Abstract
Toxoplasma gondii is an obligate intracellular parasite infecting humans and other warm-blooded animals, resulting in serious public health problems and economic losses worldwide. Rhoptries are involved in T. gondii invasion and host cell interaction and have been implicated as important virulence factors. In the present study, a DNA vaccine expressing rhoptry protein 13 (ROP13) of T. gondii inserted into eukaryotic expression vector pVAX I was constructed, and the immune protection it induced in Kunming mice was evaluated. Kunming mice were immunized intramuscularly with pVAX-ROP13 and/or with interleukin-18 (IL-18). Then, we evaluated the immune response using a lymphoproliferative assay, cytokine and antibody measurements, and the survival times of mice challenged with the virulent T. gondii RH strain (type I) and the cyst-forming PRU strain (type II). The results showed that pVAX-ROP13 alone or with pVAX/IL-18 induced a high level of specific anti-T. gondii antibodies and specific lymphocyte proliferative responses. Coinjection of pVAX/IL-18 significantly increased the production of gamma interferon (IFN-γ), IL-2, IL-4, and IL-10. Further, challenge experiments showed that coimmunization of pVAX-ROP13 with pVAX/IL-18 significantly (P < 0.05) increased survival time (32.3 ± 2.7 days) compared with pVAX-ROP13 alone (24.9 ± 2.3 days). Immunized mice challenged with T. gondii cysts (strain PRU) had a significant reduction in the number of brain cysts, suggesting that ROP13 could trigger a strong humoral and cellular response against T. gondii cyst infection and that it is a potential vaccine candidate against toxoplasmosis, which provided the foundation for further development of effective vaccines against T. gondii.
INTRODUCTION
Toxoplasma gondii is an important apicomplexan parasite that can infect nearly all warm-blooded animals and humans. Humans are infected by ingestion of raw or undercooked meat containing tissue cysts or water contaminated with sporulated oocysts from the feces of infected cats or congenitally (3, 15, 20). In addition, toxoplasmosis can cause abortions, stillbirths, and neonatal deaths in all kinds of livestock, resulting in significant economic losses (3). Therefore, development of an effective vaccine against T. gondii will be of great value for the effective control and prevention of human and animal infections with T. gondii, which might also reduce economic losses to the farming industry.
An effective vaccine preventing infection in animals used for human consumption would block the main transmission route to humans (7). Several types of vaccines against toxoplasmosis have been reported, including attenuated and inactivated vaccines and genetically engineered vaccines, but poor immunological efficacy was obtained in the study (5, 14, 22). Although a live attenuated vaccine has been used in some countries, it is inadequate and expensive, may cause side effects, and has the possibility to revert to a pathogenic strain (13). Therefore, we have focused on the development of DNA-based vaccines, hoping to achieve better immunity for preventing toxoplasmosis.
The candidate antigens involved in protective immunity against T. gondii include surface antigens (SAGs), dense granule antigens (GRAs), microneme antigens (MICs), and rhoptry (ROP) antigens. Among the above antigens, ROPs are effector proteins that modulate the host response to the parasite and play a key role in accessing the cytoplasm of host cells (21). Due to the key biological role of rhoptries, rhoptry proteins have recently become vaccine candidates for preventing several parasitic diseases, including toxoplasmosis. ROP13, a novel rhoptry protein, shows no homology to any known proteins, lacks any identifiable domains, and is a soluble protein that is proteolytically processed en route to the rhoptries (1). It is possible to detect ROP13 in the host cell in evacuoles due to the protein's effector functions involved in host response to T. gondii (21). Hence, T. gondii ROP13 may represent a good vaccine candidate.
Selection of potent cytokine adjuvants is vital for the development of T. gondii DNA vaccines. Several potential cytokines have been proven to induce enhanced immune responses in animal models and clinical tests. Murine interleukin-18 (IL-18) is a cytokine with a broad array of effector functions. Murine IL-18 activates natural killer (NK) cells; induces gamma interferon (IFN-γ) production by T cells stimulated with concanavalin A (ConA), anti-CD3 antibodies (Abs), or IL-2; and promotes their proliferation (16, 17). Since NK cells constitutively express the IL-18 receptor (4) and IL-18 is a potent enhancer of NK cell activity and synergizes with IL-12 to stimulate NK cell production of IFN-γ (24, 27), it is a likely candidate to be involved in the regulation of resistance to T. gondii.
Therefore, this study was designed to evaluate the immunogenicity and immunoprotection of T. gondii ROP13 in Kunming mice by constructing a eukaryotic plasmid, pVAX-ROP13, and evaluating the protective immune effect of pVAX-ROP13, as well as coadministration of pVAX-ROP13 with IL-18. Our results indicated that this immunization regimen is useful in enhancing immune protection against the highly virulent RH strain and the intermediately virulent PRU strain of T. gondii.
MATERIALS AND METHODS
Mice and parasites.
Six- to 8-week-old specific-pathogen-free (SPF) female Kunming mice, the most widely used outbred strain in China, were purchased from Yat-Sen University Laboratory Animal Center, Guangzhou, China. All mice were bred under specific-pathogen-free conditions and had free access to a commercial basal diet and tap water ad libitum.
Two T. gondii strains were used: the virulent RH strain (type I) and the brain cyst-forming PRU strain (type II). Tachyzoites of the highly virulent RH strain of T. gondii were preserved in our laboratory and maintained by serial intraperitoneal passage in Kunming mice. To prepare soluble tachyzoite antigens (STAg) for the detection of antibodies, the tachyzoites of the RH strain were collected from the peritoneal fluids, washed by centrifugation, and then suspended in sterile phosphate-buffered saline (PBS) and sonicated. The supernatant was considered STAg and was kept at −80°C until further use.
For the preparation of T. gondii cysts, mice were infected with 20 cysts of the PRU strain. About 1 month after infection, their brains were isolated, homogenized, and diluted in PBS (pH 7.2) in order to obtain 10 tissue cysts in 100 μl.
Construction of the eukaryotic expression plasmid.
To construct the pVAX-ROP13 expression plasmid, the coding sequence of the T. gondii ROP13 gene (GenBank accession no. DQ096560.1), 1,203 bp from sequence positions 1 to 1203, was amplified by PCR from genomic DNA of T. gondii strain RH with a pair of oligonucleotide primers (forward primer, 5′-CGCGGATCCATGAAGAGAACAGAGCTTTG-3′, and reverse primer, 5′-GCTCTAGATCACAATAGCCTCAAGGAATTC-3′), and BamH I and XbaI recognition sites were introduced. The PCR product was cloned in pGEM-T easy vector (Promega) and sequenced in both directions to ensure fidelity, generating pGEM-ROP13. The ROP13 fragment was cleaved by BamH I/XbaI from pGEM-ROP13 and cloned into the BamH I/XbaI sites of pVAX I (Invitrogen). The resulting plasmid was named pVAX-ROP13. The plasmid pVAX/IL-18 was extracted previously in our laboratory (23). The concentrations of pVAX-ROP13 and pVAX/IL-18 were determined using a spectrophotometer at optical densities at 260 and 280 nm (OD260 and OD280).
In vitro pVAX-ROP13 plasmid expression.
Marc-145 cells were transfected with pVAX-ROP13 using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Forty-eight hours after transfection, the cells were fixed with cool acetone for 15 min, and ROP13 expression was detected using the indirect immunofluorescence assay (IFA), with anti-T. gondii polyclonal antiserum (goat) and a fluorescein isothiocyanate (FITC)-labeled rabbit anti-goat IgG (Proteintech Group Inc., Chicago, IL). Evans blue (Fisher) was included in the secondary-antibody solution as a counterstain. Coverslips were rinsed three times with PBS. The monolayers binding the marker were covered with glycerol and examined for specific fluorescence under a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Germany).
Immunization and challenge infection.
Mice were divided into five groups of 40 mice per group. The mice were injected intramuscularly with 100 μg of plasmid DNA suspended in 100 μl sterile PBS (100 μl in each thigh skeletal muscle), whereas control mice received PBS alone. The mice in groups I and II were injected with PBS and empty pVAX I vector, respectively, as controls; the mice in group III and group V were injected with pVAX-ROP13 alone (100 μg) or combined with pVAX/IL-18 (50 μg/each) and those in group IV with pVAX/IL-18. The mice in all groups were vaccinated three times at weeks 0, 2, and 4.
Two weeks after the final immunization, 17 mice in all groups were challenged intraperitoneally (i.p.) with 1 × 103 tachyzoites of the virulent T. gondii RH strain, and the other 17 mice were inoculated orally with 10 cysts of the PRU strain. The mice were observed daily for mortality. One month after the challenge, the surviving mice were sacrificed and their brains were removed. Each brain was homogenized in 2 ml of PBS. The mean number of cysts per brain was determined by counting in three samples of 25-μl aliquots of each homogenized brain under an optical microscope. Blood was collected from the mouse tail vein before each immunization. Sera were separated and stored at −20°C for enzyme-linked immunosorbent assay (ELISA).
Antibody assays.
Antigen-specific antibody levels were measured with an anti-T. gondii IgG ELISA kit according to the manufacture's instructions (Combined Biotech Co., Ltd., Shenzhen, China). In brief, plates were coated overnight at 4°C with 1 μg of STAg in 100 μl of carbonate buffer, pH 9.2. The blood of three mice was collected from the mouse tail vein, and the sera diluted with PBS were applied to the wells; then, the bound antibodies were detected with horseradish peroxidase-conjugated anti-mouse IgG diluted 1:2,000. Immune complexes were revealed by incubation with orthophenylene diamine and 0.15% H2O2 for 30 min. The reaction was stopped by the addition of 1 M H2SO4, and the absorbance was measured at 450 nm using an ELISA reader (Bio-TekEL × 800). All samples were run in triplicate.
Lymphoproliferation assay.
Briefly, splenocyte suspensions were prepared from each of the three mice per group by pushing the spleens through a wire mesh 1 day before the challenge. After the red blood cells (RBC) were removed using RBC lysis solution (Sigma), splenocytes were resuspended in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). Cells were then plated in 96-well Costar plates at a density of 5 × 105 cells per well and cultured with STAg (10 μg/ml) or ConA (5 μg/ml; Sigma) or medium alone (negative control) at 37°C with 5% CO2. The proliferative activity was measured using a 3-(4,5-dimethylthylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/ml; Sigma) dye assay (16). The stimulation index (SI) was calculated as the ratio of the average OD570 value of wells containing antigen-stimulated cells to the average OD570 value of wells containing only cells with medium. All assays were performed in triplicate.
Cytokine assays.
To evaluate cytokine production, splenocytes from three immunized mice were cultured with different stimuli as described for the lymphoproliferation assay 2 weeks after the last immunization. Cell-free supernatants were harvested and assayed for IL-2 and IL-4 activities at 24 h, for IL-10 activity at 72 h, and for IFN-γ activity at 96 h. The IL-2, IL-4, IL-10, and IFN-γ concentrations were evaluated using a commercial ELISA kit according to the manufacturer's instructions (R&D Systems). Cytokine concentrations were determined by reference to standard curves constructed with known amounts of mouse recombinant IFN-γ, IL-2, IL-4, or IL-10. The sensitivity limits for the assays were 20 pg/ml for IFN-γ, 50 pg/ml for IL-2, and 10 pg/ml for IL-4 and IL-10.
Statistical analysis.
Data, including antibody responses, lymphoproliferation assays, and cytokine production, were compared between the different groups by one-way analysis of variance (ANOVA). All data were processed by analysis with SPSS13.0 Data Editor (SPSS Inc., Chicago, IL). P values of <0.05 were considered to be statistically significant.
RESULTS
Identification of the expressed product by IFA.
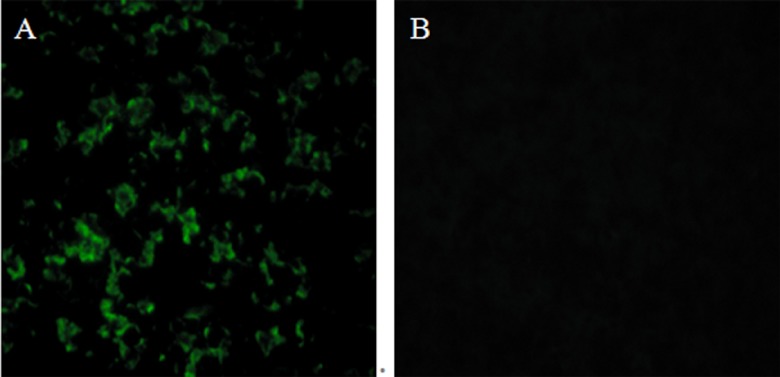
In vitro expression of pVAX-ROP13 was evaluated by IFA at 48 h posttransfection. Green fluorescence was observed in Marc-145 cells transfected with pVAX-ROP13, whereas no fluorescence was observed in the pVAX I-transfected cells using anti-T. gondii polyclonal antiserum (Fig. 1). This result showed that ROP13 protein was expressed by pVAX-ROP13 in Marc-145 cells.
Fig 1.
IFA detection of T. gondii ROP13 on Marc-145 cells. The Marc-145 cells were previously transfected with pVAX-ROP13 (A) and the empty vector (B).
Humoral response.
To determine the level of anti-T. gondii antibodies, all sera were tested by ELISA. Figure 2 shows that a significantly high level of IgG antibodies was detected in the sera of mice immunized with pVAX-ROP13 and pVAX-ROP13 plus pVAX/IL-18, especially after the third immunization (the level of antibodies increased with successive immunizations). In contrast, mice injected with PBS, pVAX I, or pVAX/IL-18 alone did not generate anti-TgROP13 antibodies (Fig. 2). There were significant differences between the five groups (P < 0.05).
Fig 2.
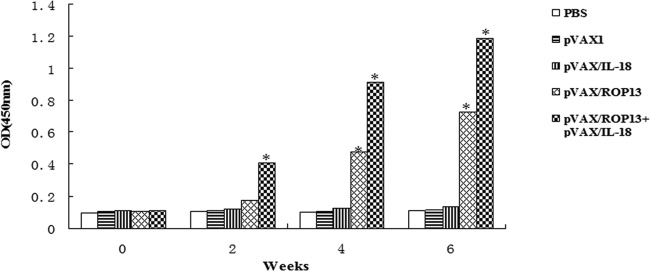
Determination of specific anti-ROP13 antibody titers in the sera of Kunming mice immunized with 100 μl of PBS; 100 μg of pVAX I, pVAX I/IL-18, or pVAX-ROP13 alone; or 50 μg of pVAX I/IL-18 and pVAX-ROP13 at weeks 0, 2, 4, and 6. Sera were collected 1 day prior to each immunization and tested by ELISA using STAg. The results are shown as means of three independent experiments. Statistically significant differences (P < 0.05) are indicated by asterisks.
Cellular proliferative response induced by DNA vaccination.
To measure the splenocyte proliferative response, the splenocytes from three mice immunized with PBS, pVAX I, or pVAX/IL-18 alone or combined with ROP13 were prepared 2 weeks after the third immunization to assess the proliferative immune responses to ROP13. As shown in Table 1, the two groups immunized with pVAX-ROP13 and without pVAX/IL-18 increased lymphocyte response compared with the control (P < 0.05). When the mice were coinjected with pVAX/IL-18, the level of splenocyte proliferation was further increased (P < 0.05). In addition, splenocytes from all experimental and control groups proliferated to comparable levels in response to the mitogen ConA (data not shown).
Table 1.
Splenocyte proliferation responses of mice immunized with plasmids pVAX-ROP13, pVAX/IL-18, and pVAX-ROP13 plus pVAX/IL-18
| Group | Mean stimulation index ± SD |
|---|---|
| pVAX-ROP13 + pVAX/IL-18 | 3.66 ± 0.12a |
| pVAX-ROP13 | 1.58 ± 0.05a |
| pVAX/IL-18 | 1.54 ± 0.04b |
| pVAX I | 0.19 ± 0.15b |
| PBS | 0.21 ± 0.03c |
Results were statistically significantly different from the PBS group (P < 0.001).
Results were statistically significantly different from the PBS group (P < 0.01).
Results were statistically significantly different from the PBS group (P < 0.05).
Cytokine production.
The supernatants of splenocytes isolated from three mice immunized with pVAX I alone or combined with pVAX-ROP13 were harvested at different times after restimulation with STAg and assessed for the production of IFN-γ, IL-2, IL-4, and IL-10 (Table 2). Very large amounts of specific IFN-γ and IL-2 were produced in the supernatants of restimulated splenocyte cultures from mice immunized with pVAX-ROP13 plus pVAX/IL-18, pVAX-ROP13, and pVAX/IL-18, but no specific production of IFN-γ was observed in other groups (PBS and pVAX I groups). Specific amounts of IL-4 and IL-10 were also synthesized by the restimulated splenocytes from the mice immunized with pVAX-ROP13 and pVAX-ROP13 plus pVAX/IL-18. There were no significant differences in the production of IL-4 or IL-10 between the pVAX/IL-18 group and the other groups immunized with pVAX I or PBS (P < 0.05).
Table 2.
Cytokine production by splenocytes of immunized Kunming mice after stimulation by STAga
| Group | Cytokine production (pg/ml)b |
|||
|---|---|---|---|---|
| IFN-γ | IL-2 | IL-4 | IL-10 | |
| pVAX-ROP13 + pVAX/IL-18 | 1,107.28 ± 26.74A | 934.52 ± 13.03A | 257.54 ± 4.17A | 246.02 ± 10.61A |
| pVAX-ROP13 | 826.75 ± 18.91B | 793.07 ± 22.09B | 163.23 ± 6.05B | 160.49 ± 3.14B |
| pVAX/IL-18 | 671.39 ± 30.82C | 367.26 ± 21.89C | 71.62 ± 9.81C | 34.59 ± 11.46C |
| pVAX I | 52.33 ± 13.14D | 48.67 ± 8.32D | 52.00 ± 19.48C | 53.64 ± 9.25C |
| PBS | 47.63 ± 4.34D | 46.33 ± 10.04D | 50.72 ± 5.73C | 51.13 ± 3.93C |
Splenocytes from mice were harvested 2 weeks after the final immunization.
Values for IFN-γ are for 96 h, values for IL-2 and IL-4 are for 24 h, and values for IL-10 are for 72 h. Values followed by the same letter showed no significant difference (P > 0.05), whereas different letters indicate significant differences (P < 0.05) in the same column.
Protection of mice against challenge with T. gondii strains RH and PRU.
To assess protective immunity, mice were challenged with T. gondii strains RH and PRU 2 weeks after the final immunization. Mortality was checked daily under challenge with T. gondii strain RH. The survival percentages of the different groups of mice are shown in Fig. 3. Mice immunized with PBS or pVAX I died before the 10th day. Immunization of Kunming mice with pVAX-ROP13 increased the survival time (24.9 ± 2.3 days; P < 0.05 versus controls) of these mice following infection with T. gondii. Coimmunization with pVAX-ROP13 and pVAX I/IL-18 enhanced the survival time of the immunized mice (32.3 ± 2.7 days; P < 0.05) (Fig. 3). The group challenged with PRU strain cysts had a significant reduction in the number of brain cysts (P < 0.05) (Table 3).
Fig 3.
Survival curves of immunized Kunming mice after lethal challenge with 1 × 103 tachyzoites of T. gondii strain RH 2 weeks after the last immunization.
Table 3.
Brain cyst reduction rate of mice immunized with plasmids pVAX-ROP13, pVAX/IL-18, and pVAX-ROP13 plus pVAX/IL-18
| Group | Cyst count (±SE)a | Rate of reduction (%) |
|---|---|---|
| pVAX-ROP13 + pVAX/IL-18 | 1,051 ± 56.0A | 66.03 |
| pVAX-ROP13 | 1,862 ± 12.50B | 39.82 |
| pVAX/IL-18 | 2,580 ± 40.62C | 16.61 |
| pVAX I | 2,972 ± 53.52D | 0.39 |
| PBS | 3,094 ± 68.83D |
Values followed by the same letter showed no significant difference (P > 0.05), whereas different letters indicate significant differences (P < 0.05).
DISCUSSION
In this study, we used a high-biosafety plasmid, pVAX I, as the vector. As one of the members of the rhoptry family, the T. gondii ROP13 gene was cloned into the same mammalian expression backbone (pVAX I), designated pVAX-ROP13. The eukaryotic expression system was used in our study, thus avoiding the problems of incorrect folding or lack of some posttranslational modifications due to prokaryotic expression of recombinant proteins. Consequently, conformational epitopes of the antigen should be effectively bound with antibody. By IFA analysis, we demonstrated that the FITC-labeled secondary antibody can react with ROP13 expressed in cells (Fig. 1).
In the past few decades, many T. gondii antigens have been assessed as potential vaccine candidates against toxoplasmosis, including the surface antigens (SAG1) (9), dense granule proteins (GRA1 to GRA7) (6), microneme proteins (MIC1, MIC6, and MIC8) (8, 10, 18), rhoptry antigens (ROP2, ROP16, and ROP18) (22, 25, 26), and matrix protein MAG1 (2). However, there had been no reported studies evaluating the immunogenicity of T. gondii ROP13. Therefore, we constructed the pVAX-ROP13 plasmid, and the results showed that the serum anti-ROP13 IgG titers were high. Significant differences were observed between the serum antibody titers for the five groups (P < 0.05) (Fig. 2).
As a potent IFN-γ-inducing factor, IL-18 can induce production of IFN-γ by T cells and enhance NK cell cytolytic activity and immunity to tumors and infection (4, 12). Similar to IL-12, the dominant functions of IL-18 facilitate Th1 immune responses (11). Therefore, we tested IL-18 as an adjuvant to improve the immunogenicity of T. gondii ROP13 plasmid DNA vaccine against T. gondii infection in mice. Coadministration of pVAX-ROP13 with IL-18 could enhance the immune response and the survival time of immunized mice (Fig. 3). This was consistent with the results of previous studies that used IL-18 as an adjuvant in DNA vaccines, enhancing the development of Th1-driven antigen-specific T helper and cytolytic immune responses (9, 19). Therefore, IL-18 appears to be a broadly effective Th1 adjuvant that could be useful in the development of vaccines against toxoplasmosis.
The present study evaluated for the first time the immunogenicity and protective potency of a DNA vaccine expressing T. gondii ROP13. Our results demonstrated that the protection afforded to Kunming mice by injection of pVAX-ROP13 with or without pVAX/IL-18 increased the survival rate (P < 0.05) compared to untreated mice or those injected with PBS or pVAX I. Moreover, mice immunized with pVAX-ROP13 can effectively reduce the number of brain cysts (P < 0.05) compared to the control groups, demonstrating the efficacy of ROP13 vaccine against chronic T. gondii infection. The survival time of mice immunized with pVAX-ROP13 (24.9 ± 2.3 days) is a little shorter than that of mice immunized with ROP18 (27.9 ± 15.1 days) but is longer than those of mice immunized with MIC6 (13.3 ± 1.2 days) and ROP16 (21.6 ± 9.9 days) (18, 25, 26).
Taken together, these results suggest that ROP13 DNA vaccine induced strong protective humoral and cellular responses against T. gondii, indicating that it has the potential to be a vaccine candidate worthy of further development. The use of an IL-18-encoding plasmid as an adjuvant successfully enhanced the immune protection and survival time of immunized mice. Further studies are warranted to evaluate the immune efficacy of this DNA vaccine construct in other animal host species against toxoplasmosis.
ACKNOWLEDGMENTS
This study was supported in part by grants from the National Program for High Technology Research (2011AA10A215); the Project of Science and Technology New Star of Zhu Jiang (2011J2200100); the National Natural Science Foundation of China (30901067, 31230073, 31172316, 31228022, and 31101812); the Open Funds of the State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (SKLVEB2009KFKT014, SKLVEB2010KFKT010, and SKLVEB2011KFKT004); the Scientific and Technological Planning Project of Guangdong Province (grant no. 2010B020307006); the Specialized Research Fund for the Doctoral Program of Higher Education (grant no. 20094404120016); and the Yunnan Provincial Program for Introducing High-Level Scientists (grant no. 2009CI125).
Footnotes
Published ahead of print 26 September 2012
REFERENCES
- 1.Bradley PJ, et al. 2005. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 280:34245–34248 [DOI] [PubMed] [Google Scholar]
- 2.Di Cristina M, et al. 2004. The Toxoplasma gondii bradyzoite antigens BAG1 and MAG1 induce early humoral and cell-mediated immune responses upon human infection. Microbes Infect. 6:164–171 [DOI] [PubMed] [Google Scholar]
- 3.Dubey JP. 2010. Toxoplasmosis of animals and humans, 2nd ed CRC Press Inc., Boca Raton, FL [Google Scholar]
- 4.Hyodo Y, et al. 1999. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J. Immunol. 162:1662–1668 [PubMed] [Google Scholar]
- 5.Innes EA, Vermeulen AN. 2006. Vaccination as a control strategy against the coccidial parasites Eimeria, Toxoplasma and Neospora. Parasitology 133(Suppl.):S145–S168 [DOI] [PubMed] [Google Scholar]
- 6.Jongert E, et al. 2008. An enhanced GRA1-GRA7 cocktail DNA vaccine primes anti-Toxoplasma immune responses in pigs. Vaccine 26:1025–1031 [DOI] [PubMed] [Google Scholar]
- 7.Kur J, Holec-Gasior L, Hiszczyńska-Sawicka E. 2009. Current status of toxoplasmosis vaccine development. Expert Rev. Vaccines 8:791–808 [DOI] [PubMed] [Google Scholar]
- 8.Liu MM, et al. 2010. Toxoplasma gondii microneme protein 8 (MIC8) is a potential vaccine candidate against toxoplasmosis. Parasitol. Res. 106:1079–1084 [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, et al. 2010. The protective effect of a Toxoplasma gondii SAG1 plasmid DNA vaccine in mice is enhanced with IL-18. Res. Vet. Sci. 89:93–97 [DOI] [PubMed] [Google Scholar]
- 10.Lourenco EV, et al. 2006. Immunization with MIC1 and MIC4 induces protective immunity against Toxoplasma gondii. Microbes Infect. 8:1244–1251 [DOI] [PubMed] [Google Scholar]
- 11.Marshall DJ, et al. 2006. Interleukin-18 enhances Th1 immunity and tumor protection of a DNA vaccine. Vaccine 24:244–253 [DOI] [PubMed] [Google Scholar]
- 12.Mastroeni P, et al. 1999. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect. Immun. 67:478–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateus-Pinilla NE, Dubey JP, Choromanski L, Weigel RM. 1999. A field trial of the effectiveness of a feline Toxoplasma gondii vaccine in reducing T. gondii exposure for swine. J. Parasitol. 85:855–860 [PubMed] [Google Scholar]
- 14.Mishima M, et al. 2002. Recombinant feline herpesvirus type 1 expressing Toxoplasma gondii ROP2 antigen inducible protective immunity in cats. Parasitol. Res. 88:144–149 [DOI] [PubMed] [Google Scholar]
- 15.Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976 [DOI] [PubMed] [Google Scholar]
- 16.Okamura H, et al. 1995. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect. Immun. 63:3966–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamura H, et al. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378:88–91 [DOI] [PubMed] [Google Scholar]
- 18.Peng GH, et al. 2009. Toxoplasma gondii microneme protein 6 (MIC6) is a potential vaccine candidate against toxoplasmosis in mice. Vaccine 27:6570–6574 [DOI] [PubMed] [Google Scholar]
- 19.Salagianni M, Wong KL, Thomas MJ, Noble A, Kemeny DM. 2007. An essential role for IL-18 in CD8 T cell-mediated suppression of IgE responses. J. Immunol. 178:4771–4778 [DOI] [PubMed] [Google Scholar]
- 20.Tenter AM, Heckeroth AR, Weiss LM. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turetzky JM, Chu DK, Hajagos BE, Bradley PJ. 2010. Processing and secretion of ROP13: a unique Toxoplasma effector protein. Int. J. Parasitol. 40:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, et al. 2007. Immune response induced by recombinant Mycobacterium bovis BCG expressing ROP2 gene of Toxoplasma gondii. Parasitol. Int. 56:263–268 [DOI] [PubMed] [Google Scholar]
- 23.Wei F, et al. 2008. Enhancement by IL-18 of the protective effect of a Schistosoma japonicum 26 kDa GST plasmid DNA vaccine in mice. Vaccine 26:4145–4149 [DOI] [PubMed] [Google Scholar]
- 24.Wei XQ, et al. 1999. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J. Immunol. 163:2821–2828 [PubMed] [Google Scholar]
- 25.Yuan ZG, et al. 2011. Protective immunity induced by Toxoplasma gondii rhoptry protein 16 against toxoplasmosis in mice. Clin. Vaccine Immunol. 18:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan ZG, et al. 2011. Protective effect against toxoplasmosis in mice induced by DNA immunization with gene encoding Toxoplasma gondii ROP18. Vaccine 29:6614–6619 [DOI] [PubMed] [Google Scholar]
- 27.Zhang T, et al. 1997. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect. Immun. 65:3594–3599 [DOI] [PMC free article] [PubMed] [Google Scholar]