI] [PubMed] [Google Scholar]
- 39. Ram AFJ, Wolters A, Hoopen RT, Klis FM. 1994. A new approach for isolating cell wall mutants in Saccharomyces cerevisiae by screening for hypersensitivity to calcofluor white. Yeast 10:1019–1030 [DOI] [PubMed] [Google Scholar]
- 40. Wood PJ, Fulcher RG. 1983. Dye interactions. A basis for specific detection and histochemistry of polysaccharides. J. Histochem. Cytochem. 31:823–826 [DOI] [PubMed] [Google Scholar]
- 41. Lopez-Saez JF, Mingo R, Gonzalez F. 1982. ATP level and caffeine efficiency on cytokinesis inhibition in plants. Eur. J. Cell Biol. 27:185–190 [PubMed] [Google Scholar]
- 42. Mazur P, Baginsky W. 1996. In vitro activity of 1,3-beta-d-glucan synthase requires the GTP-binding protein Rho1. J. Biol. Chem. 271:14604–14609 [DOI] [PubMed] [Google Scholar]
- 43. Williams C. 2003. The polybasic region of Ras and Rho family small GTPases: a regulator of protein interactions and membrane association and a site of nuclear localization signal sequences. Cell. Signal. 15:1071–1080 [DOI] [PubMed] [Google Scholar]
- 44. Bahn Y-S, Kojima K, Cox GM, Heitman J. 2005. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol. Biol. Cell 16:2285–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.