Abstract
Characterizing host immune responses to molecular targets of Mycobacterium tuberculosis is essential to develop effective immunodiagnostics and better vaccines. We investigated the immune response against a large series of M. tuberculosis antigens, including 5 classical and 64 nonclassical (39 DosR regulon-encoded, 4 resuscitation-promoting factor [RPF], and 21 reactivation-associated) antigens in active-pulmonary-tuberculosis (TB) patients. Whole blood from TB patients (n = 34) was stimulated in vitro with M. tuberculosis antigens. Gamma interferon (IFN-γ) was measured after 7 days of stimulation, using an enzyme-linked immunosorbent assay (ELISA). The majority of the study participants responded to the classical M. tuberculosis antigens TB10.4 (84.8%), early secreted antigenic target-6 kDa (ESAT-6)/CFP-10 (70.6%), and purified protein derivative (PPD) (55.9%). However, only 26.5% and 24.2% responded to HSP65 and Ag85A/B, respectively. Of the 64 nonclassical antigens, 23 (33.3%) were immunogenic (IFN-γ levels, >62 pg/ml) and 8 were strong inducers of IFN-γ (IFN-γ levels, ≥100 pg/ml). The RPF antigens were the most immunogenic. In addition, we observed distinct cytokine expression profiles in response to several M. tuberculosis antigens by multiplex immunoassay. Tumor necrosis factor alpha (TNF-α), interleukin 10 (IL-10), and IL-6 were commonly detected at high levels after stimulation with 4/15 latency antigens (Rv0081, Rv2006, Rv2629, and Rv1733c) and were found especially in supernatants of the three strong IFN-γ inducers (Rv2629, Rv1009, and Rv2389c). IL-8, IL-6, and IL-17 were exclusively detected after stimulation with Rv0574c, Rv2630, Rv1998, Rv054c, and Rv2028c. In conclusion, in active-pulmonary-TB patients, we identified 23 new immunogenic M. tuberculosis antigens. The distinct expression levels of IFN-γ, TNF-α, IL-6, and IL-10 in response to specific subsets of M. tuberculosis antigens may be promising for the development of immunodiagnostics.
INTRODUCTION
Mycobacterium tuberculosis infects one-third of the world population (21), 8 to 10 million of whom developed active tuberculosis (TB) and 1.1 million of whom died as a result of TB in 2010 (50). Due to social and economic factors (43), limitations of diagnostic tools, the lack of an effective vaccine for TB (3, 36, 52), and the emergence of alarming multidrug resistance and extensively drug-resistant M. tuberculosis (50), control of TB remains a major challenge. Effective diagnosis, drugs, and vaccines are therefore urgently required (12, 43).
To design new diagnostics or vaccines, it is necessary to enlarge our knowledge of potential immunogenic M. tuberculosis antigens (41, 6). Ideally, antigens should represent the different stages of M. tuberculosis infection and should include M. tuberculosis antigens expressed during the early onset of the infection (growth stage), during the latent/dormancy stage, and during resuscitation of the dormancy stage (19). Several immunogenic recombinant (32) and secreted (early secreted antigenic target-6 kDa [ESAT-6], Ag85B, MPT64, and MPB70) (29) antigens have been identified using advanced molecular technologies (34). However, the absence of reliable methods able to predict which M. tuberculosis antigens may lead to protective immune responses warrants further screening of M. tuberculosis antigens (41).
Secreted antigens are produced early in the course of infection (5) and can elicit protective immunity (4), which rapidly stabilizes the bacterial load in the lung (5). These antigens have been recognized as potential vaccine components (Ag85 and ESAT-6) and specific immunodiagnostic reagents (ESAT-6 and culture filtrate protein 10 [CFP-10]) (33) for TB. In vitro (31) and in vivo (52) studies have shown the capacity of the resuscitation-promoting factor (RPF) proteins to elicit both humoral and cellular immune responses that result in protection against TB infection (13, 14, 38).
Whereas latently TB-infected populations have been used as human models for the screening of M. tuberculosis antigens (8), only a few studies have been performed to assess the immunogenicity of M. tuberculosis antigens in active-TB populations (2, 28). The fact that there is heterogeneity in T-cell repertoires between TB patients and subjects with latent TB infection (LTBI) (43) and that immune recognition of M. tuberculosis antigens may vary in the course of TB infection and disease (44) reinforces the need to involve active-TB patients in the screening of M. tuberculosis antigens.
This study aimed to reassess the immunogenicity of previously well-established diagnostic TB antigens (classical antigens; n = 5) (TB10, ESAT-6, Ag85A/B, purified protein derivative [PPD], and HSP65), as well as to analyze the immune responses to a range of new M. tuberculosis antigens (nonclassical antigens; n = 64), including those for which immunogenicity has not yet been assessed.
Since the strength of the host immune response against M. tuberculosis infection is directly proportional to the level of cellular (CD4+) production of gamma interferon (IFN-γ) (19), we employed a validated whole-blood assay (WBA) (49) to measure the level of ex vivo IFN-γ induced by each antigen using an enzyme-linked immunosorbent assay (ELISA).
Although it is known that IFN-γ plays an important role against M. tuberculosis infection, a complex network of other cytokines, such as tumor necrosis factor alpha (TNF-α) (19) and interleukin 17 (IL-17) (26), IL-6, IL-8 (48), IL-2 (9), and IL-10 (9), may play a role in the immunopathogenesis of M. tuberculosis infection. Therefore, we also assessed the levels of TNF-α, IL-2, IL-6, IL-8, IL-17, and IL-10 cytokines using a Luminex assay after stimulation with immunogenic antigens based on the ability to induce IFN-γ levels of ≥62 pg/ml, as measured by ELISA.
MATERIALS AND METHODS
Study site and patients.
This cross-sectional study was performed at St. Peter Specialized TB Hospital, Addis Ababa, Ethiopia, during November and December 2006. A total of 37 pulmonary-TB patients who were naïve for TB treatment were recruited consecutively after informed and written consent was sought. The mean (±standard deviation [SD]) age was 30.0 (±11.5) years, and 72.7% were men. Two (5.8%) HIV-positive subjects and one (2.9%) subject with a Mycobacterium bovis bacillus Calmette-Guérin (BCG) scar were excluded from analysis. Patients with clinical symptoms suggestive of TB were required to have at least two positive sputum smears for acid-fast bacilli (AFB) by direct microscopy to be diagnosed and confirmed with pulmonary TB. A total of 10 ml of whole blood (WB) was collected in a heparinized tube from each participant and was processed at the Ethiopian Health and Nutrition Research Institute (EHNRI). The study nurse collected demographic data from the participants using a standardized questionnaire. Diagnosis and management of TB cases followed the national guidelines (18).
M. tuberculosis antigens.
As shown in Table 1, the 69 recombinant M. tuberculosis proteins evaluated in this study were divided into two groups: (i) 5 classical antigens for which immunogenicity and specificity for M. tuberculosis is well defined (5, 10, 11, 33), including TB10.4 (Rv0288), ESAT-6 (Rv3875)/CFP-10 (Rv3874), Ag85A/B (Rv3804c/Rv1886v), 65,000-molecular-weight (65K) heat shock protein (HSP65) (Rv0440), and PPD, and (ii) 64 nonclassical antigens for which little or no data are available on immunogenicity, which included 39 dormancy (DosR) regulon-encoded or latency antigens (45) and 21 reactivation (46) and 4 RPF (52) antigens. All the M. tuberculosis antigens were selected and produced at the Department of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands (20).
Table 1.
Five classical and 64 nonclassical M. tuberculosis recombinant antigens screened for immunogenicity based on the level of IFN-γ elicited
| Gene namea | Size (amino acids) | Description | Previously tested |
|---|---|---|---|
| Classical antigens (n = 5) | |||
| Rv3804c/RV1886c | 338 | Secreted antigen 85-A (FBPA/85-B (FBPB) | Yes |
| Rv0440 | 540 | Heat shock protein 65 (HSP65) | Yes |
| Rv3875/Rv3874 | 95/98 | 6-kDa early secretory antigenic target (ESXA; ESAT-6)/10-kDa culture filtrate antigen ESXB (LHP) (CFP10) | Yes |
| Rv0288 | 96 | Low-molecular-wt protein antigen (ESXH - TB10.4) | Yes |
| PPD | Purified protein derivative | Yes | |
| Nonclassical antigens (n = 64) | |||
| Latency antigens | |||
| Rv0079 | 273 | Hypothetical protein | Yesb,c |
| Rv0081 | 114 | Probable transcriptional regulatory protein | |
| Rv0570-C | 354 | Rv0570 C-terminal part (amino acids 1–354) | Yesb,c |
| Rv0571c | 443 | Conserved hypothetical protein | Yesb |
| Rv0572c | 113 | Hypothetical protein | Yesb,c |
| Rv0573c | 463 | Conserved hypothetical protein | Yesb |
| Rv0574c | 380 | Conserved hypothetical protein | Yesb |
| Rv1733c | 210 | Probable conserved transmembrane protein | Yesb,c |
| Rv1734c | 80 | Conserved hypothetical protein | Yesb |
| Rv1736c-C | 380 | Rv1736c C-terminal part | Yesb |
| Rv1736c-N | 308 | Rv1736c N-terminal part | Yesb |
| Rv1737c | 395 | Possible nitrate/nitrite transporter | Yesb |
| Rv1738 | 94 | Conserved hypothetical protein | Yesb,c |
| Rv1812c | 400 | Probable dehydrogenase | Yesb |
| Rv1813c | 143 | Conserved hypothetical protein | Yesb,c |
| Rv1997-C | 430 | Rv1997 C terminal | Yesb |
| Rv1997-N | 504 | Rv1997 N terminal | Yesb |
| Rv1998 | 258 | Conserved hypothetical protein | Yesb |
| Rv2003c | 285 | Conserved hypothetical protein | Yesb |
| Rv2004c | 498 | Conserved hypothetical protein | Yesb |
| Rv2006 | 1,327 | Probable trehalose-6-phosphate | Yesb |
| Rv2007c | 114 | Probable ferredoxin | Yesb,c |
| Rv2028c | 279 | Conserved hypothetical protein | Yesb |
| Rv2032 | 331 | Conserved hypothetical protein | Yesb,c |
| Rv2624c | 272 | Conserved hypothetical protein | Yesb,d |
| Rv2625c | 393 | Probable conserved transmembrane protein | Yesb,d |
| Rv2626c | 143 | Conserved hypothetical protein | Yesb,c |
| Rv2627c | 413 | Conserved hypothetical protein | Yesb,c |
| Rv2628 | 120 | Hypothetical protein | Yesb,c,d |
| Rv2629 | 374 | Conserved hypothetical protein | Yesb |
| Rv2630 | 179 | Hypothetical protein | Yesb |
| Rv2631 | 432 | Conserved hypothetical protein | Yesb |
| Rv3126c | 104 | Hypothetical protein | Yesb,c,d |
| Rv3127 | 344 | Conserved hypothetical protein | Yesb,c |
| Rv3128c | 337 | Conserved hypothetical protein | Yesb |
| Rv3131 | 332 | Conserved hypothetical protein | Yesb,c |
| Rv3132c | 578 | Two-component sensor histidine kinase | Yesb,c |
| Rv3133c | 217 | Two-component transcriptional regulatory protein | Yesb,c |
| Rv3134c | 268 | Conserved hypothetical protein | Yesb,c |
| Reactivation antigens | |||
| Rv0140 | 126 | Conserved hypothetical protein | Yesd |
| Rv0246 | 436 | Probable conserved integral membrane protein | No |
| Rv0251c | 159 | Possible heat shock protein | No |
| Rv0331 | 388 | Putative dehydrogenase | No |
| Rv0384c | 848 | Heat shock protein F84.1 | No |
| Rv0753c | 510 | Methylmalmonate semialdehyde dehydrogenase | No |
| Rv1130 | 526 | Conserved hypothetical protein | No |
| Rv1131 | 393 | Citrate synthase 3 | No |
| Rv1471 | 123 | Thioredoxin reductase | No |
| Rv1472 | 285 | Enoyl-coenzyme A hydratase/isomerase superfamily | No |
| Rv1717 | 116 | Conserved hypothetical protein | No |
| Rv1874 | 228 | Hypothetical protein | No |
| Rv1875 | 147 | Conserved hypothetical protein | No |
| Rv2465c | 162 | Phosphopentose isomerase | No |
| Rv2466c | 207 | Conserved hypothetical protein | No |
| Rv2662 | 90 | Hypothetical protein | No |
| Rv3054c | 184 | Conserved hypothetical protein | No |
| Rv3223c | 216 | ECF subfamily sigma Subunit | No |
| Rv3307 | 268 | Probable purine nucleoside phosphorylase | No |
| Rv3463 | 285 | Conserved hypothetical protein | No |
| Rv3862c | 116 | Possible transcriptional regulatory protein WHIB6 | No |
| RPF antigens | |||
| Rv0867c | 407 | Possible resuscitation-promoting factor A | Yesd |
| Rv1009 | 362 | Possible resuscitation-promoting factor B | Yesd |
| Rv1884c | 176 | Possible resuscitation-promoting factor C | Yesd |
| Rv2389c | 154 | Possible resuscitation-promoting factor D | Yese |
Annotations are from http://genolist.pasteur.fr/TubercuList/.
Reference 7.
Reference 28.
Reference 44.
Reference 13.
The 64 M. tuberculosis antigens were randomly grouped into 4 batches of 16, and each antigen batch was tested on whole blood from a group of, on average, 9 randomly divided TB patients. Thus, blood samples from the TB patients in each study group were stimulated with a total of 21 antigens, always including the 5 classical antigens plus 16 nonclassical antigens. All M. tuberculosis antigens were reconstituted in sterile phosphate-buffered saline (PBS) to a concentration of 20 μg/ml and PPD to 10 μg/ml before stimulation.
WBA.
For each stimulus, coated plates were prepared. To this end, 100 μl of each stimulus, with culture medium (RPMI 1640; Sigma; catalog no. R0883) as a negative control, was transferred in triplicate to 96-well round-bottom culture plates (Nunc; catalog no. 163320) and stored at −80°C until it was used for WBA. Plates were coated with phytohemagglutinin (PHA) (10 μg/ml; Sigma; lot 33306) on the date of blood culture as a positive control. After dilution of the blood (1 in 5) with complete RPMI 1640 supplemented with 10% fetal calf serum (FCS) (Invitrogen; catalog no. 10106169) and 1% penicillin/streptomycin (PS), 100 μl of blood/well was transferred to the thawed antigen-coated plates to reach a final volume of 200 μl. The final concentration of all the recombinant antigens was 10 μg/ml, while it was 5 μg/ml for PPD (Statenus Seruminstitute; PPD RT48) and PHA. Culture plates were incubated in a 5% CO2 incubator at 37°C for 7 days, and the supernatants were collected and stored at −80°C for further analysis.
IFN-γ measurement in supernatants using ELISA.
The level of IFN-γ induced by each of the 69 M. tuberculosis antigens was quantified from the supernatants using an IFN-γ ELISA. In brief, purified anti-human IFN-γ monoclonal antibody (MAb) (Pharmingen 551221, lot 72093) was diluted to a final concentration of 2 μg/ml using coating buffer (0.1 M NaHCO3, pH 8.2), and 50 μl of the MAb was transferred to each well (except well A1, the blank) of a 96-well flat-bottom ELISA plate (ThermoLab Immulon 4 HBX) and incubated overnight at 4°C in a CO2 incubator. After washing, 150 μl blocking solution (PBS plus 3% bovine serum albumin [BSA]) was added per well and incubated for 2 h at room temperature. Finally, 50 μl of the supernatant samples and positive control (PHA) was added in triplicate to each well. Moreover, 100 μl/well of the IFN-γ standards (Pharmingen; lot 33306) was transferred to the wells following serial dilution (4,000 down to 31 pg/ml) using standard diluent (RPMI plus 5% FCS). The plates were incubated overnight. After washing, 100 μl of the detection antibody (biotin mouse anti-human IFN-γ MAb; Pharmingen 554550, lot 48629) with a final concentration of 1 μg/ml was transferred to each well and incubated for 45 min at room temperature (RT). Then, 50 μl of the enzyme StrepABC complex (DakoCytomation; lot K0377) was added per well and incubated at RT for 1 h. Finally, 150 μl of the substrate ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] (Roche Applied Science; catalog no. 11112422001) was added to each well, and the plates were incubated at 37°C for 15 min in the dark and read at 405 nm.
The IFN-γ response for individual antigens was considered positive or negative for each participant based on the cutoff value (IFN-γ at 62.0 pg/ml), which was calculated as the mean plus 2 times the standard deviation of the negative control (7).
Measurement of additional cytokines using an MIA.
The levels of Th1 (IL-2), Th2 (IL-10), and inflammatory (TNF-α, IL-6, IL-17, and IL-8) cytokines (pg/ml) were measured from the same culture supernatant for a subset of 29 immunogenic antigens using a multiplex immunoassay (MIA) (LabMAP technology; Luminex Corporation, Austin, TX) at the University Medical Center, Utrecht (UMCU), The Netherlands, as described previously (16). The lower limits of detection of the Luminex assay for IL-2, IL-4, IL-10, IL-17, TNF-α, IL-8, and IL-6 were 0.25, 0.12, 0.50, 0.75, 0.25, 0.5, and 0.75 pg/ml, respectively.
Statistical analysis.
The mean cytokine value (pg/ml) of the negative control (RPMI 1640 medium) was subtracted from the value induced by each M. tuberculosis antigen. The levels of each cytokine induced among the classical antigens were compared. The expression levels of cytokines induced by the newly evaluated M. tuberculosis antigens were compared to those of the classical antigens. A Mann-Whitney U test was used for statistical analysis. A P value of <0.05 was considered significant. Data were analyzed using STATA software version 11.0 (Stata Corporation, College Station, TX).
Ethical approval.
This study was approved by the Research and Ethical Clearance Committee, EHNRI, and by the National Health Research Ethics Review Committee, Ethiopia.
RESULTS
Magnitude of the immune response (IFN-γ production) to M. tuberculosis antigens in TB patients.
To analyze the immunogenicities of the 5 classical and the 64 new M. tuberculosis antigens in active-pulmonary-TB patients, we assessed the level of IFN-γ (pg/ml) induced by each antigen after 7 days of whole-blood culture. All the study participants (100%) responded to PHA (3,389.9 ± 2,999.2 [SD] pg/ml IFN-γ) (Table 2 and Fig. 1), whereas only 2 (5.9%) individuals produced a positive IFN-γ response (>62 pg/ml) in unstimulated cultures (26.2 ± 19.9 pg/ml IFN-γ).
Table 2.
IFN-γ elicited by the negative control, PHA, and the 5 classical and 23 identified immunogenic antigens
| Stimulus/antigen | No. of study participants | % Positive respondersa | IFN-γ (pg/ml) (mean ± SD) |
|---|---|---|---|
| Negative control (RPMI medium) | 34 | 5.9 | 26.2 ± 19.9 |
| PHA | 33 | 100 | 3,389.9 ± 2,999.2 |
| Classical | |||
| PPD | 34 | 55.9 | 242.0 ± 297.5b |
| Rv0288 (TB10.4) | 33 | 84.8 | 373.2 ± 606.3b |
| Rv3875/RV3874 (fusion) | 34 | 70.6 | 161.9 ± 162.1b |
| Rv0440 (HSP65) | 34 | 26.5 | 38.2 ± 57.9 |
| Rv3804c/RV1886c (Ag85A/B) | 34 | 24.2 | 32.2 ± 42.5 |
| Nonclassical | |||
| Reactivation | |||
| Rv0140 | 9 | 22.0 | 73.0 ± 134.8 |
| Rv0384c | 9 | 22.2 | 90.5 ± 190.1 |
| Rv2662 | 9 | 44.4 | 65.7 ± 70.3 |
| Rv3223cd | 9 | 22.2 | 125.4 ± 213.2b |
| Rv3307 | 9 | 22.2 | 76.9 ± 151.1 |
| Rv3862c | 6 | 33.3 | 90.8 ± 127.0 |
| Latency | |||
| Rv0079 | 9 | 11.1 | 76.3 ± 175.7 |
| Rv0081 | 6 | 33.3 | 61.3 ± 84.3 |
| Rv0574c | 9 | 22.2 | 97.5 ± 204.1 |
| Rv1733cc | 6 | 33.3 | 81.0 ± 83.3 |
| Rv1734c | 9 | 33.3 | 57.3 ± 84.8 |
| Rv1998 | 9 | 22.2 | 67.4 ± 101.7 |
| Rv2006 | 6 | 16.7 | 72.0 ± 127.5 |
| Rv2007c | 10 | 30.0 | 62.6 ± 85.7 |
| Rv2028c | 10 | 20.0 | 83.5 ± 189.1 |
| Rv2627cc | 6 | 33.3 | 100.9 ± 147.5b |
| Rv2629c,d | 6 | 50.0 | 121.9 ± 121.0b |
| Rv2630c | 9 | 33.3 | 113.1 ± 228.0b |
| Rv3132c | 9 | 22.2 | 97.5 ± 191.4 |
| RPF | |||
| Rv0867cc,d | 6 | 66.7 | 223.6 ± 234.6b |
| Rv1009c,d | 6 | 50.0 | 154.0 ± 152.1b |
| Rv1884cc,d | 6 | 66.7 | 126.1 ± 128.0b |
| Rv2389cc,d | 6 | 33.3 | 182.4 ± 307.8b |
Percentage of respondents able to elicit IFN-γ at >62 pg/ml.
Strong IFN-γ inducer (able to elicit IFN-γ at ≥100 pg/ml).
Antigen that induced significantly higher IFN-γ levels than Ag85A/B (P < 0.05; Student's t test).
Antigen that induced significantly higher IFN-γ levels than HSP65 (P < 0.05; Student's t test).
Fig 1.
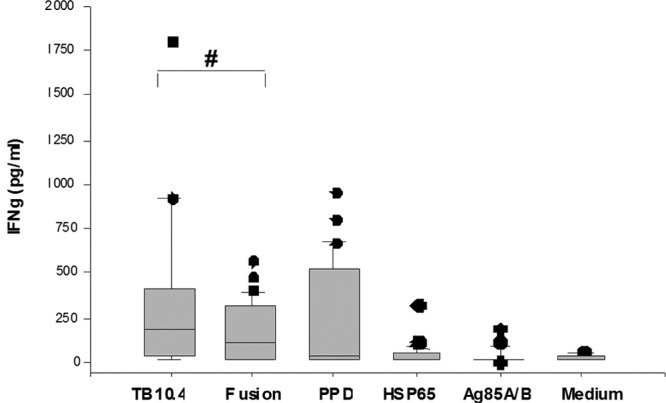
Production of IFN-γ by WB obtained from 34 active-pulmonary-TB patients. WB was stimulated in vitro with the classical M. tuberculosis antigens (TB10.4, PPD, ESAT-6/CFP-10 [fusion], HSP65, and Ag85A/B) and a negative control (RPMI medium). An IFN-γ ELISA analysis was done on a 7-day supernatant. The box plots show the 25th, 50th, and 75th percentiles, and the whiskers represent the minimum and maximum levels of IFN-γ (pg/ml) induced by each stimulus. The responses were compared using a Mann-Whitney U test. #, P < 0.05.
(i) IFN-γ response to the classical antigens.
The highest level of IFN-γ production was induced after stimulation with TB10.4 (373.2 pg/ml) compared to PPD (242.0 pg/ml) (P = 0.26) and ESAT-6/CFP-10 (161.9 pg/ml) (P = 0.02). The level of IFN-γ induced by PPD was not significantly different from that induced by ESAT-6/CFP-10 (P = 0.17). In contrast, the level of IFN-γ induced by Ag85A/B and HSP65 was below the cutoff value (62 pg/ml) (Table 2 and Fig. 1).
The proportion of study participants who responded to the M. tuberculosis antigens is essential for further prioritizing the antigens. TB10.4, ESAT-6/CFP-10, and PPD were recognized by 81.8%, 67.7%, and 58.8% of the TB patients, respectively, while the antigens HSP65 and Ag85A/B were recognized by only 29.1% and 21.2% of the study group (Fig. 1).
Thus, of the classical antigens, TB10.4, followed by ESAT-6/CFP-10, was the most immunogenic antigen with respect to the level of IFN-γ induced and the rate of recognition by TB patients.
(ii) IFN-γ response to the nonclassical antigens.
To identify new immunogenic antigens, we tested 64 nonclassical antigens for recognition by TB patients. Four of the 64 antigens (Rv2004, Rv3128, Rv0251, and Rv1717) were not recognized by any of the patients, whereas 23 antigens (4 RPF, 13 latency, and 6 reactivation) were able to induce a positive response (eliciting IFN-γ at >62 pg/ml). Of these identified immunogenic antigens, 15 were intermediate inducers of IFN-γ (≥62 but <100 pg/ml), and eight (Rv1884c, Rv2389c, Rv1009, Rv0867c, Rv2629, Rv2627c, Rv2630, and Rv3223c) were strong inducers of IFN-γ (IFN-γ at ≥100 pg/ml). Furthermore, 10 of these 23 immunogenic antigens (Rv1884c, Rv1009, Rv0867c, Rv2629, Rv1733, Rv1736-C, Rv2024, Rv3223c, Rv2662, and Rv0246) were recognized by 55.6 to 66.7% of the study participants. The results are summarized in Table 2.
None of the 23 immunogenic antigens induced higher levels of IFN-γ than stimulation with TB10.4, ESAT-6/CFP-10, or PPD. However, all the RPF (Rv1009, Rv2389c, Rv1884c, and Rv0867c) and 4 latency (Rv2627c, Rv2629, Rv2630, and Rv1733c) antigens elicited higher IFN-γ levels than Ag85A/b and HSP65 (P < 0.05 for all).
Taken together, of the families of antigens evaluated, 28.6%, 33.3%, and 100% of the reactivation, latency, and RPF antigens, respectively, were found to be immunogenic. The RPF antigens, in particular, were found to be the most immunogenic with respect to the level of IFN-γ induced and the number of study participants who responded to the antigens (Table 2).
Measurement of pro- and anti-inflammatory cytokines after WB stimulation with M. tuberculosis antigens.
To further characterize the immune responses to the 26 immunogenic M. tuberculosis antigens, which included 3 classical (TB10.4, ESAT-6/CFP-10, and PPD) and 23 nonclassical antigens, the levels of pro- and anti-inflammatory cytokines (IL-7, IL-2, IL-8, IL-6, TNF-α, and IL-10) were quantified from the same WB culture supernatants using a multiplex immunoassay. The results are shown in Fig. 2.
Fig 2.
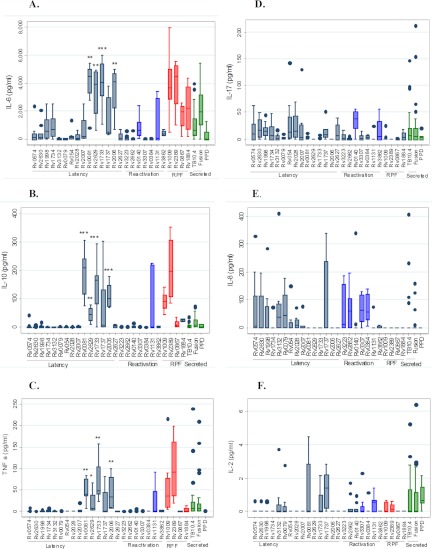
Concentrations of IL-6 (A), IL-10 (B), TNF-α (C), IL-17 (D), IL-8 (E), and IL-2 (F) cytokines in culture supernatants of whole blood stimulated with mycobacterial antigens. Whole blood obtained from active-pulmonary-TB patients (average, n = 9) was stimulated with a wide range of mycobacterium antigens (n = 29), including latency (n = 15) (gray bars), reactivation (n = 7) (blue bars), RPF (n = 4) (red bars), and secreted (TB10.4; fusion) antigens and PPD (green bars). The concentrations of the cytokines were measured from the 7th-day supernatants using the Luminex assay. The box plots show the 25th, 50th, and 75th percentiles, and the whiskers represent the minimum and maximum levels of IFN-γ (pg/ml) induced by each stimulus. Responses were compared using a Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Shown is a comparison of the highly expressed IL-6, IL-10, and TNF-α cytokines induced by 4 latency antigens (RV0081, RV2629, RV1733c, and RV2006) with those of the 11 other latency antigens.
Overall, the potent inflammatory cytokines (IL-6 and IL-17) were most commonly detected in WB culture supernatants (Fig. 2). IL-6, in particular, was detected after stimulation of all 29 (100%) antigens and had the highest level of expression (mean values ranging from 12.9 to 4,127.1 pg/ml per antigen). Likewise, IL-17 was detected in 26 (89.7%) of the antigen stimulations, with mean values ranging from 0.01 to 21.5 pg/ml per antigen.
More interestingly, whereas most cytokines were expressed in response to Rv1737 and Rv1131, we observed distinct expression of a cytokine(s) toward a subset of antigens (or an antigen) within the different families of M. tuberculosis antigens, as described below.
(i) Immune responses to the latency-associated antigens.
As shown in Fig. 2, IL-6, TNF-α, and IL-10, but not IL-17 and IL-8, were exclusively induced, and at a higher level, by 4 (Rv0081, Rv2629, Rv1733c, and Rv2006) of the 15 latency antigens evaluated (P < 0.05 for all). In contrast, only the potent inflammatory cytokines (IL-8, IL-6, and IL-17), but not IL-2, TNF-α, and IL-10, were commonly detected after stimulation with the 6 antigens Rv0574, Rv2630, Rv1998, Rv1734c, Rv054, and Rv2028c.
Immune responses to the reactivation antigens.
After stimulation with the 7 immunogenic reactivation antigens (Rv1131, Rv3223c, Rv2662, Rv0140, Rv3307, Rv0384c, and Rv3862c), except for RV1131, neither IL-10 nor TNF-α was produced, while only IL-2, IL-6, and IL-17 were induced by Rv0140.
(ii) Immune responses to the RPF antigens.
After stimulation with the 4 highly immunogenic RPF antigens (Rv1009, Rv2389c, Rv0867c, and Rv1884c), only IL-2, IL-10, TNF-α, and IL-6 were induced by Rv1009 and Rv2389c, but no detectable IL-8 and IL-17 was induced by any of the RPF antigens.
(iii) Responses to the classical antigens (TB10.4, ESAT-6/CFP-10 fusion protein, and PPD).
There was no detectable amount of TNF-α, IL-8, IL-17, and IL-10 after stimulation with PPD. However, the levels of IL-2, TNF-α, IL-17, and IL-6 were elevated, whereas low levels of IL-8 and IL-10 were found in response to TB10.4 and ESAT-6/CFP-10 fusion protein.
In summary, our results revealed distinct expression of pro- and anti-inflammatory cytokines in response to a subset of M. tuberculosis antigens.
DISCUSSION
The challenges in TB diagnosis (33) and the absence of an effective vaccine against TB (52) support the need to identify immunogenic M. tuberculosis antigens. In this study, we screened a large series of M. tuberculosis antigens for the ability to elicit Th1 cellular immune responses characterized by IFN-γ.
Secreted M. tuberculosis antigens, which are expressed early during the course of infection (5), have been widely reported as potential vaccine candidates (hsp60, Ag85A/Ag85B, and ESAT-6) and/or immunodiagnostic reagents (ESAT-6 and CFP-10) for TB (33, 39). We included these antigens as classical controls. An additional 64 new M. tuberculosis antigens were tested for their immunogenicities relative to these antigens. Of the 64 nonclassical antigens, 23 were immunogenic, 8 of which were strong inducers of IFN-γ.
Of further interest, IL-10, IL-6, and TNF-α, but no (or little) IL-17, IL-18, and IL-2, were detected in culture supernatants of WB assays stimulated with three (Rv2629, Rv1009, and Rv2389c) of the eight strong IFN-γ inducers.
IFN-γ response to the classical antigens.
The strong immunogenicity of TB10.4 reported previously (1, 7, 23) is also supported by this study, in which 84.4% of the donors recognized the antigen. Moreover, ESAT-6/CFP-10 was recognized by 67.2% of the donors in this study. In agreement with our results, other studies showed recognition of ESAT-6 in 80% of LTBI populations (41) and in 65% of TB patients in Ethiopia (37). Black et al. (7) also showed recognition of ESAT-6/CFP-10 in >75% of TB patients.
Although Ag85A/B is a promising candidate for subunit TB vaccines (39), in contrast to reports by Launois, et al. (27), but similar to others (7, 40), we found low antigenicity of Ag85A/B compared to ESAT-6/CFP-10, TB10, and PPD. The lowest proportion of responding study participants and the smallest amount of IFN-γ produced were observed after stimulation with HSP65. This could be due to the nature of the antigen, which may be expressed to a lesser extent and thus lead to less immune responsiveness in the active-TB patient population we studied (17).
Overall, results in the literature regarding IFN-γ responses to the classical antigens in active-TB patients are inconsistent. Among the likely explanations for these inconsistencies are differences in host genetic makeup (24), in M. tuberculosis strains (47), in study methodologies (15), and in the extent of TB disease progression, with diminished IFN-γ production during advanced disease (51). The results of our recent study performed in the same geographical location also point to diminished IFN-γ production in HIV-negative TB patients compared to LTBI populations (D. Kassa, unpublished data).
IFN-γ response to the 64 nonclassical M. tuberculosis antigens.
The main objective of this study was to identify additional immunogenic M. tuberculosis antigens. We were able to prioritize 23 promising antigens, eight of which (Rv2627c, Rv2629, Rv3223c, Rv1884c, Rv2630, Rv2389c, Rv054, and Rv0867c) induced high levels of IFN-γ (≥100 pg/ml). Four of these (Rv1009, Rv0867c, Rv2389c, and Rv1884c) were RPF antigens (Table 2).
An extensive study done by Zvi et al. (53) identified 189 antigens from the complete M. tuberculosis genome; of these, 45 were ranked as top hits and 20 as the most immunodominant T-cell antigens. Of the 23 immunogenic antigens we identified, 16 were in the list of these 189 antigens and 10 (Rv0079, Rv1733, Rv2028c, Rv2627c, Rv2629, Rv3132c, Rv1009, Rv0867c, Rv2389, and Rv1884c) were in the list of 45 top-hit antigens, while two (Rv0867 and Rv2627) were in the list of the 20 most immunodominant antigens identified by Zvi et al. (53).
In agreement with our study, which involved active-TB patients, other studies from South Africa, The Gambia, and Uganda that involved LTBI-infected populations also showed that Rv0081, Rv1733c, and Rv2006 were the most frequently recognized antigens (7). Rv1733c, Rv0140, and Rv1009, in contrast, induced significantly stronger IFN-γ responses in LTBI than in TB patients (44). Interestingly, stronger IFN-γ responses to Rv2031, Rv1733c, and Rv2626c antigens were also reported in BALB/c mice persistently infected with M. tuberculosis than in acutely infected mice (39).
It is also known that the majority of TB disease development occurs due to reactivation of LTBI, in which RPF antigens may be secreted and are involved in reactivation of the dormant bacteria (31). Interestingly, in this study, we observed the highest immunogenicity for the RPF antigens compared to the latency and reactivation antigens, which may correlate with the characteristics of the study groups and the intrinsic nature of the antigens (52).
While the recognition of the latency-associated antigens by cells from active TB patients could reflect the fact that most TB patients undergo a latent infection prior to TB disease (44), it might also indicate the involvement of latency antigens in the pathogenesis of TB. However, whether these antigens are differently recognized by cells from LTBI populations warrants further study.
In summary, we identified additional immunogenic M. tuberculosis antigens composed of latency, reactivation, and RPF families, which can serve as additional antigens for further evaluation as supplementary diagnostic reagents and vaccine subunits.
Of special interest, five (Rv2662, Rv3223c, Rv3307, Rv3862c, and Rv2630) of the immunogenic antigens we identified were not in the list of 189 antigens identified by Zvi et al. (53), nor were they previously evaluated by other studies, to our knowledge. Because they induced a very good immune response, they may be potential candidates for future evaluations.
Pro- and anti-inflammatory cytokine responses to a subset of immunogenic M. tuberculosis antigens.
Although IFN-γ plays a pivotal role in controlling M. tuberculosis infection, the level of inflammatory versus anti-inflammatory cytokines determines the clinical outcome of M. tuberculosis infection. It is also known that, while antigens that evoke strong IFN-γ responses are candidates for TB vaccine development (19), others have argued that care should be taken when antigens that induce high levels of IL-10 are considered for vaccine formulations for TB, as IL-10 downregulates the production of protective cytokines, including IFN-γ, TNF-α, IL-1, and IL-12 (34, 48). On the other hand, combinations of antigen-specific cytokines with IFN-γ are known to strengthen the diagnostic potential of M. tuberculosis antigens (5, 33). Therefore, we also characterized the levels of pro- and anti-inflammatory cytokines in the culture supernatants of the immunogenic M. tuberculosis antigen using the Luminex assay.
The detection of IL-6 and IL-17 in 100% and 82.8% of supernatants (Fig. 2) may confirm nonspecific inflammation during active TB (35). Likewise, the detection of only IL-6, IL-17, and IL-8 in supernatants of cultures with 5 latency antigens (Rv0574c, Rv2630, Rv1998, Rv054, and Rv2028c) might confirm the roles of these antigens in inflammation and the pathogenesis of TB.
In agreement with Yeremeev, et al. (52), we observed common secretion of TNF-α and IL-10 in only 5/15 latency, 1/6 reactivation, and 2/4 RPF antigens (Fig. 2). This might indicate a role for these antigens in TB pathogenesis (42, 9), but could also support reports that a combination of TNF-α and IL-10 with specific M. tuberculosis antigens (5, 33) and IL-6, TNF-α, and transforming growth factor beta (TGF-β) (35) may be used as potential immunodiagnostics for active TB. Studies have also shown increased Th1 and Th2 cytokine production in patients with active TB and that a configuration of IFN-γ, IL-2R, and IL-10 may predict (60 to 70% probability) susceptibility to acquiring or not acquiring acute TB (22). Others have also reported the combination of IL-6, TNF-α, and IFN-γ as effective markers to monitor TB treatment success (30).
The elevated levels of IL-6, IL-17, and TNF-α in response to TB10.4 and ESAT-6/CFP-10 were in agreement with other reports (30). This might support previous reports that ESAT-6- and CFP-10-induced IFN-γ, TNF-α, and IL-6, together with immunoglobulin G (IgG), are useful biomarkers of TB disease (30). On the other hand, the production of these inflammatory cytokines by TB10.4 and ESAT-6/CFP-10 might raise questions about utilizing the antigens for vaccine development (41).
Overall, the induction of multiple cytokines by a subset of antigens reflects the involvement of multifunctional subsets of T-cell or leukocyte populations (15) and multiple cytokines (35) in TB pathogenesis. More importantly, our results might indicate a link between antigen-specific T-cell subsets and cytokine signatures during active TB, which might lead to new applications for the diagnosis of active TB and correlates of TB disease progression (51). However, although it is possible that the type and magnitude of M. tuberculosis antigen-specific cytokine production in vitro could be affected by the extent and phase of TB disease (primary or reactivated TB disease) or by the circulating memory T cells, it was not possible to confirm whether the TB patients in this study had presented to the health facilities with primary or reactivated TB.
In conclusion, in this study, we confirmed the immunogenicity of ESAT-6/CFP-10 and TB-10.4, but in addition we also identified 23 immunogenic antigens that could serve as additional candidates for immunodiagnostics and vaccines for TB. Whereas the distinct expression levels of IFN-γ, TNF-α, IL-6, and IL-10 in response to specific subsets of M. tuberculosis antigens (Rv0081, Rv2629, Rv1733c, and Rv2006) suggest a promising potential path for the development of immunodiagnostics, those antigens that favor Th1 rather than Th2 or proinflammatory responses could be more relevant for the design of new vaccines against TB.
ACKNOWLEDGMENTS
This work, which is part of an ongoing longitudinal study entitled Biomarkers of Protective Immunity against Tuberculosis in the Context of Human Immunodeficiency Virus/AIDS (HIV/AIDS) in Africa (25), was supported by the Bill and Melinda Gates Foundation through the Grand Challenges in Global Health Initiative, grant no. 37772. The study also received support from the European-Developing Countries Clinical Trials Programme (EDCTP) (African European Tuberculosis Consortium [AE-TBC] project) and the European Union's Seventh Framework Programme (FP7/2007-2013) for the project NEWTBVAC.
Footnotes
Published ahead of print 26 September 2012
REFERENCES
- 1. Aagaard C, et al. 2006. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalence of bovine tuberculosis: ESAT-6-CRP-10 mixture shows optimal sensitivity and specificity. J. Clin. Microbiol. 44:4326–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Attiyah RA, Mustafa S, Abal A, El-Shamy M, Dalemans AS, Skeiky YAW. 2004. In vitro cellular immune responses to complex and newly defined recombinant antigens of Mycobacterium tuberculosis. Clin. Exp. Immunol. 138:139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Thoracic Society 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 161:1376–1395 [DOI] [PubMed] [Google Scholar]
- 4. Andersen P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andersen P, Andersen AB, Sorensen AL, Nagai S. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359–3372 [PubMed] [Google Scholar]
- 6. Bertholet S, et al. 2008. Reed identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J. Immunol. 181:7948–7957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Black GF, et al. 2009. Immunogenicity of novel DosR regulon-encoded candidate antigens of Mycobacterium tuberculosis in three high-burden populations in Africa. Clin. Vaccine Immunol. 16:1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boesen H, Jensen BN, Wilcke T, Andersen P. 1995. Human T cell responses to secreted antigen fraction of Mycobacterium tuberculosis. Infect. Immun. 63:1491–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boussiotis V, et al. 2000. IL-10 producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Invest. 105:1317–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brodin P, et al. 2005. Functional analysis of early secreted antigenic target-6, the dominant T-cell antigen of Mycobacterium tuberculosis, reveals key residues involved in secretion, complex formation, virulence, and immunogenicity. J. Biol. Chem. 280:33953–33959 [DOI] [PubMed] [Google Scholar]
- 11. Brodin P, Rosenkrands I, Andersen P, Cole ST, Brosch R. 2004. ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol. 12:500–508 [DOI] [PubMed] [Google Scholar]
- 12. Colditz GA, Brewer TF, Berkey CS. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698–702 [PubMed] [Google Scholar]
- 13. Commandeur S, et al. 2011. Double- and monofunctional CD4+ and CD8+ T-cell responses to Mycobacterium tuberculosis DosR antigens and peptides in long-term latently infected individuals. Eur. J. Immunol. 41:2925–2936 [DOI] [PubMed] [Google Scholar]
- 14. Commandeur S, et al. 2011. Identification of human T-cell responses to Mycobacterium tuberculosis resuscitation-promoting factors in long-term latently infected individuals. Clin. Vaccine Immunol. 18:676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Day CL, et al. 2011. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J. Immunol. 187:2222–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Jager W, Velthuis H, Prakken BJWK, Rijkers GT. 2003. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin. Diagn. Lab. Immunol. 10:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Demissie A, et al. 2006. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 13:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Federal Ministry of Health 2005. Manual of tuberculosis, leprosy and TB/HIV prevention and control programme, 3rd ed Federal Ministry of Health, Addis Ababa, Ethiopia [Google Scholar]
- 19. Feng C, Bean A, Hooi H, Briscoe H, Britton W. 1999. Increase in gamma-interferon secreting CD8+, as well as CD4+ T cells in lungs following aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 67:3242–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franken KL, et al. 2000. Purification of His-tagged proteins by immobilized chelate affinity chromatography: the benefits from the use of organic solvent. Protein Expr. Purif. 18:95–99 [DOI] [PubMed] [Google Scholar]
- 21. Frieden TR, Sterling SS, Munsiff Watt CJ, Dye C. 2003. Tuberculosis. Lancet 362:887–899 [DOI] [PubMed] [Google Scholar]
- 22. Handzel ZT, et al. 2007. Increased Th1 and Th2 type cytokine production in patients with active tuberculosis. IMAJ 9:479–483 [PubMed] [Google Scholar]
- 23. Hervas-Stubbs S, et al. 2006. High frequency of CD4+ T cells specific for the TB10.4 protein correlates with protection against Mycobacterium tuberculosis infection. Infect. Immun. 74:3396–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jabado N, Philippe G. 2005. Tuberculosis: the genetics of vulnerability. Nature 434:709–711 [DOI] [PubMed] [Google Scholar]
- 25. Kaufmann SHE, Parida SK. 2008. Tuberculosis in Africa: learning from pathogenesis for biomarker identification. Cell Host Microbe 4:219–228 [DOI] [PubMed] [Google Scholar]
- 26. Khader SA, Cooper AM. 2008. IL-23 and IL-17 in tuberculosis. Cytokine 41:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Launois P, et al. 1994. T-cell epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect. Immun. 62:3679–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leyten EM, et al. 2006. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis. Microbes Infect. 8:2052–2060 [DOI] [PubMed] [Google Scholar]
- 29. Madi NM, Al-Attiyah R, Fahaban Mustafa AS, Atbal Fftung Hgiker Ravn P, Andersen P. 1998. Effective chemotherapy restores T-cell responses to M. tuberculosis antigens in tuberculosis patients, p 439–442 In Talwar GP, Nath I, Ganguly NK, Rao KVS. (ed), Proceedings of the 10th International Immunology Congress. Monduzzi Editore, Bologna, Italy [Google Scholar]
- 30. Mattos AM, et al. 2010. Increased IgG1, IFN-{gamma}, TNF-{alpha} and IL-6 responses to Mycobacterium tuberculosis antigens in patients with tuberculosis are lower after chemotherapy. Int. Immunol. 22:775–782 [DOI] [PubMed] [Google Scholar]
- 31. Mukamolova GV, et al. 2002. The rpf gene of Micrococcus luteus encodes an essential secreted growth factor. Mol. Microbiol. 46:611–621 [DOI] [PubMed] [Google Scholar]
- 32. Mustafa AS. 1996. Recombinant mycobacterial antigens/epitopes recognized by human T-cells, p 201–211 In Mustafa AS, Al-Attiyah R, Nath I, Chugh TD. (ed), T-cell subsets and cytokines interplay in infectious diseases. Karger, Basel, Switzerland [Google Scholar]
- 33. Mustafa AS. 2002. Development of new vaccines and diagnostic reagents against tuberculosis. Mol. Immunol. 39:113–119 [DOI] [PubMed] [Google Scholar]
- 34. Mustafa AS, Al-Saidi F, El-Shamy AS, Al-Attiyah R. 2011. Cytokines in response to proteins predicted in genomic regions of difference of Mycobacterium tuberculosis. Microbiol. Immunol. 55:267–278 [DOI] [PubMed] [Google Scholar]
- 35. Nemeth J, et al. 2011. Specific cytokine patterns of pulmonary tuberculosis in Central Africa. Clin. Immunol. 138:50–59 [DOI] [PubMed] [Google Scholar]
- 36. Ottenhoff THM, Kaufmann SHE. 2012. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 8:e1002607 doi:10.1371/journal.ppat.1002607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ravn P, et al. 1999. Human T-cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637–645 [DOI] [PubMed] [Google Scholar]
- 38. Romano M, et al. 2012. Potential of Mycobacterium tuberculosis resuscitation-promoting factors as antigens in novel tuberculosis sub-unit vaccines. Microbes Infect. 14:86–95 [DOI] [PubMed] [Google Scholar]
- 39. Roupie V, et al. 2007. Immunogenicity of eight dormancy regulon-encoded proteins of Mycobacterium tuberculosis in DNA-vaccinated and tuberculosis-infected mice. Infect. Immun. 75:941–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sable SB, Verma I, Behera D, Khuller GK. 2005. Human immune recognition-based multicomponent subunit vaccines against tuberculosis. Eur. Respir. J. 25:902–910 [DOI] [PubMed] [Google Scholar]
- 41. Sable SB, Kalra M, Verma I, Khuller GK. 2007. Tuberculosis subunit vaccine design: the conflict of antigenicity and immunogenicity. Clin. Immunol. 122:239–251 [DOI] [PubMed] [Google Scholar]
- 42. Schindler R, et al. 1990. Correlation and interactions in the production of IL-6, IL-1 and TNF in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 75:40–47 [PubMed] [Google Scholar]
- 43. Schoel B, Gulle H, Kaufmann SHE. 1992. Heterogeneity of the repertoire of T cells of tuberculosis patients and healthy contacts of Mycobacterium tuberculosis antigens separated by high-resolution techniques. Infect. Immun. 60:1717–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schuck SD, et al. 2009. Identification of T-cell antigens specific for latent Mycobacterium tuberculosis infection. PLoS One 4:e5590 doi:10.1371/journal.pone.0005590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sherman DR, et al. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. U. S. A. 98:7534–7539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Talaat AM, et al. 2007. Mycobacterial bacilli are metabolically active during chronic tuberculosis in murine lungs: insights from genome-wide transcriptional profiling. J. Bacteriol. 189:4265–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsenova L, et al. 2007. BCG vaccination confers poor protection against M. tuberculosis HN878-induced central nervous system disease. Vaccine 25:5126–5132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Van Crevel R, Ottenhoff T, van der Meer J. 2002. Innate immunity to M. tuberculosis. Clin. Microbiol. Rev. 15:294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weire RE, Morgan AR, Britton WJ, Butlin CR, Dockrell HM. 1994. Development of a WB assay to measure T cell responses to leprosy: a new tool for immuno-epidemiological field studies of leprosy immunity. J. Immunol. Methods 176:93–101 [DOI] [PubMed] [Google Scholar]
- 50. WHO 2010. Global tuberculosis control 2010. WHO, Geneva, Switzerland [Google Scholar]
- 51. Winek J, et al. 2008. Interferon gamma production in the course of Mycobacterium infection. J. Physiol. Pharmacol. 59(Suppl. 6):751–775 [PubMed] [Google Scholar]
- 52. Yeremeev VV, et al. 2003. Proteins of the Rpf family: immune cell reactivity and vaccination efficacy against tuberculosis in mice. Infect. Immun. 71:4789–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zvi A, Arie N, Fulkerson J, Sadoff JC, Shafferman A. 2008. Whole genome identification of Mycobacterium tuberculosis vaccine candidates by comprehensive data mining and bioinformatic analyses. BMC Med. Genomics 1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]