Abstract
Preparedness against an A/H5N1 influenza pandemic requires well-tolerated, effective vaccines which provide both vaccine strain-specific and heterologous, cross-clade protection. This study was conducted to assess the immunogenicity and safety profile of an MF59-adjuvanted, prepandemic influenza vaccine containing A/turkey/Turkey/01/2005 (H5N1) strain viral antigen. A total of 343 participants, 194 adults (18 to 60 years) and 149 elderly individuals (≥61 years), received two doses of the investigational vaccine given 3 weeks apart. Homologous and heterologous antibody responses were analyzed by hemagglutination inhibition (HI), single radial hemolysis (SRH), and microneutralization (MN) assays 3 weeks after administration of the first vaccine dose and 3 weeks and 6 months after the second dose. Immunogenicity was assessed according to European licensure criteria for pandemic influenza vaccines. After two vaccine doses, all three European licensure criteria were met for adult and elderly subjects against the homologous vaccine strain, A/turkey/Turkey/1/2005, when analyzed by HI and SRH assays. Cross-reactive antibody responses were observed by HI and SRH analyses against the heterologous H5N1 strains, A/Indonesia/5/2005 and A/Vietnam/1194/2004, in adult and elderly subjects. Solicited local and systemic reactions were mostly mild to moderate in severity and occurred less frequently in the elderly than in adult vaccinees. In both adult and elderly subjects, MF59-adjuvanted vaccine containing 7.5 μg of A/Turkey strain influenza virus antigen was highly immunogenic, well tolerated, and able to elicit cross-clade, heterologous antibody responses against A/Indonesia and A/Vietnam strains 6 weeks after the first vaccination.
INTRODUCTION
Avian A/H5N1 influenza remains a potential pandemic threat to humans worldwide. Since the reemergence of the virus in 2003, bird populations across Asia, Africa, the Middle East, and Europe have been affected (38). At the time of writing, a total of 604 human cases of avian influenza disease had been reported to the World Health Organization, and 357 of those cases were fatal (36). Ongoing efforts to protect the human population against A/H5N1 influenza are essential. Vaccination is a highly effective and financially viable method of disease control and is, therefore, a key element of current international prepandemic preparedness strategy (37).
Due to viral evolution and antigenic shift, the exact subclade of virus responsible for any future pandemic cannot accurately be predicted. Therefore, an adequate prepandemic vaccine must induce the production of cross-reactive antibodies able to provide the individual with a degree of heterologous, cross-clade immunity. Several clinical trials of A/H5N1 vaccines containing A/Vietnam/1194/2004 strain antigen have shown that, as well as decreasing the amount of antigenic material required per dose (7), the oil-in-water adjuvant MF59 (Novartis Vaccines and Diagnostics) increases the production of cross-reactive, neutralizing antibodies (13, 14, 18–20, 24, 28). The ability of MF59 to enhance antigen-specific and cross-reactive antibody responses has been demonstrated in vaccinees of all ages, including the elderly (2, 12, 33, 34) and other high-risk populations (1, 8, 9, 17, 22, 30, 39).
This open-label clinical trial was the first to evaluate immunogenicity and safety profiles in response to MF59-adjuvanted influenza vaccine containing clade 2 A/H5N1 viral strain antigen (A/turkey/Turkey/01/2005). Vaccine antigen-specific and cross-reactive antibody responses were assessed in healthy adult and elderly subjects by hemagglutination inhibition (HI), single radial hemolysis (SRH), and microneutralization (MN) assays 3 weeks after immunization according to the European licensure criteria for pandemic influenza vaccines.
MATERIALS AND METHODS
Study design and objectives.
The trial registration number was NCT00841646 (www.clinicaltrials.gov). This phase II, open-label trial was conducted at two study sites in Hungary between December 2008 and November 2009. The study protocol was approved by the institutional review board of each institution, and the trial was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all participants prior to enrollment. Healthy adult and elderly subjects were enrolled to receive two vaccine doses given 3 weeks apart. The main exclusion criteria were receipt of any A/H5N1 influenza vaccine or any investigational agent 4 weeks prior to enrollment, acute illness requiring systemic antibiotic or antiviral therapy within 1 week prior to enrollment, receipt of any vaccine 3 weeks before enrollment, hypersensitivity to any vaccine component, an impaired or altered immune system, pregnancy, an axillary temperature of ≥38°C within 3 days prior to enrollment, and a body mass index of >35 kg/m2. The primary objective of this study was to evaluate homologous antibody responses against the clade 2 vaccine strain A/turkey/Turkey/01/2005 (H5N1) in adult and elderly subjects, according to European licensure criteria established by the European Committee for Medicinal Products for Human Use (CHMP) (10). The secondary objective of this study was the assessment of cross-reactive antibody responses.
Vaccine.
One 0.5-ml dose of the investigational, inactivated, egg-derived, MF59-adjuvanted, prepandemic vaccine contained 7.5 μg of A/turkey/Turkey/1/2005 (H5N1; clade 2.2.1) influenza virus strain hemagglutinin surface antigen and a standard dose (9.75-mg squalene) of MF59 adjuvant, as found in the European licensed seasonal influenza vaccine Fluad (Novartis Vaccines and Diagnostics). Vaccine was supplied in prefilled monodose (0.5 ml) syringes and administered in the deltoid muscle of the nondominant arm.
Immunogenicity assessment.
Blood samples (∼20 ml per sample) were collected for immunogenicity analysis at baseline (day 1), 3 weeks after administration of the first vaccine dose (day 22), and 3 weeks (day 43) and approximately 6 months (day 202) after administration of the second dose. Serum aliquots were stored at −18°C and shipped to the Novartis Vaccines Clinical Serology Laboratory in Marburg, Germany, and the Department of Physiopathology, Experimental Medicine and Molecular Epidemiology, University of Siena, Siena, Italy. Antibody levels were determined by hemagglutination inhibition (HI) and microneutralization (MN) assays in Marburg and by single radial hemolysis (SRH) in Siena. The HI assay was performed using horse erythrocytes and based on methods described by Stephenson and colleagues (29); HI titer is expressed as the reciprocal of the highest dilution at which hemagglutination was totally inhibited. MN assays were performed according to methods described by Nicholson and colleagues (23); serial dilutions of serum started at 10; the reciprocals of 2-fold dilutions that achieved ≥50% neutralization of viral growth were considered to be a positive result. The protocol for SRH was based on methods described by Schild and colleagues (27). Seroconversion in the HI assay was defined as a negative prevaccination antibody titer of <10 to a positive postvaccination titer of ≥40; in the MN assay, a titer of <20 becoming ≥40; and in the SRH assay, an area of ≤4 mm2 becoming ≥25 mm2. A significant increase in antibody titer in HI and MN assays was defined as a ≥4-fold increase and in the SRH assay as a ≥50% increase in area. HI and MN titers below the detection limits of 10 and 20, respectively, were arbitrarily assigned to half that limit for the purpose of analysis. SRH areas below the detection limit were given a value of 4 mm2. Homologous antibody titers were measured by HI, SRH, and MN assays against the vaccine antigen strain A/turkey/Turkey/1/2005. Cross-reactive antibody titers were measured by HI, SRH, and MN assays against the heterologous H5N1 strains A/Vietnam/1194/2004 (clade 1) and A/Indonesia/5/2005 (clade 2.1).
Safety assessment.
Subjects were observed for a minimum of 30 min after vaccine administration to monitor for possible immediate reactions. Vaccinees were provided with diary cards and asked to record any specified local or systemic reaction occurring within 1 week of vaccination. Solicited local reactions were pain at the site of injection, erythema, induration, swelling, and ecchymosis. Solicited systemic reactions were fever (≥38°C), chills, malaise, myalgia, arthralgia, headache, sweating, nausea, fatigue, vomiting, and diarrhea. Unsolicited adverse events (AEs) were recorded for 3 weeks after each vaccination. All serious adverse events (SAEs) and AEs requiring the attention of a physician or leading to withdrawal were recorded throughout the study period (days 1 to 202). The investigator used a standard scale to grade AEs, which were defined as mild, moderate, or severe if resulting in no limitation of, some limitation of, or an inability to perform normal daily activities, respectively.
Statistical analyses.
There was no formal statistical hypothesis tested. Immunogenicity endpoints were based on the following HI (CHMP) licensure criteria: the number of subjects achieving seroconversion or significantly increased antibody titers should be >40% and >30% for adult and elderly subjects, respectively; geometric mean ratios (GMRs) should be >2.5 for adults and >2.0 for the elderly; and for seroprotection, the proportion of subjects achieving an HI titer of ≥40 or an SRH area of >25 mm2 should be >70% and >60% for adults and the elderly, respectively. Log10-transformed geometric mean titer (GMT), geometric mean area (GMA), and GMR values generated by HI, MN, and SRH assays were determined and compared using an analysis of variance (ANOVA) model containing a factor for vaccine group and study center. Safety data were evaluated descriptively, and the frequency and severity of AEs were summarized.
RESULTS
A total of 343 healthy volunteers participated, 194 adults (18 to 60 years) and 149 elderly (≥61 years) subjects, of whom 99% completed the study (Fig. 1). All participants were Caucasian. Adult and elderly subject groups were of similar weights and heights. More elderly (43%) than adult (20%) subjects had previously received influenza vaccine (Table 1). The Full Analysis (FAS) and Per Protocol (PPS) data sets differed by <10%; PPS immunogenicity data are reported throughout.
Fig 1.
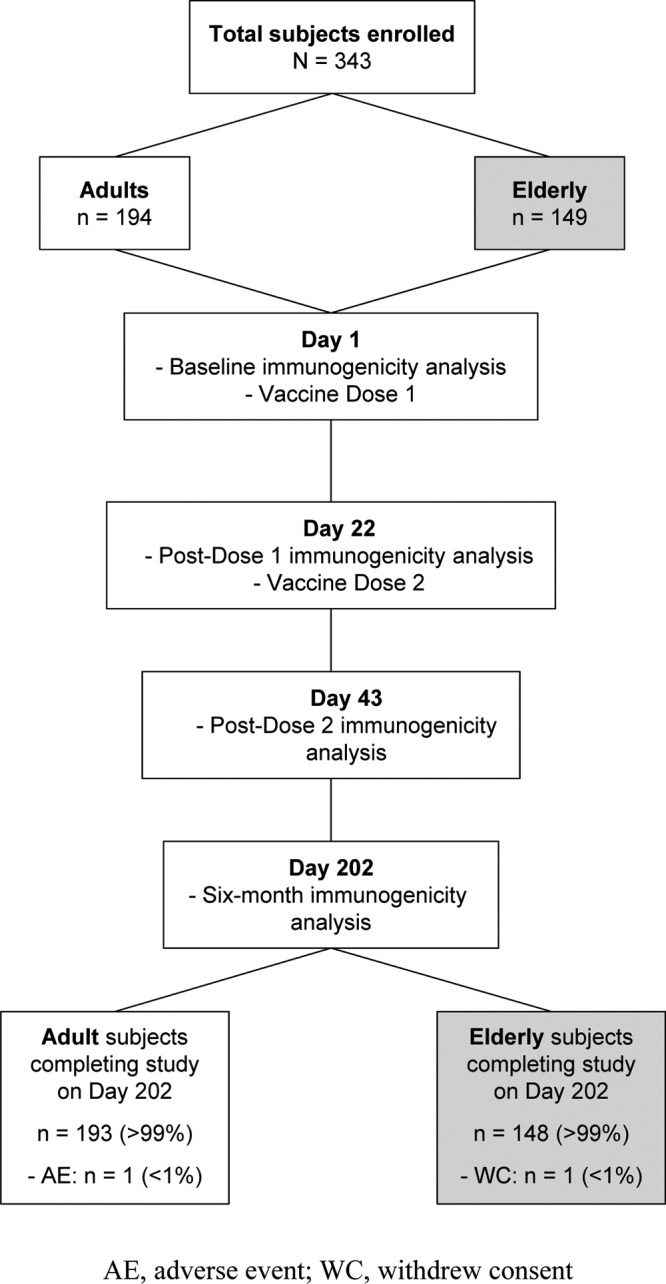
Study design and subject disposition. A total of 343 adult and elderly subjects were enrolled in the study. All subjects received two vaccine doses administered 3 weeks apart. Vaccine antigen-specific and cross-reactive antibody responses were analyzed 3 weeks after receipt of the first vaccine dose and 3 weeks and ∼6 months after the second dose.
Table 1.
Study population demographics
| Characteristic | Adults (n = 194) | Elderly (n = 149) |
|---|---|---|
| Age (yr, mean ± SD) | 41.1 ± 13.1 | 68.4 ± 6.5 |
| Male (%) | 55 | 42 |
| Caucasian (%) | 100 | 100 |
| Weight (kg, mean ± SD) | 78.5 ± 15.4 | 75.5 ± 13.0 |
| Height (cm, mean ± SD) | 172.0 ± 10.1 | 166.0 ± 9.3 |
| Previous seasonal influenza vaccine (%) | 20 | 43 |
Immunogenicity analysis.
Antibody responses against the vaccine strain antigen (A/turkey/Turkey/1/2005) are shown in Table 2. After the first vaccine dose (day 22), there were few licensure criteria met in either group in any of the three assays—specifically, elderly subjects met the licensure criterion for seroconversion by HI assay (32%); adults achieved GMRs of 2.6 and 2.5 in HI and SRH, respectively; and elderly subjects had an HI GMR of 2.8. After the second vaccine dose (day 43), adult and elderly groups met all three CHMP licensure criteria. Adult and elderly groups achieved HI seroconversion rates of 69% and 62% and SRH seroconversion rates of 85% and 70%, respectively. The CHMP criterion for GMR was met in both age groups by HI and SRH analyses. Adult subjects met the criterion for seroprotection by SRH alone (91%), while both HI (64%) and SRH (82%) assays found elderly subjects to be seroprotected. These data were supported by MN analyses throughout.
Table 2.
Immunogenicity analysis against the vaccine strain A/turkey/Turkey/1/2005 (H5N1)a
| Assay (no. of adults/no. of elderly) | Parameter | Day(s) | Value (95% CI) |
|
|---|---|---|---|---|
| Adults | Elderly | |||
| HI (194/148) | GMT | 1 | 5.5 (5.1–5.9) | 5.9 (5.3–6.5) |
| 22 | 14 (11–18) | 17 (13–22) | ||
| 43 | 107 (79–145) | 61 (44–82) | ||
| 202 | 11 (9.1–13) | 11 (9.1–14) | ||
| GMR | 22/1 | 2.6 (2.1–3.3) | 2.8 (2.2–3.6) | |
| 43/1 | 19 (14–26) | 10 (7.5–14) | ||
| 202/1 | 2.0 (1.7–2.4) | 1.9 (1.5–2.3) | ||
| SP (%) | 1 | 3.1 (1.1–6.6) | 4.7 (1.9–9.5) | |
| 22 | 29 (23–36) | 35 (27–43) | ||
| 43 | 70 (63–77) | 64 (56–72) | ||
| 202 | 24 (18–31) | 23 (16–31) | ||
| SC (%) | 22 | 28 (22–35) | 32 (24–40) | |
| 43 | 69 (62–76) | 62 (54–70) | ||
| 202 | 22 (16–28) | 20 (14–28) | ||
| SRH (187/143) | GMA | 1 | 7.4 (6.6–8.3) | 8.7 (7.5–10) |
| 22 | 19 (16–21) | 15 (12–17) | ||
| 43 | 44 (41–48) | 34 (30–39) | ||
| 202 | 13 (11–14) | 12 (10–14) | ||
| GMR | 22/1 | 2.5 (2.2–2.9) | 1.7 (1.5–2.0) | |
| 43/1 | 6.0 (5.2–6.9) | 4.0 (3.4–4.7) | ||
| 202/1 | 1.7 (1.5–1.9) | 1.4 (1.2–1.5) | ||
| SP (%) | 1 | 11 (7–17) | 14 (9–21) | |
| 22 | 51 (43–58) | 39 (31–48) | ||
| 43 | 91 (85–94) | 82 (74–88) | ||
| 202 | 27 (21–34) | 25 (18–33) | ||
| SC (%) | 22 | 40 (32–47) | 28 (20–36) | |
| 43 | 85 (79–90) | 70 (61–77) | ||
| 202 | 20 (14–26) | 12 (7–18) | ||
| MN (194/148) | GMT | 1 | 5.2 (5.0–5.5) | 5.3 (5.0–5.5) |
| 22 | 19 (16–22) | 12 (10–15) | ||
| 43 | 137 (116–163) | 56 (46–68) | ||
| 202 | 19 (16–22) | 12 (11–14) | ||
| GMR | 22/1 | 3.5 (3.0–4.2) | 2.3 (1.9–2.8) | |
| 43/1 | 26 (22–31) | 11 (8.7–13) | ||
| 202/1 | 3.6 (3.1–4.2) | 2.3 (2.0–2.7) | ||
| Titer ≥40 (%) | 1 | 1.0 (0–3.0) | 1.0 (0–4.0) | |
| 22 | 25 (19–32) | 16 (11–23) | ||
| 43 | 85 (79–90) | 68 (59–75) | ||
| 202 | 22 (17–29) | 8 (4–14) | ||
| ≥4-fold increase (%) | 22 | 46 (39–54) | 26 (19–33) | |
| 43 | 93 (89–96) | 81 (74–87) | ||
| 202 | 49 (41–56) | 26 (19–34) | ||
Bold text indicates that CHMP licensure criteria were met. SP, seroprotection (HI titer of ≥40; SRH area of ≥25 mm2); SC, seroconversion (HI titer of <10 to ≥40; SRH area of ≤4 mm2 to ≥25 mm2) or significant increase (≥4-fold increase in HI titer; ≥50% increase in SRH area); CI, confidence interval.
Cross-reactive antibody responses against the heterologous influenza strains A/Indonesia/5/2005 and A/Vietnam/1194/2004 are presented in Tables 3 and 4, respectively. HI assay antibody responses against the two heterologous strains were similar within each age group. In adults, two vaccine doses resulted in HI GMRs of 4.71 and 4.25, seroconversion rates of 49% and 44%, and seroprotection rates of 50% and 47% against A/Indonesia and A/Vietnam strains, respectively. HI antibody responses were weaker in the elderly than in adults. The CHMP licensure criteria for GMR and seroconversion were met in adult and elderly subjects by HI assay. Assessment of cross-reactive antibody responses by SRH assay found higher titers against the A/Indonesia strain than against the A/Vietnam strain in adult and elderly subjects. All licensure criteria were met by both age groups, apart from the seroprotection criterion against A/Vietnam in adult and elderly subjects. In adults, two vaccine doses resulted in SRH GMRs of 6.24 and 4.45, seroconversion rates of 79% and 60%, and seroprotection rates of 83% and 62% against A/Indonesia and A/Vietnam strains, respectively. SRH antibody responses were weaker in the elderly than in adults. Analysis by MN assay after two vaccine doses found adult (58%, ≥4-fold increase) and elderly (30%, ≥4-fold increase) subjects to have low heterologous responses against the A/Indonesia strain. No heterologous response was observed by MN assay against the A/Vietnam strain.
Table 3.
Immunogenicity analysis against the heterologous strain A/Indonesia/5/2005 (H5N1)a
| Assay (no. of adults/no. of elderly) | Parameter | Day(s) | Value (95% CI) |
|
|---|---|---|---|---|
| Adults | Elderly | |||
| HI (194/148) | GMT | 1 | 5.4 (5.1–5.6) | 5.4 (5.1–5.6) |
| 43 | 25 (20–32) | 14 (12–18) | ||
| 202 | 7.1 (6.3–7.9) | 6.9 (6.3–7.7) | ||
| GMR | 43/1 | 4.7 (3.7–5.9) | 2.7 (2.2–3.3) | |
| 202/1 | 1.3 (1.2–1.5) | 1.3 (1.2–1.4) | ||
| SP (%) | 1 | 0.5 (0–2.9) | 0 (0–2.5) | |
| 43 | 50 (43–57) | 34 (26–42) | ||
| 202 | 11 (6.9–16) | 4.1 (1.5–8.6) | ||
| SC (%) | 43 | 49 (42–56) | 32 (25–41) | |
| 202 | 8.3 (4.8–13) | 4.1 (1.5–8.6) | ||
| SRH (187/143) | GMA | 1 | 6.2 (5.5–6.9) | 6.6 (5.8–7.6) |
| 43 | 38 (35–43) | 26 (23–30) | ||
| 202 | 11 (9.7–12) | 11 (9.2–12) | ||
| GMR | 43/1 | 6.2 (5.4–7.2) | 3.9 (3.3–4.5) | |
| 202/1 | 1.8 (1.6–2.0) | 1.6 (1.4–1.8) | ||
| SP (%) | 1 | 11 (7–16) | 9 (5–15) | |
| 43 | 83 (77–88) | 61 (52–69) | ||
| 202 | 22 (16–28) | 21 (15–29) | ||
| SC (%) | 43 | 79 (72–85) | 64 (56–73) | |
| 202 | 19 (14–26) | 18 (12–25) | ||
| MN (194/148) | GMT | 1 | 5.0 (5.0–5.1) | 5.0 (5.0–5.0) |
| 43 | 24 (20–28) | 11 (9.3–13) | ||
| 202 | 7.2 (6.5–7.9) | 6.0 (5.5–6.4) | ||
| GMR | 43/1 | 4.7 (3.9–5.6) | 2.2 (1.9–2.6) | |
| 202/1 | 1.4 (1.3–1.6) | 1.2 (1.1–1.3) | ||
| Titer ≥40 (%) | 1 | 0 (0–2.0) | 0 (0–2.0) | |
| 43 | 38 (31–45) | 14 (8.0–20) | ||
| 202 | 4.0 (1.0–7.0) | 1.0 (0–5.0) | ||
| ≥4-fold increase (%) | 43 | 58 (50–65) | 30 (23–38) | |
| 202 | 11 (7.0–16) | 5.0 (2.0–10) | ||
Bold text indicates that CHMP licensure criteria were met. SP, seroprotection (HI titer of ≥40; SRH area of ≥25 mm2); SC, seroconversion (HI titer of <10 to ≥40; SRH area of ≤4 mm2 to ≥25 mm2) or significant increase (≥4-fold increase in HI titer; ≥50% increase in SRH area); CI, confidence interval.
Table 4.
Immunogenicity analysis against the heterologous strain A/Vietnam/1194/2004 (H5N1)a
| Assay (no. of adults/no. of elderly) | Parameter | Day(s) | Value (95% CI) |
|
|---|---|---|---|---|
| Adults | Elderly | |||
| HI (194/148) | GMT | 1 | 5.9 (5.5–6.5) | 6.9 (6.0–7.9) |
| 43 | 25 (20–33) | 19 (15–25) | ||
| 202 | 9.2 (7.9–11) | 11 (8.9–13) | ||
| GMR | 43/1 | 4.3 (3.4–5.4) | 2.8 (2.2–3.6) | |
| 202/1 | 1.6 (1.4–1.8) | 1.5 (1.4–1.9) | ||
| SP (%) | 1 | 3.1 (1.1–6.6) | 5.4 (2.4–10) | |
| 43 | 47 (40–55) | 39 (31–48) | ||
| 202 | 18 (13–24) | 17 (11–24) | ||
| SC (%) | 43 | 44 (37–51) | 34 (26–42) | |
| 202 | 12 (7.7–17) | 12 (6.8–18) | ||
| SRH (187/143) | GMA | 1 | 5.1 (4.8–5.5) | 5.3 (4.9–5.9) |
| 43 | 23 (20–26) | 16 (14–19) | ||
| 202 | 7.3 (6.5–8.0) | 8.2 (7.2–9.4) | ||
| GMR | 43/1 | 4.5 (3.9–5.1) | 3.0 (2.6–3.6) | |
| 202/1 | 1.4 (1.3–1.6) | 1.5 (1.4–1.7) | ||
| SP (%) | 1 | 4.0 (2.0–8.0) | 5.0 (2.0–10) | |
| 43 | 62 (54–69) | 45 (37–54) | ||
| 202 | 10 (6.0–15) | 14 (9.0–21) | ||
| SC (%) | 43 | 60 (53–68) | 44 (35–53) | |
| 202 | 10 (6.0–15) | 14 (9.0–21) | ||
| MN (194/148) | GMT | 1 | 5.0 (5.0–5.1) | 5.2 (5.0–5.4) |
| 43 | 9.4 (8.2–11) | 6.9 (5.9–8.0) | ||
| 202 | 5.7 (5.3–6.0) | 6.3 (5.6–7.2) | ||
| GMR | 43/1 | 1.9 (1.6–2.1) | 1.3 (1.2–1.5) | |
| 202/1 | 1.1 (1.1–1.2) | 1.2 (1.1–1.4) | ||
| Titer ≥40 (%) | 1 | 0 (0–2.0) | 1.0 (0–4.0) | |
| 43 | 10 (6.0–16) | 6.0 (3.0–11) | ||
| 202 | 1.0 (0–4.0) | 4.0 (2.0–9.0) | ||
| ≥4-fold increase (%) | 43 | 19 (13–25) | 7.0 (4.0–13) | |
| 202 | 4.0 (2.0–8.0) | 18 (4.0–14) | ||
Bold text indicates that CHMP licensure criteria were met. SP, seroprotection (HI titer of ≥40; SRH area of ≥25 mm2); SC, seroconversion (HI titer of <10 to ≥40; SRH area of ≤4 mm2 to ≥25 mm2) or significant increase (≥4-fold increase in HI titer; ≥50% increase in SRH area); CI, confidence interval.
Safety analysis.
Solicited local and systemic reactions occurring within 1 week of vaccination are summarized in Table 5. The majority of solicited reactions were mild to moderate in severity and were more frequent in adult than elderly subjects. More local and systemic reactions were reported after the first (55%) than the second (41%) dose in adults and in elderly subjects (first, 36%; second, 26%). The most frequently reported local reaction in adults was pain, experienced by 43% and 31% of subjects after first and second doses, respectively, of which only one case, after the first dose in an elderly subject, was described as severe. Myalgia was the most common systemic reaction in adults, reported by 13% and 7% of subjects after first and second doses, respectively. Only 1% of adults experienced fever (≥38°C), and no cases of severe fever (≥40°C) were reported. Vaccine-related AEs were experienced by 2% of adult subjects after both first and second doses; all were of mild to moderate severity and usually local or systemic reactions, such as pain. No vaccine-related AEs were reported after day 43. One adult subject did not complete the study due to an AE which was not considered to be vaccine related by the investigator (fatal heart and liver failure, day 46). The most common local reaction in the elderly was erythema, experienced by 19% and 17% of subjects after first and second doses, respectively. The profile of systemic reactions in elderly subjects was similar to that in adults, myalgia being the most frequently reported systemic reaction, in 7% and 5% of the elderly population after first and second doses, respectively, and 1% experiencing fever (≥38°C), with no cases of severe fever (≥40°C). The percentage of elderly subjects reporting mild to moderate vaccine-related AEs after first (3%) and second (2%) doses was similar to that in adults. No vaccine-related AEs were reported after day 43. One elderly subject withdrew consent after receiving the first vaccine dose; no specific reason for this decision was given.
Table 5.
Percentages of subjects experiencing mild to moderate (and severe) solicited local and systemic reactions within 1 week of vaccination
| Reaction | % of subjects with mild to moderate (% with severe) reactions |
|||
|---|---|---|---|---|
| Adults |
Elderly |
|||
| 1st dose (n = 194) | 2nd dose (n = 194) | 1st dose (n = 149) | 2nd dose (n = 148) | |
| Local | ||||
| Erythema | 12 (0) | 14 (1) | 18 (1) | 17 (0) |
| Induration | 7 (0) | 6 (1) | 7 (0) | 7 (0) |
| Swelling | 9 (0) | 9 (1) | 12 (1) | 7 (0) |
| Ecchymosis | 3 (0) | 2 (0) | 3 (0) | 1 (0) |
| Pain | 43 (0) | 31 (0) | 14 (1) | 14 (0) |
| Systemic | ||||
| Chills | 4 (0) | 1 (0) | 4 (1) | 2 (0) |
| Malaise | 7 (0) | 3 (0) | 5 (1) | 2 (1) |
| Myalgia | 13 (0) | 7 (0) | 6 (1) | 4 (1) |
| Arthralgia | 8 (0) | 3 (0) | 4 (0) | 2 (1) |
| Headache | 8 (0) | 6 (0) | 5 (0) | 4 (1) |
| Sweating | 1 (0) | 1 (0) | 0 (1) | 1 (0) |
| Nausea | 3 (0) | 2 (0) | 2 (1) | 2 (0) |
| Vomiting | 0 (0) | 1 (0) | 1 (0) | 0 (0) |
| Diarrhea | 2 (0) | 2 (0) | 1 (0) | 1 (0) |
| Fatigue | 6 (0) | 6 (0) | 5 (0) | 1 (0) |
| Fever (≥38°C) | 1 (0) | 1 (0) | 1 (0) | 1 (0) |
DISCUSSION
The pandemic threat posed by the A/H5N1 (avian) influenza virus is significant, and ongoing efforts to protect the human population against the possible emergence of a highly virulent strain capable of human-to-human transmission are essential. A recent study by Imai et al. investigated the molecular features which could render H5 viruses transmissible in mammals; animal studies indicate that genetic reassortment involving as few as four mutations in the hemagglutinin surface protein are sufficient for efficient viral transmission to occur (15). These findings emphasize the need to prepare for potential avian influenza pandemics. Proactive priming in those who have not encountered the A/H5N1 influenza virus serves to equip the individual with A/H5N1-specific memory lymphocyte populations and decrease the number of vaccinations required in the event of a pandemic (16, 26). This clinical trial evaluated the immunogenicity and safety profiles of an MF59-adjuvanted, prepandemic influenza vaccine containing clade 2 A/H5N1 viral strain antigen (A/turkey/Turkey/01/2005). Vaccine antigen-specific and cross-reactive antibody responses were assessed. The results of this study support previously published data from clinical trials of the investigational vaccine (12).
Antibody responses against the vaccine antigen strain were sufficient to meet the European licensure criteria after two doses in both adult and elderly subjects. The results of this study are consistent with similar trials of MF59-adjuvanted A/H5N1 vaccine containing A/Vietnam/1194/2004 strain antigen, which also found that adult and elderly subjects require two vaccine doses (2, 4). Modeling studies suggest that A/H5N1 vaccines conferring even moderate heterologous protection can substantially mitigate the impact of a pandemic (11). The levels of cross-reactive antibody production observed throughout this study were uncharacteristically low when measured by MN assay. However, cross-reactive data generated by HI and SRH assays demonstrate that the MF59-adjuvanted vaccine induced seroprotective heterologous antibody titers. These data are consistent with previous clinical trials which also show that MF59 enhances the production of cross-reactive antibodies (2, 12, 13, 18–20). A study by Galli et al. showed that MF59-adjuvanted vaccine induced a 3-fold expansion in A/H5N1-specific CD4+ T cells able to react with clade 0-like and clade 2 A/H5N1 proteins, confirming that MF59 promotes the development of broadly cross-reactive T cells and thereby heterologous B cell antibody responses (14).
MF59 has a well-established safety record in vaccinees of all ages (2–5, 21, 25, 30, 31). Use of a similar oil-in-water emulsion adjuvant in A/H1N1 pandemic vaccines has been associated with narcolepsy in young children (35), but a recent investigation found no evidence of an association between MF59 adjuvant and increased risk of narcolepsy (6, 31). The investigational vaccine was generally well tolerated by adult and elderly subjects, with the majority of reactions being of mild to moderate severity and rapidly resolved. The tolerability profiles generated during this study were similar to those observed in other trials of MF59-adjuvanted A/H5N1 vaccines in adults and the elderly (2, 4, 21). MF59 has been shown to promote long-term antibody persistence (14, 32), an element essential to successful pandemic vaccination strategy. Immunogenicity analysis 1 year after vaccination would be beneficial in order to evaluate MF59-enhanced long-term antibody persistence. All study participants were deemed to be healthy on enrollment; therefore, the findings of this investigation should not be applied to the immunosuppressed or those affected by chronic conditions.
The MF59-adjuvanted, prepandemic, investigational vaccine was shown to be well tolerated and adequately immunogenic in terms of both vaccine antigen-specific and cross-reactive antibody responses. These data and a previous report (12) demonstrate the investigational vaccine, containing 7.5 μg of clade 2, H5N1, A/turkey/Turkey/1/2005 strain influenza antigen per dose, to be suitable for prepandemic use in adult and elderly populations.
ACKNOWLEDGMENTS
All authors participated in the conception, design and implementation of this trial. All authors were involved in the interpretation of analyzed data and the decision to submit for publication.
J.K., J.B., and E.F. are permanent employees of Novartis Vaccines and Diagnostics. All other authors declare no conflicts of interest.
This study was supported by funds provided by Novartis Vaccines and Diagnostics.
We thank Jamie Stirling and Shivani Vadapalli (both of Novartis Vaccines and Diagnostics) and Clem Weinberger (New York, NY) for editorial assistance in the preparation of the manuscript.
Footnotes
Published ahead of print 17 October 2012
REFERENCES
- 1.Alghisi F, et al. 2011. Immunogenicity and safety profile of the monovalent A/H1N1 MF59-adjuvanted vaccine in patients affected by cystic fibrosis. Thorax 66:259–260 [DOI] [PubMed] [Google Scholar]
- 2.Banzhoff A, et al. 2009. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 4:e4384 doi:10.1371/journal.pone.0004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banzhoff A, Haertel S, Praus M. 2011. Passive surveillance of adverse events of an MF59-adjuvanted H1N1v vaccine during the pandemic mass vaccinations. Hum. Vaccin. 7:539–548 [DOI] [PubMed] [Google Scholar]
- 4.Beran J, Abdel-Messih IA, Raupachova J, Hobzova L, Fragapane E. 2010. A phase III, randomized, open-label study to assess the tolerability and immunogenicity of an H5N1 influenza vaccine administered to healthy adults with a 1-, 2-, 3-, or 6-week interval between first and second doses. Clin. Ther. 32:2186–2197 [DOI] [PubMed] [Google Scholar]
- 5.Black S, et al. 2010. Safety of MF59-adjuvanted versus non-adjuvanted influenza vaccines in children and adolescents: an integrated analysis. Vaccine 28:7331–7336 [DOI] [PubMed] [Google Scholar]
- 6.Crucitti A, Tsai TF. 2011. Explorations of clinical trials and pharmacovigilance databases of MF59(R)-adjuvanted influenza vaccines for associated cases of narcolepsy: a six-month update. Scand. J. Infect. Dis. 43:993. [DOI] [PubMed] [Google Scholar]
- 7.El Sahly H. 2010. MF59 as a vaccine adjuvant: a review of safety and immunogenicity. Expert Rev. Vaccines 9:1135–1141 [DOI] [PubMed] [Google Scholar]
- 8.Esposito S, et al. 2010. Immunogenicity, safety and tolerability of monovalent 2009 pandemic influenza A/H1N1 MF59-adjuvanted vaccine in patients with beta-thalassemia major. Vaccine 28:7825–7828 [DOI] [PubMed] [Google Scholar]
- 9.Esposito S, et al. 2011. An open-label, randomized clinical trial assessing immunogenicity, safety and tolerability of pandemic influenza A/H1N1 MF59-adjuvanted vaccine administered sequentially or simultaneously with seasonal virosomal-adjuvanted influenza vaccine to paediatric kidney transplant recipients. Nephrol. Dial. Transplant. 26:2018–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Agency for the Evaluation of Medicinal Products 1997. Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines. European Agency for the Evaluation of Medicinal Products, London, United Kingdom: http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf [Google Scholar]
- 11.Ferguson NM, et al. 2006. Strategies for mitigating an influenza pandemic. Nature 442:448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fragapane E, et al. 2010. A heterologous MF59-adjuvanted H5N1 prepandemic influenza booster vaccine induces a robust, cross-reactive immune response in adults and the elderly. Clin. Vaccine Immunol. 17:1817–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli G, et al. 2009. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc. Natl. Acad. Sci. U. S. A. 106:7962–7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galli G, et al. 2009. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc. Natl. Acad. Sci. U. S. A. 106:3877–3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai M, et al. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings LC, Monto AS, Chan PK, Szucs TD, Nicholson KG. 2008. Stockpiling prepandemic influenza vaccines: a new cornerstone of pandemic preparedness plans. Lancet Infect. Dis. 8:650–658 [DOI] [PubMed] [Google Scholar]
- 17.Kajaste-Rudnitski A, et al. 2011. Induction of protective antibody response by MF59-adjuvanted 2009 pandemic A/H1N1v influenza vaccine in HIV-1-infected individuals. AIDS 25:177–183 [DOI] [PubMed] [Google Scholar]
- 18.Khurana S, et al. 2010. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci. Transl. Med. 2:15ra5 doi:10.1126/scitranslmed.3000624. [DOI] [PubMed] [Google Scholar]
- 19.Khurana S, et al. 2011. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 3:85ra48 doi:10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leroux-Roels I, et al. 2007. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 370:580–589 [DOI] [PubMed] [Google Scholar]
- 21.Lopez P, et al. 2011. Combined, concurrent, and sequential administration of seasonal influenza and MF59-adjuvanted A/H5N1 vaccines: a phase II randomized, controlled trial of immunogenicity and safety in healthy adults. J. Infect. Dis. 203:1719–1728 [DOI] [PubMed] [Google Scholar]
- 22.Meier S, et al. 2011. Antibody responses to natural influenza A/H1N1/09 disease or following immunization with adjuvanted vaccines, in immunocompetent and immunocompromised children. Vaccine 29:3548–3557 [DOI] [PubMed] [Google Scholar]
- 23.Nicholson KG, et al. 2001. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 357:1937–1943 [DOI] [PubMed] [Google Scholar]
- 24.O'Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. 2011. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev. Vaccines 10:447–462 [DOI] [PubMed] [Google Scholar]
- 25.Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. 2009. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 27:6959–6965 [DOI] [PubMed] [Google Scholar]
- 26.Rockman S, Brown L. 2010. Pre-pandemic and pandemic influenza vaccines. Hum. Vaccin. 6:792–801 [DOI] [PubMed] [Google Scholar]
- 27.Schild GC, Pereira MS, Chakraverty P. 1975. Single-radial-hemolysis: a new method for the assay of antibody to influenza haemagglutinin. Applications for diagnosis and seroepidemiologic surveillance of influenza. Bull. World Health Organ. 52:43–50 [PMC free article] [PubMed] [Google Scholar]
- 28.Stephenson I, et al. 2008. Antigenically distinct MF59-adjuvanted vaccine to boost immunity to H5N1. N. Engl. J. Med. 359:1631–1633 [DOI] [PubMed] [Google Scholar]
- 29.Stephenson I, Nicholson KG, Wood JM, Zambon MC, Katz JM. 2004. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect. Dis. 4:499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai T, et al. 2010. Exposure to MF59-adjuvanted influenza vaccines during pregnancy—a retrospective analysis. Vaccine 28:1877–1880 [DOI] [PubMed] [Google Scholar]
- 31.Tsai TF, et al. 2011. Explorations of clinical trials and pharmacovigilance databases of MF59(R)-adjuvanted influenza vaccines for associated cases of narcolepsy. Scand. J. Infect. Dis. 43:702–706 [DOI] [PubMed] [Google Scholar]
- 32.Vesikari T, Groth N, Karvonen A, Borkowski A, Pellegrini M. 2009. MF59-adjuvanted influenza vaccine (FLUAD) in children: safety and immunogenicity following a second year seasonal vaccination. Vaccine 27:6291–6295 [DOI] [PubMed] [Google Scholar]
- 33.Vesikari T, et al. 2010. Immunogenicity and safety of MF59-adjuvanted H5N1 influenza vaccine from infancy to adolescence. Pediatrics 126:e762–e770 [DOI] [PubMed] [Google Scholar]
- 34.Vesikari T, et al. 2011. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N. Engl. J. Med. 365:1406–1416 [DOI] [PubMed] [Google Scholar]
- 35.Watts G. 2011. New findings on H1N1 vaccine prompt revised prescribing advice. BMJ 342:d2524 doi:10.1136/bmj.d2524. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization 2012. Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. World Health Organization, Geneva, Switzerland: http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html. Accessed 31 May 2012 [Google Scholar]
- 37.World Health Organization 2009. Pandemic influenza preparedness and response. WHO guidance document. World Health Organization, Geneva, Switzerland: http://www.who.int/influenza/resources/documents/pandemic_guidance_04_2009/en/index.html. [PubMed] [Google Scholar]
- 38.Zhao ZM, Shortridge KF, Garcia M, Guan Y, Wan XF. 2008. Genotypic diversity of H5N1 highly pathogenic avian influenza viruses. J. Gen. Virol. 89:2182–2193 [DOI] [PubMed] [Google Scholar]
- 39.Zuccotti GV, et al. 2011. Long-lasting immunogenicity and safety of a 2009 pandemic influenza A(H1N1) MF59-adjuvanted vaccine when coadministered with a 2009–2010 seasonal influenza vaccine in young patients with type 1 diabetes mellitus. Diabet. Med. 28:1530–1536 [DOI] [PubMed] [Google Scholar]


