Abstract
This study describes a Toxoplasma gondii IgG enzyme-linked immunosorbent assay based on a new chimeric antigen containing three immunodominant regions from the MIC1, MAG1, and SAG1 proteins of the parasite and shows that this test is useful for diagnostic purposes and may replace the lysed and whole-cell antigens.
TEXT
Toxoplasmosis is one of the most common parasitic diseases among humans and warm-blooded animals (8). Although human toxoplasmosis in healthy adults is usually asymptomatic, a serious disease can occur in patients with congenital infections or immunocompromised individuals. Furthermore, despite the exact recognition of its etiology, it remains a serious problem for diagnostics. Toxoplasmosis is commonly diagnosed on the basis of results from serological tests that detect anti-Toxoplasma gondii specific antibodies in a patient's serum sample. The specificities and sensitivities of serology testing rely primarily upon the diagnostic antigen(s) used. Currently, the commercially available serological kits in most cases utilize Toxoplasma lysate antigens (TLAs). Thus, recombinant antigenic proteins of T. gondii could be an alternative source of antigens useful for serodiagnosis of toxoplasmosis. An advantage of their application would be lower costs in testing due to the lower costs in the production and purification of recombinant antigens. Moreover, the use of these antigens would allow better standardization of diagnostic tests.
Over the past 30 years, many different recombinant antigens have been used for detection of T. gondii-specific antibodies in human serum samples (6). However, to date only a few papers have involved the use of chimeric antigens in serodiagnosis of toxoplasmosis (1, 5, 7). Thus, in this study we evaluated the diagnostic usefulness of the new recombinant chimeric antigens containing three immunodominant regions of the proteins MIC1 (residues 25 to 182), MAG1 (residues 30 to 222), and SAG1 (residues 49 to 198) of T. gondii. The DNA encoding the above-mentioned fragments of antigens was amplified from the pUET1/MIC1-MAG1 (5) and pUET1/SAG1 (2) recombinant plasmids by PCR with oligonucleotides M1, M2, S1, and S2 (Table 1). The PCR products of mic1/mag1 and sag1 were mixed and then used as the templates in a PCR with oligonucleotides M1 and S2, which were designed to contain BglII and HindIII sequences. Then, the PCR product was inserted into the pUET1 vector (Blirt S.A., Poland). The MIC1-MAG1-SAG1 recombinant antigen was expressed in Escherichia coli as a fusion protein containing six histidyl residues in the N- and C-terminal ends with a calculated molecular mass of 57.6 kDa and purified by means of one-step metal affinity chromatography. The yield of purified MIC1-MAG1-SAG1 was 20 mg per liter of induced bacterial culture, with a purity of over 96% (data not shown). Furthermore, the reactivity of the new chimeric protein was compared to the reactivity of a mixture of three recomddbinant antigens (designated M; rMIC1ex2 plus rMAG1 plus rSAG1) and a previously studied MIC1-MAG1 chimeric protein (5). These antigens were obtained by the methods described previously (2–5). In order to determine the diagnostic utility of the antigens, an in-house immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) was used. The IgG ELISA was carried out as described previously (5). Each of the antigens was used at a concentration of 2.5 μg per ml. A total of 270 serum samples received from a routine toxoplasmosis screening were analyzed and divided into four groups according to the results obtained with commercial tests (Vidas Toxo-IgG II, Vidas Toxo-IgG avidity, and Vidas Toxo-IgM): group I (IgM positive, IgG low or borderline avidity), based on 47 serum samples from patients suspected to have acute toxoplasmosis; group II (IgM negative, IgG low or borderline avidity), based on 19 serum samples from patients with postacute toxoplasmosis; group III (IgM negative, IgG high avidity), based on 96 serum samples from patients with chronic toxoplasmosis, which were further divided into three subgroups (IIIA, 19 with high titers of IgG of >300 IU/ml; IIIB, 48 with titers between 51 and 300 IU/ml; IIIC, 29 with low titers of ≤50 IU/ml); group IV, 108 serum samples from seronegative individuals. The reactivities of all antigen preparations against a total pool of seropositive sera were tested on the same day. Each serum sample was examined twice, and the results were determined for each serum sample by calculating the mean value of the optical density (OD) for duplicate wells. A positive result was any value higher than the average OD reading plus 2 standard deviations (cutoff) obtained with 23 sera from group IV. Moreover, reference sera (positive and negative) for each ELISA plate were used in all experiments as controls.
Table 1.
Oligonucleotide primers used for construction of the MIC1-MAG1-SAG1 chimeric antigen
| T. gondii gene(s) | Primer name | Primer sequence | Underlined sequence | Template for amplification |
|---|---|---|---|---|
| mic1/mag1 | M1 (forward) | 5′-GTGCCAGATCTAGCGTCGCATTCTCATTCG-3′ | BglII | pUET1/MIC1-MAG1 |
| M2 (reverse) | 5′-GGCAACAAGAGGGGGATCACCAGATCCCTGAACCC-3′ | Fragment of sag1 | ||
| sag1 | S1 (forward) | 5′-GGGTTCAGGGATCTGGTGATCCCCCTCTTGTTGCC-3′ | Fragment of mag1 | pUET1/SAG1 |
| S2 (reverse) | 5′-GCGGCCGCAAGCTTAAGAGTGCTGTCTGCAC-3′ | HindIII |
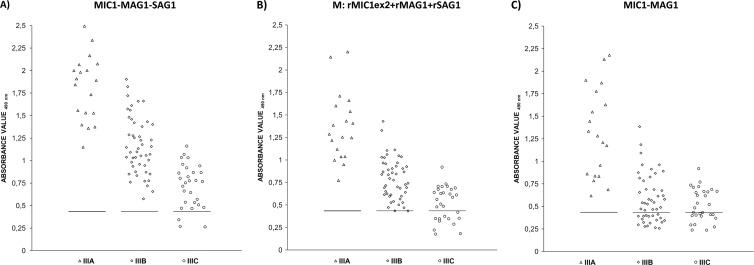
The MIC1-MAG1-SAG1 chimeric antigen, the mixture of three antigens (M), and the MIC1-MAG1 protein reacted with 98.1%, 90.7%, and 81.5% of the positive sera, respectively (Table 2). None of the 85 negative serum samples from group IV reacted above the cutoff values for the MIC1-MAG1-SAG1 chimeric protein or the mixture, resulting in a specificity of 100% for the ELISAs, whereas for the MIC1-MAG1 antigen, one result above the cutoff was observed (specificity, 98.8%). IgG antibodies in group I sera reacted significantly (100%) with the new chimeric protein, the MIC1-MAG1 recombinant antigen, and the mixture of proteins, whereas IgG antibodies in group II sera reacted with the same sensitivity with the two chimeric proteins but insignificantly (78%) with the mixtures of antigens. The most diverse reactivity was observed for the antibodies of sera from III group, which contained various titers of specific IgG (Fig. 1). However, the highest reactivity (96.9%) with this group of sera was obtained only with the preparation of the new chimeric protein, while for the mixture or MIC1-MAG1 antigen, reactivity was 88.5% and 68.8%, respectively. Moreover, in our previous study employing an IgG ELISA with a MIC1-MAG1chimeric antigen, we found that IgG antibodies to this protein were not detected in some chronic sera (85.1%; 57 positive sera from a group consisting of 67 samples) (5). When the same sera were examined in the IgG ELISA with the new MIC1-MAG1-SAG1 chimeric antigen, all of these sera gave readings suggestive of toxoplasmosis (Table 3).
Table 2.
Immunoreactivities of the MIC1-MAG1 chimeric antigen, the mixture of recombinant antigens,a and the MIC1-MAG1-SAG1 chimeric antigen using sera from individuals in the acute, postacute, or chronic phase of toxoplasmosis and sera from healthy patients
| Serum sample group and antigen | No. (%) of reactive serum samples | Mean OD (range)b |
|---|---|---|
| Ic | ||
| MIC1-MAG1 | 47 (100) | 1.246 (0.490–2.765) |
| M | 47 (100) | 1.324 (0.598–2.873) |
| MIC1-MAG1-SAG1 | 47 (100) | 1.677 (0.670–2.961) |
| IId | ||
| MIC1-MAG1 | 19 (100) | 0.634 (0.436–1.071) |
| M | 15 (78.9) | 0.648 (0.279–1.108) |
| MIC1-MAG1-SAG1 | 19 (100) | 0.868 (0.482–1.487) |
| IIIe | ||
| MIC1-MAG1 | 66 (68.8) | 0.707 (0.237–2.178) |
| M | 85 (88.5) | 0.823 (0.177–2.202) |
| MIC1-MAG1-SAG1 | 93 (96.9) | 1.159 (0.264–2.494) |
| Totalf | ||
| MIC1-MAG1 | 132 (81.5) | 0.855 (0.237–2.765) |
| M | 147 (90.7) | 0.948 (0.177–2.873) |
| MIC1-MAG1-SAG1 | 159 (98.1) | 1.275 (0.264–2.961) |
| IVg | ||
| MIC1-MAG1 | 1 (1.2) | 0.339 (0.237–0.497) |
| M | 0 | 0.265 (0.140–0.417) |
| MIC1-MAG1-SAG1 | 0 | 0.308 (0.151–0.421) |
M, rMIC1ex2 plus rMAG1 plus rSAG1.
The cutoff values were 0.428 for MIC1-MAG1, 0.428 for M, and 0.434 for MIC1-MAG1-SAG1.
Acute phase of toxoplasmosis (n = 47).
Postacute phase of toxoplasmosis (n = 19).
Chronic phase of toxoplasmosis (n = 96).
Total results for samples from patients with any of the three stages of toxoplasmosis (n = 162).
Control group, negative for anti-T. gondii antibodies (n = 85).
Fig 1.
Immunoreactivities of the MIC1-MAG1-SAG1 chimeric antigen (A), the mixture of recombinant antigens (M; rMIC1ex2 plus rMAG1 plus rSAG1) (B), and the MIC1-MAG1 chimeric antigen (C) with IgG antibodies in serum samples from patients with chronic toxoplasmosis, divided according to IgG titers, as follows: >300 IU/ml (Δ); 51 to 300 IU/ml (◇); ≤50 IU/ml (○). The horizontal lines represent the cutoff values.
Table 3.
Immunoreactivities of the two chimeric antigens (MIC1-MAG1 and MIC1-MAG1-SAG1) or the mixture of recombinant proteins (M)a with IgG antibodies in selected sera from group III (subgroup IIIC)
| Sample no. | IgG level (IU/ml)b | IgG ELISA (OD492)c |
||
|---|---|---|---|---|
| MIC1-MAG1 | M | MIC1-MAG1-SAG1 | ||
| 1 | 18 | 0.397 | 0.510 | 0.767 |
| 2 | 31 | 0.391 | 0.374 | 0.775 |
| 3 | 10 | 0.297 | 0.349 | 0.468 |
| 4 | 28 | 0.409 | 0.561 | 0.866 |
| 5 | 12 | 0.272 | 0.420 | 0.533 |
| 6 | 9 | 0.404 | 0.320 | 0.662 |
| 7 | 8 | 0.408 | 0.345 | 0.508 |
| 8 | 26 | 0.420 | 0.622 | 0.820 |
| 9 | 50 | 0.282 | 0.525 | 1.035 |
| 10 | 44 | 0.263 | 0.547 | 0.871 |
M antigen is a mixture of rMIC1ex2 plus rMAG1 plus rSAG1.
Determined using a commercial test (Vidas; bioMérieux). Positive results were defined as values above or equal to 8 IU/ml.
Positive results (indicated in bold type) were defined as ODs above cutoff values (0.428 for MIC1-MAG1, 0.428 for M, and 0.434 for MIC1-MAG1-SAG1).
The results of our study showed that in the case of antigen construction for diagnostic utility, a rational selection of protein fragments is of great importance. The MIC1-MAG1-SAG1 chimeric proteins containing different immunodominant regions from three T. gondii antigens yielded better results than the chimeric antigen, which contained only two fragments, from the MIC1 and MAG1 proteins. The addition to the chimeric protein of the fragment of surface antigen (SAG1), which is one of the most immunogenic proteins of the parasite, resulted in an increase in the reactivity with specific IgG antibodies from sera of patients with chronic toxoplasmosis. Thus, the sensitivity of the IgG ELISA with a new chimeric antigen was nearly 100% (an absorbance value below the cutoff was reported for only three serum samples). Furthermore, the results obtained in this study confirm our previous studies, which indicated that a properly constructed chimeric antigen containing several different immunodominant regions performs better than a mixture of three proteins and can replace the preparation of TLA for serodiagnosis of human toxoplasmosis (5). Therefore, the new chimeric antigen could provide the basis for commercial immunoassays for detection of T. gondii infection in humans, based on serum samples. Moreover, this antigen is the most promising and effective among all published chimeric proteins. However, further work is needed before an immunoassay with this recombinant product will be available for clinical proposes.
Footnotes
Published ahead of print 3 October 2012
REFERENCES
- 1. Beghetto E, Spadoni A, Bruno L, Buffolano W, Gargano N. 2006. Chimeric antigens of Toxoplasma gondii: toward standardization of toxoplasmosis serodiagnosis using recombinant products. J. Clin. Microbiol. 44:2133–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hiszczyńska-Sawicka E, et al. 2003. High yield expression and single-step purification of Toxoplasma gondii SAG1, GRA1, and GRA7 antigens in Escherichia coli. Protein Expr. Purif. 27:150–157 [DOI] [PubMed] [Google Scholar]
- 3. Holec L, Gąsior A, Brillowska-Dąbrowska A, Kur J. 2008. Toxoplasma gondii: enzyme-linked immunosorbent assay using different fragments of recombinant microneme protein 1 (MIC1) for detection of immunoglobulin G antibodies. Exp. Parasitol. 119:1–6 [DOI] [PubMed] [Google Scholar]
- 4. Holec L, Hiszczyńska-Sawicka E, Gąsior A, Brillowska-Dąbrowska A, Kur J. 2007. Use of MAG1 recombinant antigen for detection of Toxoplasma gondii infection In humans. Clin. Vaccine Immunol. 14:220–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holec-Gąsior L, Ferra F, Drapała D, Lautenbach D, Kur J. 2012. A new MIC1-MAG1 recombinant chimeric antigen can be used instead of the Toxoplasma gondii lysate antigen in serodiagnosis of human toxoplasmosis. Clin. Vaccine Immunol. 19:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kotresha D, Noordin R. 2010. Recombinant proteins in the diagnosis of toxoplasmosis. APMIS 118:529–542 [DOI] [PubMed] [Google Scholar]
- 7. Lau YL, Thiruvengadam G, Lee WW, Fong MY. 2011. Immunogenic characterization of the chimeric surface antigen 1 and 2 (SAG1/2) of Toxoplasma gondii expressed in the yeast Pichia pastoris. Parasitol. Res. 109:871–878 [DOI] [PubMed] [Google Scholar]
- 8. Tenter AM, Heckeroth AR, Weiss LM. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]