Abstract
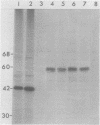
Tumors were produced in quails about 2 months after injection with a transformation-defective mutant of the Schmidt-Ruppin strain of Rous sarcoma virus, subgroup A (SR-A), that retains a small portion of the src gene. Sarcoma viruses were isolated from each of five such tumors. A transformation-defective mutant which has a nearly complete deletion of the src gene was unable to induce tumors. The avian sarcoma viruses recovered from quail tumors (rASV-Q) had biological properties similar to those of the avian sarcoma viruses previously acquired from chicken tumors (rASV-C); these chicken tumors had been induced by the same transformation-defective mutants. Both rASV-Q and rASV-C transformed cells in culture with similar focus morphology and produced tumors within 7 to 14 days after injection into chickens or quails. The size of rASV-Q genomic RNA was indistinguishable from that of SR-A by polyacrylamide gel electrophoresis. The sequences of rASV-Q RNA genomes were analyzed and compared with those of the parental transformation-defective virus, SR-A and of rASV-C by RNase T1 fingerprinting and oligonucleotide mapping. We found that the src sequences of all five isolates of rASV-Q were identical to each other but different from those of SR-A and rASV-C. Of 13 oligonucleotides of rASV-Q identified as src specific, two were not found in either SR-A or rASV-C RNA. Furthermore, some oligonucleotides present in SR-A or rASV-C or both were absent in rASV-Q. No differences were found for the sequences outside the src region in any of the viruses examined. In addition, rASV-Q-infected cells possessed a 60,000-dalton protein specifically precipitable by rabbit serum raised against SR-D-induced tumors. The facts that the src sequences are essentially the same for rASV's recovered from one animal species and different for rASV's obtained from different species provide conclusive evidence that cellular sequences of normal birds were inserted into the viral genome and supplied to the resulting recombinant viruses genetic information for cell transformation.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Tumor viruses: 1974. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1187–1200. doi: 10.1101/sqb.1974.039.01.137. [DOI] [PubMed] [Google Scholar]
- Biggs P. M., Milne B. S., Graf T., Bauer H. Oncogenicity of non-transforming mutants of avian sarcoma viruses. J Gen Virol. 1973 Mar;18(3):399–403. doi: 10.1099/0022-1317-18-3-399. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Brugge J. S., Erikson R. L. Characterization of a normal avian cell protein related to the avian sarcoma virus transforming gene product. Cell. 1978 Dec;15(4):1363–1369. doi: 10.1016/0092-8674(78)90061-2. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Purchio A. F., Brugge J. S., Erikson R. L. A normal cell protein similar in structure and function to the avian sarcoma virus transforming gene product. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3159–3163. doi: 10.1073/pnas.76.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Erikson E., Collett M. S., Erikson R. L. In vitro synthesis of a functional avian sarcoma virus transforming-gene product. Nature. 1978 Aug 31;274(5674):919–921. doi: 10.1038/274919a0. [DOI] [PubMed] [Google Scholar]
- Halpern C. C., Hayward W. S., Hanafusa H. Characterization of some isolates of newly recovered avian sarcoma virus. J Virol. 1979 Jan;29(1):91–101. doi: 10.1128/jvi.29.1.91-101.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Halpern C. C., Buchhagen D. L., Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Hayward W. S., Hanafusa H. Cellular information in the genome of recovered avian sarcoma virus directs the synthesis of transforming protein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3154–3158. doi: 10.1073/pnas.76.7.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Duesberg P. H., Hanafusa H. Transformation-defective mutants of Rous sarcoma virus with src gene deletions of varying length. J Virol. 1977 Dec;24(3):910–914. doi: 10.1128/jvi.24.3.910-914.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Genetic recombination with avian tumor virus. Virology. 1972 Jul;49(1):37–44. doi: 10.1016/s0042-6822(72)80005-9. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Hu S. S., Vogt P. K. Occurrence of partial deletion and substitution of the src gene in the RNA genome of avian sarcoma virus. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4781–4785. doi: 10.1073/pnas.74.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchase H. G., Okazaki W., Vogt P. K., Hanafusa H., Burmester B. R., Crittenden L. B. Oncogenicity of avian leukosis viruses of different subgroups and of mutants of sarcoma viruses. Infect Immun. 1977 Feb;15(2):423–428. doi: 10.1128/iai.15.2.423-428.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins T., Duesberg P. Specific RNA sequences of Rous sarcoma virus (RSV) recovered from tumors induced by transformation-defective RSV deletion mutants. Virology. 1979 Mar;93(2):427–434. doi: 10.1016/0042-6822(79)90246-0. [DOI] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsamen H., Friis R. R., Bauer H. Src Gene product from different strains of avian sarcoma virus: Kinetics and possible mechanism of heat inactivation of protein kinase activity from cells infected by transformation-defective, temperature-sensitive mutant and wild-type virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):967–971. doi: 10.1073/pnas.76.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMIZU T., RUBIN H. THE DUAL ORIGIN OF NONINFECTIVE ROUS SARCOMAS. J Natl Cancer Inst. 1964 Jul;33:79–91. [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Product of in vitro translation of the Rous sarcoma virus src gene has protein kinase activity. J Virol. 1979 Apr;30(1):311–318. doi: 10.1128/jvi.30.1.311-318.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Smith K., Padgett T., McCombe P., Roulland-Dussoix D., Moscovici C., Varmus H. E., Bishop J. M. Uninfected avian cells contain RNA related to the transforming gene of avian sarcoma viruses. Cell. 1978 Feb;13(2):371–379. doi: 10.1016/0092-8674(78)90205-2. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Vigne R., Breitman M. L., Moscovici C., Vogt P. K. Restitution of fibroblast-transforming ability in src deletion mutants of avian sarcoma virus during animal passage. Virology. 1979 Mar;93(2):413–426. doi: 10.1016/0042-6822(79)90245-9. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Halpern C. C., Nadel M., Hanafusa H. Recombination between viral and cellular sequences generates transforming sarcoma virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5812–5816. doi: 10.1073/pnas.75.12.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H. The gene order of avian RNA tumor viruses derived from biochemical analyses of deletion mutants and viral recombinants. Annu Rev Microbiol. 1978;32:561–592. doi: 10.1146/annurev.mi.32.100178.003021. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Ikawa Y. Induction of some transformation-related properties by a transformation-defective mutant of avian sarcoma virus. Virology. 1977 Dec;83(2):444–448. doi: 10.1016/0042-6822(77)90192-1. [DOI] [PubMed] [Google Scholar]