Abstract
We are expanding the use of the MIT-MANUS robotics to persons with impairments due exclusively to orthopedic disorders, with no neurological deficits. To understand the reliability of repeated measurements of the robotic tasks and the potential for registering changes due to learning is critical. Purposes of this study were to assess the learning effect of repeated exposure to robotic evaluations and to demonstrate the ability to detect a change in protocol in outcome measurements. Ten healthy, unimpaired subjects (mean age = 54.1 ± 6.4 years) performed six repeated evaluations consisting of unconstrained reaching movements to targets and circle drawing (with and without a visual template) on the MIT-MANUS. Reaching outcomes were aiming error, mean and peak speed, movement smoothness and duration. Outcomes for circle drawing were axis ratio metric and shoulder–elbow joint angles correlation metric (was based on a two-link model of the human arm and calculated hand path during the motions). Repeated-measures ANOVA (p ≤ .05) determined if difference existed between the sessions. Intraclass correlations (R) were calculated. All variables were reliable, without learning across testing sessions. Intraclass correlation values were good to high (reaching, R ≥ .80; circle drawing, R ≥ .90). Robotic measurement ability to differentiate between similar but distinct tasks was demonstrated as measured by axis ratio metric (p < .001) and joint correlation metric (p = .001). Outcome measures of the MIT-MANUS proved to be reliable yet sensitive to change in healthy adults without motor learning over the course of repeated measurements.
Keywords: outcomes, rehabilitation, orthopedic impairments
Robotic-assisted interventions and evaluations have been applied to persons with chronic upper limb neurological disorders (Fasoli et al., 2003; Fasoli, Krebs, Stein, et al., 2004; Ferraro et al., 2003; Kahn et al., 2001; Lum et al., 2002, 2004; Reinkensmeyer et al., 2000; Volpe et al., 1999, 2000, 2001). These interventions consisted of reaching tasks that employed models of motor learning engendering principles of task specificity, repetition, progression, and feedback (Salmoni et al., 1984; Schmidt & Lee, 1998). In particular, we previously have employed the MIT-MANUS, a robotic device capable of moving, guiding, or perturbing movements of a patient’s paretic upper limb to delivery therapy (Dipietro et al., 2007; Fasoli et al., 2003; Fasoli, Krebs, Ferraro, et al., 2004; Finley et al., 2005). In addition, the device has been used as an evaluation tool for recording the patient’s self-generated motions, thereby quantifying different features of motor performance (e.g., accuracy speed, coordination, smoothness) (Finley et al., 2005; Rohrer et al., 2002) However, most studies evaluating robotic-assisted therapies have relied primarily on traditional clinical measures of motor impairment such as the Fugl-Meyer as their primary outcome (Fasoli, et al., 2003; Fasoli, Krebs, Stein, et al., 2004; Ferraro et al., 2003; Lum et al., 2002, 2004; Reinkensmeyer et al., 2000). Only a few studies have reported on the robot-derived outcomes (Dipietro et al., 2007; Finley et al., 2005; Rohrer et al., 2002). The MIT-MANUS robot–generated outcome measures have demonstrated the ability to detect significant improvements in motor performance even when the clinical measures revealed only small changes (Finley et al., 2005; Rohrer et al., 2002). They can also provide means to quantify aspects that have traditionally relied exclusively on qualitative observation, such as smoothness of movement (Rohrer et al., 2002), tone (Palazzolo et al., 2007), and synergies (Dipietro et al., 2007).
We are expanding the use of the MIT-MANUS to interventions for persons with impairments due exclusively to musculoskeletal and orthopedic disorders with no neurological deficits. In particular, we are employing the robotic device to deliver therapy following postoperative tendon repairs. In this patient population, clinical scales are limited and with questionable reliability and validity (Cook et al., 2001; Michener & Leggin, 2001; Placzek et al., 2004). Therefore, robotic measurement of patient unconstrained movements could be employed as the primary outcome. If these robot outcome measures are to be used as primary outcomes to evaluate patients with impairments due to other than neurological causes, it is important to demonstrate that repeated measures of these repetitive robot tasks are insensitive to learning effects. In other words, we need to demonstrate stability of the measures of these nonnovel tasks when evaluated via the robot and exclude the potential for registering changes solely due to adaptation and learning the task and not to impairment reduction. Furthermore, we must be sure that the stability of these results is not due to the coarse or insensitive nature of the measurement. To test for the sensitivity or responsiveness of the robotic measurement, we must demonstrate that even an apparent minor protocol modification can be captured by the measurement.
Therefore, the purpose of this study was twofold: first to assess the potential learning effect of repeated exposure to robotic testing on the MIT-MANUS outcome measures and second to concomitantly demonstrate the ability of these robotic outcome measures to detect subtle change in protocol in normal, healthy individuals. It was hypothesized that the robotic outcome measures would be stable (no learning effect) in the absence of an intervention and that robot measurements are sensitive enough to capture even an apparent minor modification.
Methods
Participants
A convenience sample of 10 healthy, unimpaired, right-hand-dominant subjects (mean age = 54.1 ± 6.4 years; 6 females, 4 males) participated. Hand dominance was established as the hand that participants used for writing (observed during consent signing) and personal activities (subject self-report on feeding, grooming, and throwing). All subjects were naïve to the robot and in an effort to create a more novel task with the potential for learning, the nondominant arm (left) was tested. The Baltimore Veterans Administration Medical Center Research and Development Committee via the Institutional Review Board of the University of Maryland School of Medicine and the Committee on the Use of Human Experimental Subjects of the Massachusetts Institute of Technology approved this protocol, and each subject provided informed consent before participation.
Protocol
To establish that the participants had no upper extremity impairment, and to establish that they performed within normal performance of healthy adults, each subject underwent repeated upper extremity clinical evaluations consisting of goniometrically measured range of motion, strength via hand-held dynamometry (Kendall et al., 2005), Jebsen Hand Function Test (Jebsen et al., 1969), and the nine-hole peg test (Oxford Grice et al., 2003).
To evaluate if a learning effect results from repeated exposure to robotic testing and to test the reliability and stability of the robotic outcomes, subjects performed six robot evaluation sessions, two sessions per day over three separate days. The majority of these sessions took place within 2 weeks but two subjects extended into the third week owing to illness and vacation. A robot evaluation session consisted of one reaching task and four circle-drawing tasks. The evaluations were performed on the highly back-driveable low-friction MIT-MANUS (Hogan et al., 1995; Krebs et al., 1998). Back-driveable robots have low end-point impedance, thus ensuring a gently compliant behavior of the robot when interacting with the subject. Therefore, the machine does not interfere with motion and allows the individual to move freely. Back-driveability is a feature particularly important for rehabilitation robots, and it is not usually found in traditional factory robots (Krebs & Hogan, 2006). The testing protocol consisted of a reaching task and circle-drawing tasks (with and without a circular template). For all robot tasks, the subjects were seated in a chair, centered in front of the robot support table. A waist strap with vertical straps positioned anterior-medially to the shoulders was applied during all training and evaluation sessions to limit/prevent forward trunk compensation without impeding scapular motions (Figure 1). The robot reaching evaluation required the subject to reach from the center target to eight peripheral targets evenly distributed on a circle with radius of 14 cm and moving clockwise or counterclockwise around the circle pattern—neither task with movement assistance. Twenty trials of circle drawing (10 clockwise and 10 counterclockwise) were performed without a template, with subjects instructed to “draw a medium sized circle,” and with instruction on start point, direction, and end point (i.e., “If you think of a clock, begin at 9:00 and trace a medium circle, going clockwise, and end at 9:00.”). A circular template of 14-cm radius was then placed on the tabletop and participants were instructed to “trace the circle” for an additional 20 trials. All subjects performed the tasks in the same order at each session (reaching, circle drawing without template–clockwise/counterclockwise, circle drawing with template–clockwise/counterclockwise).
Figure 1.
Photograph of a participant performing the point-to-point reaching task of the evaluation.
Robotic Outcome Variables
For robotic reaching tasks, movement initiation was defined as the time when the speed first became greater than 2% of the peak speed, and termination was defined when it dropped and remained below the 2% threshold again. Outcome variables assessed for the reaching task were aiming error (mean absolute angle between actual direction and a straight line between start and target), mean speed (total displacement traveled over total movement duration), peak speed, mean-to-peak speed ratio (mean speed divided by the peak speed is a metric of movement smoothness; Rohrer et al., 2002), and movement duration.
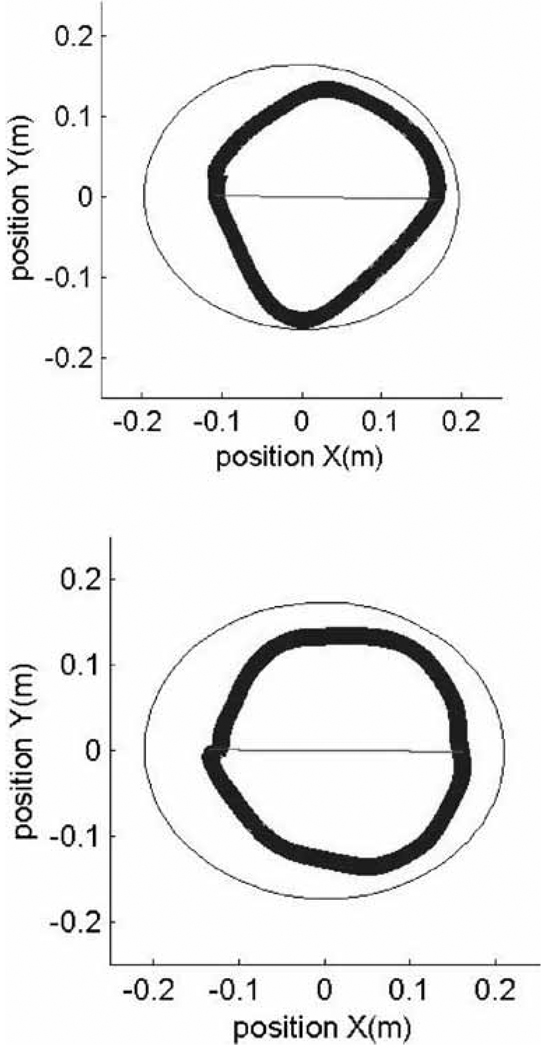
Outcomes for the circle-drawing tasks were an axis ratio metric and shoulder–elbow joint angles correlation metric. The goodness of a circle was characterized by the axis ratio metric, and its computation is described in detail elsewhere (Dipietro et al., 2007). The axis ratio is a value from 0 to 1 and increases as the fitted ellipse approximates a circle (refer to x-position and y-position of Figure 2) (Oliveira et al., 1996). The joint angle correlation metric was based on the correlation between shoulder and elbow angles calculated from the recorded hand paths and a two-link model of the human arm to calculate the inverse kinematics (Yoshikawa, 1990). The model took into account the anthropometric characteristics of the upper extremities of the participants. This metric characterizes the independence of the subject’s shoulder and elbow joint movements and is described in detail elsewhere (Dipietro et al., 2007). The joint correlation is a number between 0 and 1, with lower values indicating greater isolation or independence of the shoulder and elbow joint movements.
Figure 2.
Circle drawing of one representative subject, one trial. Thin circle (outside) calculated ellipse, thicker (inner circle) is the subject’s drawing. Top panel: Circle drawing without template. Bottom panel: Circle drawing with template.
Analysis
Repeated-measures ANOVA (p ≤ .05) was used to determine if differences existed in the functional evaluation measures (Jebsen hand function test; Jebsen et al., 1969), nine-hole peg test (Oxford Grice et al., 2003) and clinical measures (range of motion and strength) between the two baseline tests. Descriptive statistics of mean and standard error of the mean were then calculated (Tables 1 and 2).
Table 1.
Active Range of Motion and Strength of Participants (Mean ± SE)
| Active Range of Motion (degrees) |
Strength (kilograms) |
|||||
|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Mean | Test 1 | Test 2 | Mean | |
| Wrist flexion | 83.30±2.11 | 80.70±2.25 | 82.00±2.16 | 12.50±1.23 | 12.09±1.13 | 12.30±1.15 |
| Wrist extension | 62.40±3.48 | 66.10±3.63 | 64.25±3.51 | 15.05±1.77 | 15.18±1.61 | 15.11±1.65 |
| Elbow flexion | 148.80±1.69 | 147.50±1.63 | 148.15±1.63 | 18.93±1.83 | 18.09±1.78 | 18.51±1.76 |
| Elbow extension | −1.20±1.20 | −1.40±1.40 | −1.30±1.27 | 14.61±1.55 | 14.71±1.44 | 14.66±1.46 |
| Thumb opposition | 2.00± 0.00 | 2.00±0.00 | 2.00±0.00 | 7.80±0.65 | 7.77±0.62 | 7.78±0.62 |
| Shoulder flexion | 153.50±2.68 | 152.90±2.46 | 153.20±2.50 | 16.02±1.76 | 15.39±1.66 | 15.71±1.67 |
| Shoulder extension | 70.80±3.45 | 72.50±2.46 | 71.65±3.34 | 15.25±1.53 | 15.23±2.18 | 15.24±1.83 |
| Shoulder abduction | 167.20±5.73 | 171.3±3.58 | 169.40±4.71 | 14.59±1.33 | 13.64±1.73 | 14.11±1.51 |
| Grip | N/A | N/A | N/A | 27.60±2.62 | 26.80±2.73 | 27.20±2.60 |
Table 2.
Jebsen Hand Function and Nine-Hole Peg Test (n = 10; in Seconds, Mean ± SE)
| Dominant | Nondominant | |||||
|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Mean | Test 1 | Test 2 | Mean | |
| Writing | 10.42±0.15 | 11.34±0.15 | 10.88±0.49 | 27.76±0.56 | 25.85±0.48 | 26.79±1.62 |
| Card turning | 3.97±0.05 | 4.07±0.07 | 4.43±0.14 | 4.35±0.06 | 4.28±0.06 | 4.70±0.15 |
| Small objects | 6.15±0.08 | 6.33±0.11 | 6.47±0.36 | 6.66±0.08 | 7.14±0.16 | 7.31±0.51 |
| Simulated feeding | 7.56±0.14 | 6.92±0.10 | 7.83±0.36 | 8.44±0.11 | 8.17±0.11 | 8.87±0.38 |
| Checkers | 4.68±0.14 | 4.26±0.09 | 4.67±0.28 | 4.99±0.13 | 5.60±0.11 | 5.76±0.42 |
| Large light objects | 3.41±0.03 | 3.44±0.04 | 3.68±0.09 | 3.60±0.04 | 3.50±0.04 | 3.83±0.08 |
| Large heavy objects | 3.41±0.04 | 3.38±0.04 | 3.68±0.10 | 3.64±0.05 | 3.62±0.06 | 4.01±0.13 |
| Nine-hole peg | 17.94±0.16 | 17.62±0.17 | 17.78±0.53 | 19.90±0.34 | 19.50±0.25 | 19.7±0.92 |
Repeated-measures ANOVA (p ≤ .05) determined if differences existed between the six testing sessions. Tukey post hoc analysis was performed with significant F-ratios. To determine the reproducibility of measures (relative reliability), the intraclass correlation coefficient (ICC) was calculated as the ratio of variance between subjects and the total variance (two-way ANOVA model, ICC) (Beckerman et al., 2001): ICC ≥ 0.60 was accepted as fair reliability, ICC ≥ 0.75 as good reliability, and ICC ≥ 0.90 as high reliability (Baumgartner, 1989; Shrout & Fleiss, 1979).
To determine if learning occurred within the initial test session (intrasession) repeated-measures ANOVA (p ≤ .05) was performed on the initial 20 repetitions of circle drawing. Two-way repeated-measures ANOVA (p ≤ .05) determined if differences existed in the axis ratio metric and joint correlation metric between the testing sessions (Tests 1–6) and between the two visual conditions (with and without template). Test–retest reliability (ICC) was evaluated as described above for reaching variables (Baumgartner, 1989; Shrout & Fleiss, 1979)
Results
The clinical measures and functional tests were all stable between the repeated testing sessions (all variables, p ≥ .09). Upper extremity ranges of motion and strength measures for all subjects were found to be within normal limits for healthy adults (Table 1) (Kendall et al., 2005). These values are the outcome goals following rehabilitation of most upper extremity orthopedic disorders. Functional measures were also found to be within ranges of normal healthy adults (Jebsen et al., 1969; Oxford Grice et al., 2003).
Results indicated that the reaching and circle-drawing variables were reliable, without a learning effect occurring within the initial testing session or across numerous testing sessions (Table 3). No difference in any of the remaining variables among any of the six test sessions occurred. All ICC values for reaching task outcomes were good with the circle variables, demonstrating high reliability (Table 3). Both outcome variables for circle drawing were found to be better (higher axis ratio, lower joint correlation) for the template condition (p < .001) as compared with the task without a template.
Table 3.
Robotic Outcome Measures by Test (n = 10, Mean ± SE)
| Reaching Task | Test 1 | Test 2 | Test 3 | Test 4 | Test 5 | Test 6 | Mean | ICC |
|---|---|---|---|---|---|---|---|---|
| Aiming error (rad) | 0.493 (0.023) | 0.484 (0.023) | 0.458 (0.023) | 0.487 (0.023) | 0.512 (0.023) | 0.529 (0.023) | 0.494 (0.026) | 0.87 |
| Mean speed (m/s) | 0.105 (0.007) | 0.112 (0.007) | 0.107 (0.007) | 0.110 (0.007) | 0.121 (0.007) | 0.130 (0.007) | 0.114 (0.004) | 0.86 |
| Peak speed (m/s) | 0.224 (0.018) | 0.234 (0.018) | 0.220 (0.018) | 0.236 (0.018) | 0.264 (0.018) | 0.285 (0.018) | 0.244 (0.010) | 0.86 |
| Mean-peak speed ratio | 0.479 (0.008) | 0.483 (0.008) | 0.497 (0.008) | 0.486 (0.008) | 0.475 (0.008) | 0.471 (0.008) | 0.482 (0.005) | 0.88 |
| Movement duration (s) | 1.582 (0.095) | 1.512 (0.095) | 1.518 (0.095) | 1.527 (0.095) | 1.423 (0.095) | 1.255 (0.095) | 1.470 (0.051) | 0.85 |
| Circle-Drawing Tasks | Test 1 | Test 2 | Test 3 | Test 4 | Test 5 | Test 6 | Mean | ICC |
| Axis ratio without template# | 0.738* (0.009) | 0.771 (0.009) | 0.771 (0.009) | 0.793* (0.009) | 0.757 (0.009) | 0.775 (0.009) | 0.768 (0.004) | 0.98 |
| Axis ratio With template# | 0.840 (0.009) | 0.836 (0.009) | 0.842 (0.009) | 0.825 (0.009) | 0.829 (0.009) | 0.833 (0.009) | 0.834 (0.004) | 0.91 |
| Joint correlation without template## | 0.324 (0.013) | 0.281 (0.013) | 0.280 (0.013) | 0.249 (0.013) | 0.300 (0.013) | 0.262 (0.013) | 0.283 (0.005) | 0.95 |
| Joint correlation with template## | 0.146 (0.013) | 0.153 (0.013) | 0.158 (0.013) | 0.151 (0.013) | 0.131 (0.013) | 0.153 (0.013) | 0.149 (0.013) | 0.92 |
Significant difference Test 1 and Test 4 (p = 0.001).
Significant difference between with and without template conditions (p < 0.001).
Significant difference between with and without template conditions (p < 0.001).
Discussion
As we continue to expand the use of rehabilitation robotics, we must understand the reliability and sensitivity of repeated measurements of repetitive tasks measured by the robot. In particular, it will be important that a positive change recorded can be attributed to impairment reduction and not to a learning effect. In normal unimpaired healthy adults, there was no learning effect due to repeated use of the robot. The highly back-driveable and low-friction MIT-MANUS was “transparent” to the user, showing that the robot dynamics and adaptation, as measured by the specific metrics, was invariant to repeated measurements. In other words, its use did not interfere with the repetitive point-to-point movements and no adaptation or learning was observed. The robotic-derived outcomes are stable in repeated measurements, within and across testing sessions.
Past studies of patients with stroke have used clinical scales as primary outcomes to evaluate roboticassisted intervention (Aisen et al., 1997; Fasoli, Krebs, Ferraro, et al., 2004; Volpe et al., 2000). Future studies will be using the MIT-MANUS and other highly back-driveable low-friction robots as an evaluation tool in patients with musculoskeletal or orthopedic impairments for which the robot measures will be critical. The assessment of robot “transparency” is critical in drawing conclusions regarding the benefits of the robotic interventions based on these robotic evaluations. The findings of this study allow an investigator to conclude that observed changes are due to the particular robotic intervention employed and not due to participants “simply adapting or learning” how to perform the task better with the robot.
In addition to the above findings of no learning effect and sensitivity to change, another application of our results is that our tests were performed in a population that was age-matched to a population of adults with musculoskeletal impairments to be studied in the near future. Thus the current data provides profiles of comparison values for the reaching task variables in unimpaired adults to be used in comparisons to patient populations.
Zelaznik and Lantero reported that a loss of vision resulted in variability of circle size, shape, and location (Zelaznik & Lantero, 1996). Although our conditions were not exactly the same (use of a visual guidance template and without a template versus with vision and removal of vision; Zelaznik & Lantero, 1996), our findings supported our hypothesis that the robot outcomes would be able to detect the change in these conditions. Specifically, the joint correlation, which is a measure of ability to isolate movements of the shoulder and the elbow, was different between the conditions in all tests. This outcome variable and the axis ratio could provide quantification of movement substitutions following injury or surgery for tendon repairs. Contrary to coarse clinical scales, robotic measurements excel in sensitivity with great resolution. Even an apparently small change in the evaluation protocol, as in the presence of a template on the table during the circle-drawing task, can be detected and discriminated by our robotic measurements. This supports the potential for the robotic outcomes to detect changes in advance of clinically measured changes.
In an effort to create a novel task, participants performed the testing using their nondominant limb. Although hand dominance was determined by observation of patients writing and in agreement with questions on hand preference for performance of personal activities (feeding, grooming, and throwing), the lack of using a valid and reliable tool for determination of hand dominance is a limitation within this study. In addition, the small variation in time intervals between testing sessions may have altered the results and in future studies should be tightly controlled to reduce potential bias. The lack of randomization in the template condition is a limitation of the study. Future studies on patients and unimpaired individuals should have a random presentation of the circle drawing conditions.
The outcome measures with a highly back-driveable robot, the MIT-MANUS, are reliable and stable in normal, unimpaired healthy adults when performing the repetitive tasks of reaching and circle drawing. Motor adaptation or learning does not occur over the course of repeated exposure to the testing procedures. The MIT-MANUS-derived robotic metrics are sensitive to even slight changes in protocol, therefore, potentially able to determine changes in advance of clinical measures. Since the clinical scales for orthopedic impairments are limited and these robot outcome measures are shown to be reliable and sensitive, they can be used in future investigations as primary outcomes to evaluate patients with orthopedic conditions. In addition, the present data set serves as a small comparison group to future orthopedic populations.
Acknowledgments
This research was funded by VA Rehabilitation and Development Service Merit grant V512(P)P-521-02, VA Rehabilitation and Development Service Merit grant # B2436T, and VA Rehabilitation and Development Service MS Center of Excellence Grant and Career Development Grant #B3827V from the VA Rehabilitation and Development Service.
Footnotes
Disclosure: Hermano I. Krebs is a co-inventor of the MIT-held patent for the robotic device used in this work and holds equity positions in Interactive Motion Technologies, Inc., a company that manufactures this type of technology under license to MIT.
References
- Aisen ML, Krebs HI, Hogan N, McDowell F, Volpe BT. The effect of robot-assisted therapy and rehabilitative training on motor recovery following stroke. Archives of Neurology. 1997;54(4):443–446. doi: 10.1001/archneur.1997.00550160075019. [DOI] [PubMed] [Google Scholar]
- Baumgartner TA. Norm-referenced measurement: Reliability. In: Safrit MJ, Woods TM, editors. Measurement Concepts in Physical Education and Exercise Science. Champaign, IL: Human Kinetics, Inc.; 1989. pp. 45–56. [Google Scholar]
- Beckerman H, Roebroeck ME, Lankhorst GJ, Becher JG, Bezemer PD, Verbeek AL. Smallest real difference, a link between reproducibility and responsiveness. Quality of Life Research. 2001;10(7):571–578. doi: 10.1023/a:1013138911638. [DOI] [PubMed] [Google Scholar]
- Cook KF, Gartsman GM, Roddey TS, Olson SL. The measurement level and trait-specific reliability of 4 scales of shoulder functioning: an empiric investigation. Archives of Physical Medicine and Rehabilitation. 2001;82(11):1558–1565. doi: 10.1053/apmr.2001.26622. [DOI] [PubMed] [Google Scholar]
- Dipietro L, Krebs HI, Fasoli S, Volpe B, Stein J. Motor Synergies are Tuned During Stroke Recovery. Journal of Neurophysiology. 2007;98(2):757–768. doi: 10.1152/jn.01295.2006. [DOI] [PubMed] [Google Scholar]
- Fasoli SE, Krebs HI, Ferraro M, Hogan N, Volpe BT. Does shorter rehabilitation limit potential recovery poststroke? Neurorehabilitation and Neural Repair. 2004;18(2):88–94. doi: 10.1177/0888439004267434. [DOI] [PubMed] [Google Scholar]
- Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Archives of Physical Medicine and Rehabilitation. 2003;84(4):477–482. doi: 10.1053/apmr.2003.50110. [DOI] [PubMed] [Google Scholar]
- Fasoli SE, Krebs HI, Stein J, Frontera WR, Hughes R, Hogan N. Robotic therapy for chronic motor impairments after stroke: Follow-up results. Archives of Physical Medicine and Rehabilitation. 2004;85(7):1106–1111. doi: 10.1016/j.apmr.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Ferraro M, Palazzolo JJ, Krol J, Krebs HI, Hogan N, Volpe BT. Robot-aided sensorimotor arm training improves outcome in patients with chronic stroke. Neurology. 2003;61(11):1604–1607. doi: 10.1212/01.wnl.0000095963.00970.68. [DOI] [PubMed] [Google Scholar]
- Finley MA, Fasoli S, Dipietro L, Ohlhoff J, MacClellan L, Meister C, et al. Short Duration Robotic Therapy in Stroke Patients with Severe Upper-Limb Impairment. Journal of Rehabilitation Research and Development. 2005;42(5):683–692. doi: 10.1682/jrrd.2004.12.0153. [DOI] [PubMed] [Google Scholar]
- Hogan N, Krebs HI, Sharon A, Charnnarong J. No. 5,466,213. USA Patent. 1995
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Archives of Physical Medicine and Rehabilitation. 1969;50(6):311–319. [PubMed] [Google Scholar]
- Kahn LE, Averbuch M, Rymer WZ, Reinkensmeyer J. Comparison of Robot-Assisted Reaching to Free Reaching in Promoting Recovery from Chronic Stroke. In: Mokhtari M, editor. Integration of Assistive Technology in the Information Age. Amsterdam: IOS Publisher; 2001. [Google Scholar]
- Kendall FP, McCreary EK, Provance PG. Muscles-testing and function. 5th ed. Baltimore: Williams & Wilkins; 2005. [Google Scholar]
- Krebs HI, Hogan N. Therapeutic Robotics: A Technology Push. Proceedings of the IEEE. 2006;94(9):1727–1738. doi: 10.1109/JPROC.2006.880721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs HI, Hogan N, Aisen ML, Volpe BT. Robot-aided neurorehabilitation. IEEE Transactions on Rehabilitation Engineering. 1998;6(1):75–87. doi: 10.1109/86.662623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC. Evidence for improved muscle activation patterns after retraining of reaching movements with the MIME robotic system in subjects with post-stroke hemiparesis. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2004;12(2):186–194. doi: 10.1109/TNSRE.2004.827225. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC, Majmundar M, Van der Loos M. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Archives of Physical Medicine and Rehabilitation. 2002;83(7):952–959. doi: 10.1053/apmr.2001.33101. [DOI] [PubMed] [Google Scholar]
- Michener LA, Leggin BG. A review of self-report scales for the assessment of functional limitation and disability of the shoulder. Journal of Hand Therapy. 2001;14(2):68–76. doi: 10.1016/s0894-1130(01)80036-3. [DOI] [PubMed] [Google Scholar]
- Oliveira LF, Simpson DM, Nadal J. Calculation of are of stabilometric signals using principle component analysis. Physiological Measurement. 1996;17:305–312. doi: 10.1088/0967-3334/17/4/008. [DOI] [PubMed] [Google Scholar]
- Oxford Grice K, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available Nine Hole Peg Test for finger dexterity. The American Journal of Occupational Therapy. 2003;57(5):570–573. doi: 10.5014/ajot.57.5.570. [DOI] [PubMed] [Google Scholar]
- Palazzolo JJ, Ferraro M, Krebs HI, Lynch D, Volpe BT, Hogan N. Stochastic Estimation of Arm Mechanical Impedance During Robotic Stroke Rehabilitation. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2007;15(1):94–103. doi: 10.1109/TNSRE.2007.891392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek JD, Lukens SC, Badalanmenti S, Roubal PJ, Freeman DC, Walleman KM, et al. Shoulder outcome measures: a comparison of 6 functional tests. American Journal of Sports Medicine. 2004;32(5):1270–1277. doi: 10.1177/0363546503262193. [DOI] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Kahn LE, Averbuch M, McKenna-Cole A, Schmit BD, Rymer WZ. Understanding and treating arm movement impairment after chronic brain injury: progress with the ARM guide. Journal of Rehabilitation Research and Development. 2000;37(6):653–662. [PubMed] [Google Scholar]
- Rohrer B, Fasoli S, Krebs HI, Hughes R, Volpe B, Frontera WR, et al. Movement smoothness changes during stroke recovery. The Journal of Neuroscience. 2002;22(18):8297–8304. doi: 10.1523/JNEUROSCI.22-18-08297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmoni AW, Schmidt RA, Walter CB. Knowledge of results and motor learning: a review and reappraisal. Psychological Bulletin. 1984;95:355–386. [PubMed] [Google Scholar]
- Schmidt RA, Lee TD. Motor Control and Learning. 3rd ed. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- Shrout P, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–427. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Krebs HI, Hogan N. Is robot-aided sensorimotor training in stroke rehabilitation a realistic option? Current Opinion in Neurology. 2001;14(6):745–752. doi: 10.1097/00019052-200112000-00011. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Krebs HI, Hogan N, Edelstein OL, Diels C, Aisen M. A novel approach to stroke rehabilitation: robot-aided sensorimotor stimulation. Neurology. 2000;54(10):1938–1944. doi: 10.1212/wnl.54.10.1938. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Krebs HI, Hogan N, Edelsteinn L, Diels CM, Aisen ML. Robot training enhanced motor outcome in patients with stroke maintained over 3 years. Neurology. 1999;53(8):1874–1876. doi: 10.1212/wnl.53.8.1874. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T. Foundations of Robotics. Analysis and Contr. Cambridge: MIT Press; 1990. [Google Scholar]
- Zelaznik HN, Lantero D. The role of vision in repetitive circle drawing. Acta Psychologica. 1996;92(1):105–118. doi: 10.1016/0001-6918(95)00007-0. [DOI] [PubMed] [Google Scholar]