Abstract
Circulating immune complexes (ICs) are associated with the pathogenesis of several diseases. Very little is known about the effect of ICs on the host immune response in patients with tuberculosis (TB). The effects of ICs isolated from patients with TB in modulating the release of calcium, cytokines, and granular proteins were studied in normal granulocytes, as were their chemotactic, phagocytic, and oxidative burst processes. ICs from TB patients induced decreased production of cytokines and platelet-activating factor (PAF) from normal granulocytes. ICs from TB patients also induced enhanced chemotaxis and phagocytosis but caused diminished oxidative burst. This was accompanied by an increased release in intracellular calcium. On the other hand, ICs from TB patients induced increased release of the granular proteins human neutrophil peptides 1 to 3 (HNP1–3). Thus, ICs from patients with TB exhibit a profound effect on granulocyte function with activation of certain effector mechanisms and dampening of others.
INTRODUCTION
Several reports have shown the prevalence of high levels of immune complexes (ICs) in pulmonary tuberculosis (PTB) (32). Mycobacterial antigens, antimycobacterial antibodies, and C3 and C4 components have been demonstrated in ICs isolated from sera from patients with active TB (8). Similarly, the mean levels of circulating immune complex (CIC) in children with TB were found to be significantly higher than those in healthy children (35). A longitudinal study done by Johnson et al. (16) suggested that the levels of CIC are related to disease progression, as elevated CIC levels decreased to normal limits following treatment in patients with active TB.
Apart from circulating immune complexes, those deposited in tissues might also modulate disease pathogenesis in patients with TB, as suggested by previous studies. One of these studies reported the presence of extravascular immune complexes with high bacterial load and low cell-mediated immunity in experimental TB (26). Another study concluded that the antigen/antibody ratio within the lesions might be crucial in modulating the balance between tissue destruction and healing (27). Another study of humans showed that the occurrence of Henoch-Schönlein purpura nephritis in patients with pulmonary tuberculosis was associated with the deposition of circulating immune complexes (18). While the presence of ICs is established in both circulation and in tissues in many inflammatory responses, including TB, the triggers and mediators downstream of the IC have been less well studied.
The critical role of humoral immune responses has been relatively less extensively studied than has the T cell response in TB. Antibodies can have a significant impact on host immunity and disease outcome in TB by engagement of Fc gamma (Fcγ) receptors that can influence both Th1 activation and mycobacterial containment. ICs are known to modulate cellular functions by several mechanisms, including induction of activating or inhibitory signals (25). Through Fcγ receptor binding, ICs link the specificity of the adaptive immune system and the powerful effector functions triggered by innate immune effector cells (24). In active infections, including TB, large numbers of ICs are generated owing to the priming of antigen-specific B cells. It has been reported that ICs trigger activation cascades in Mycobacterium tuberculosis infection that limit susceptibility to infection (19).
Several lines of evidence support a role for neutrophils in the immune response to M. tuberculosis. First, neutrophils are recruited early to the lungs of mice with TB, and depletion of neutrophils results in increased bacterial growth. Second, in vitro studies suggest that human neutrophils are capable of inhibiting the growth of M. tuberculosis. Third, neutrophils are readily detected in sputum and bronchoalveolar lavage fluid samples from humans with active pulmonary tuberculosis (22). Also, a number of studies have shown the critical role played by granulocytes in mouse models of IC-mediated diseases (39, 40). Phagocytosis in neutrophils has been shown to be an active process, often dependent on the presence of functionally active Fcγ (33). In addition, generation of oxidative burst, degranulation, and changes in cytoplasmic calcium have also been shown to be induced by ICs in granulocytes (1).
As mentioned above, there are very few data on the functional role played by ICs in patients with TB. We hypothesized that one mechanism by which ICs could potentially influence disease activity in TB is by modulating granulocyte activation and function. To test our hypothesis, we examined the effect of ICs from patients with TB on granulocyte function. We demonstrate that ICs from individuals with TB modulate granulocyte activation and effector function, a process that could potentially augment pathology or contribute to protection by activating innate immune effectors.
MATERIALS AND METHODS
Study subjects.
Serum samples were obtained from 10 healthy Indian subjects (hereafter referred to as UNINF [for uninfected]; mean age ± standard deviation [SD], 35 ± 12.3 years; 7 males and 3 females) and 10 patients with active TB (hereafter referred to as INF [for infected]; mean age ± SD, 30 ± 7.3 years; 7 males and 3 females). Responder granulocytes were obtained from a separate set of 10 healthy, uninfected volunteers. All INF individuals were culture positive for M. tuberculosis. Patients with active tuberculosis were excluded if they were pregnant or had concomitant diabetes mellitus, autoimmunity or immunodeficiency, or any other acute or chronic infections by thorough medical history and clinical examination. Subjects were enrolled prior to commencement of anti-TB therapy. The patients included were all newly diagnosed for PTB, and none had extrapulmonary involvement. The healthy volunteers were matched on the basis of ethnicity and geographic location. On the basis of a detailed clinical history, controls included in the study did not have a history of TB or current signs or symptoms consistent with TB. None of the subjects included in the study had evidence of HIV infection. The study was approved by the Institutional Ethical Committee of the Tuberculosis Research Center, and informed written consent was obtained from all participants.
Isolation of serum.
Blood samples were collected, left for 1 h at room temperature to clot, and then subjected to centrifugation at 2,200 × g for 20 min at 4°C. The serum devoid of clots was then transferred to serum storage vials and stored at −80°C.
CIC purification.
Serum (50 μl) was incubated with an equal volume of 5% polyethylene glycol 6000 (PEG 6000) (final concentration of 2.5% in phosphate-buffered saline [PBS]) at 4°C overnight. The serum was centrifuged at 2,000 rpm for 30 min at 4°C. The precipitate was washed twice with PBS and suspended in 500 μl of PBS (pH 7.4). The precipitate was undisturbed for 30 min at room temperature. The absorbance of the precipitate was read at 280 nm using a spectrophotometer. CIC levels were determined by interpolation from a standard curve plotted using aggregated human gamma globulins as a standard. The isolated ICs were diluted to the initial serum volume in sterile PBS and were used at a concentration of 10% (vol/vol) in in vitro culture assays.
Whole-blood culture and granulocyte isolation.
Granulocytes were isolated as described previously (3). Briefly, the anticoagulated whole blood was treated with Ficoll-Hypaque, which allowed the separation of peripheral blood mononuclear cells (PBMC) and the granulocytes were layered over the erythrocytes. After the PBMC and dextran layers were removed, dextran was added to the granulocyte and erythrocyte layers, which were left undisturbed for 45 min at room temperature. Once the erythrocytes were removed, the granulocytes were sedimented using centrifugation, washed with RPMI 1640, and then used for analysis. Flow cytometric analysis was performed to assess the purity of the isolated granulocytes. Granulocytes were first gated using forward and side scatter and then by selecting CD15+ cells. The purity of granulocytes within the sorted CD15 cell population was typically >95% (data not shown).
In vitro culture.
Either whole blood or isolated granulocytes used as the responder cells to study the effect of immune complex admixture were obtained from 10 healthy volunteers. The whole blood or granulocytes from each healthy volunteer were stimulated with ICs from one UNINF subject and one INF subject individually. Either whole blood or granulocytes were cultured with RPMI 1640 in the presence of PEG 6000-precipitated ICs (10% [vol/vol]) isolated from INF and UNINF individuals in 24-well tissue culture plates (Corning, Corning, NY) using 2 × 106 cells per well (isolated granulocytes). PEG 6000 precipitates isolated for cell culture assays were prepared fresh and used immediately without freezing or thawing. Unstimulated cells without the presence of IC (as negative controls) from both the study groups and cells stimulated with formyl-Met-Leu-Phe (fMLP) (as positive controls) were also included for all 10 healthy volunteers.
Multiplex cytokine enzyme-linked immunosorbent assay (ELISA).
Normal granulocytes were stimulated with isolated ICs along with appropriate culture controls. After a stimulation period of 18 h, the culture supernatants were used for analysis of cytokines using the Bioplex multiplex cytokine assay system (Bio-Rad). The cytokines analyzed were interleukin-1β (IL-1β), IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-17, granulocyte-macrophage colony-stimulating factor (GM-CSF), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α).
HNP1–3 and platelet-activating factor ELISA.
Supernatants from whole blood stimulated with or without isolated ICs for 4 h at 37°C were collected and stored at −80°C. The levels of human neutrophil peptides 1 to 3 (HNP1–3) (Hycult Biotech, Canton, MA) and platelet-activating factor (PAF) (Uscn Life Science Inc., Wuhan, China) were assessed using commercially available ELISA kits.
Chemotaxis.
Migration of granulocytes was assessed using the Migratest kit (Orpegen Pharma, Heidelberg, Germany). The number of granulocytes from whole blood stimulated with or without ICs (10 min at 37°C) from UNINF and INF individuals that migrated toward the chemotactic peptide fMLP was determined.
Phagocytosis.
Whole blood was assayed for phagocytic activity by flow cytometry using the Phagotest kit (Orpegen Pharma). Escherichia coli commercially labeled with fluorescein isothiocyanate (Orpegen Pharma) was added to aliquots of heparinized blood in the presence or absence of ICs (blood samples from UNINF and INF individuals) and incubated for 10 min at 37°C. The percentage of cells that phagocytosed the fluorescein isothiocyanate-labeled bacteria was then determined by flow cytometry using a BD FACSCalibur instrument (BD Biosciences).
Oxidative burst.
Whole blood was assayed for oxidative burst by flow cytometry using a BD FACSCalibur instrument (BD Biosciences) based on the cleavage of dihydrorhodamine 123 by oxidative species to rhodamine using a Bursttest kit (Orpegen Pharma) according to the manufacturer's instructions.
Calcium levels.
The effect of IC admixture on intracellular calcium mobilization was assessed using the Screen Quest Fluo-8 no-wash calcium assay kit (AAT Bioquest, Inc., Sunnyvale, CA). Isolated granulocytes were incubated with 100 μl of Fluo-8 NW dye-loading solution. The cells were incubated in a 5% CO2 incubator for 30 min and then at room temperature for another 30 min. Fluorescence at 490 nm was recorded for 2 min, at which point immune complexes were added (10 μl). Fluorescence was then recorded for an additional 5 min. The data are presented as percentages of nontreated control for which calcium-associated fluorescence was measured in parallel cultures at the same time points. Cell counts were performed, and densities were adjusted for each culture before dye loading to ensure exactly the same cell densities for all study conditions.
Nitric oxide levels.
Normal granulocytes were stimulated with isolated ICs along with appropriate controls. After a stimulation period of 18 h, the culture supernatants were collected and used to measure nitric oxide (NO) using a colorimetric nonenzymatic assay for NO (Oxford Biomedical Research, Rochester Hills, MI) exactly as described by the manufacturer. Briefly, 200-μl portions of supernatants were incubated at room temperature overnight with 0.5 g of dry granulated cadmium while being agitated. Following a 5-min incubation at room temperature with the colorimetric reagents provided, absorbance at 540 nm was read.
Statistical analysis.
Geometric mean (GM) was used as the measure of central tendency. Comparisons were made using the nonparametric Mann-Whitney U test. P values less than 0.05 were taken to indicate statistical significance. All statistics were performed using GraphPad Prism (V5.0 for Windows; GraphPad Software, Inc., San Diego, CA).
RESULTS
Levels of circulating immune complexes.
The levels of circulating immune complexes were significantly higher (P = 0.004) in TB patients (geometric mean [GM], 12.8 μg/ml; 95% confidence interval [95% CI], 9.0 to 17.8 μg/ml) than in healthy volunteers (GM, 5.5 μg/ml; 95% CI, 3.6 to 8.4 μg/ml). The data are shown in Fig. 1.
Fig 1.
Levels of ICs in serum samples from patients with TB and healthy volunteers. Serum samples from UNINF and INF subjects (10 subjects in each group) were treated with PEG 6000, and the absorbance of the precipitate was read at 280 nm using a spectrophotometer. CIC levels were determined by interpolation from a standard curve plotted using aggregated human gamma globulins as a standard. Each symbol represents the value for one individual. The horizontal line shows the geometric mean. The P values were calculated using the Mann-Whitney U test. UNINF, uninfected individuals; INF, infected patients (patients with TB).
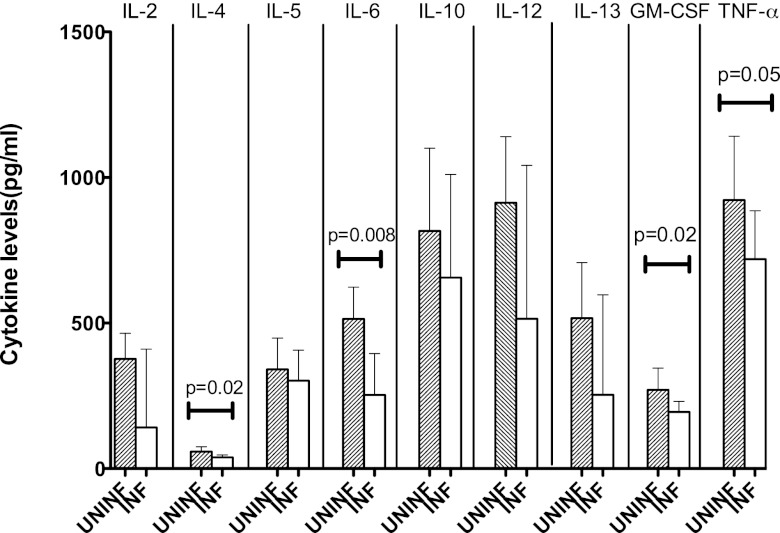
ICs from INF individuals induce decreased release of IL-4, IL-6, GM-CSF, and TNF-α from normal granulocytes.
Immune complexes are known to modulate release of cytokines (15, 31). To determine the effect of ICs from TB patients, we assessed the production of a panel of proinflammatory and anti-inflammatory cytokines from normal granulocytes stimulated with ICs from INF or UNINF subjects (Fig. 2). Expression of cytokines on negative control (unstimulated) and positive control (stimulated with fMLP) was also measured. While the release of cytokines was barely detected in the unstimulated granulocytes (negative control), stimulation with fMLP resulted in increased production of the cytokines (all values in picograms/milliliter) (for IL-4, GM of 20 [95% CI, 1.5 to 30]; for IL-6, GM of 600 [95% CI, 15 to 620]; for GM-CSF, GM of 450 [95% CI, 25 to 525]), and for TNF-α, GM of 490 [95% CI, 12 to 550]). The levels of IL-4 (all values in picograms/milliliter) (for INF subjects, GM of 38.67 [95% CI, 31.54 to 47.40]; for UNINF subjects, GM of 58.72 [95% CI, 45.81 to 75.26]), IL-6 (for INF subjects, GM of 253.2 [95% CI, 162.1 to 395.7]; for UNINF subjects, GM of 514.0 [95% CI, 423.7 to 623.7]), GM-CSF (for INF subjects, GM of 195.0 [95% CI, 164.4 to 231.3]; for UNINF subjects, GM of 270.4 [95% CI, 211.6 to 345.5], and TNF-α (for INF subjects, GM of 719.8 [95% CI, 585.0 to 885.6]; for UNINF subjects, GM of 923.2 [95% CI, 746.3 to 1,142]) were significantly lower in granulocytes that were stimulated in the presence of ICs from INF subjects compared with those cells stimulated with ICs from UNINF subjects. The levels of IL-2, IL-5, IL-10, IL-12, and IL-13 were not significantly different (Table 1), while IL-1β, IL-17, and IFN-γ were not detected in the supernatants. Thus, ICs from TB patients predominantly induced diminished secretion of both proinflammatory cytokines (IL-6, GM-CSF, and TNF-α) and anti-inflammatory cytokines (IL-4) from granulocytes.
Fig 2.
PEG 6000-precipitated plasma ICs from patients with TB induce diminished production of cytokines from granulocytes. Granulocytes from healthy volunteers (n = 10) were stimulated with PEG 6000 precipitates of plasma ICs from UNINF and INF subjects (10 subjects in each group) for 18 h, and cytokines were measured by multiplex ELISA. The P values were calculated using the Mann-Whitney U test. UNINF, uninfected individuals; INF, infected patients (patients with TB). The geometric means (bars) and 95% confidence interval (error bars) are shown.
Table 1.
Levels of interleukins in TB patients and UNINF subjectsa
| Interleukin | GM (pg/ml) (range) of interleukin in: |
|
|---|---|---|
| INF subjects | UNINF subjects | |
| IL-2 | 141.3 (48.69–409.9) | 376.4 (304.6–465.1) |
| IL-5 | 302.0 (224.0–407.1) | 341.1 (259.4–448.5) |
| IL-10 | 655.7 (425.3–1,011) | 816.6 (605.8–1,100) |
| IL-12 | 514.9 (254.6–1,042) | 913.2 (731.0–1,141) |
| IL-13 | 253.8 (377.3–707.3) | 516.6 (377.3–707.3) |
The release of IL-2, IL-5, IL-10, IL-12, and IL-13 was not significantly altered when granulocytes were treated with PEG 6000-precipitated plasma ICs from patients with TB compared to ICs from UNINF subjects. Granulocytes from healthy volunteers (n = 10) were stimulated with PEG precipitates of plasma ICs from UNINF and INF subjects (10 subjects per group) for 18 h, and cytokines were measured by multiplex ELISA. UNINF, uninfected individuals; INF, infected patients (TB patients); GM, geometric mean.
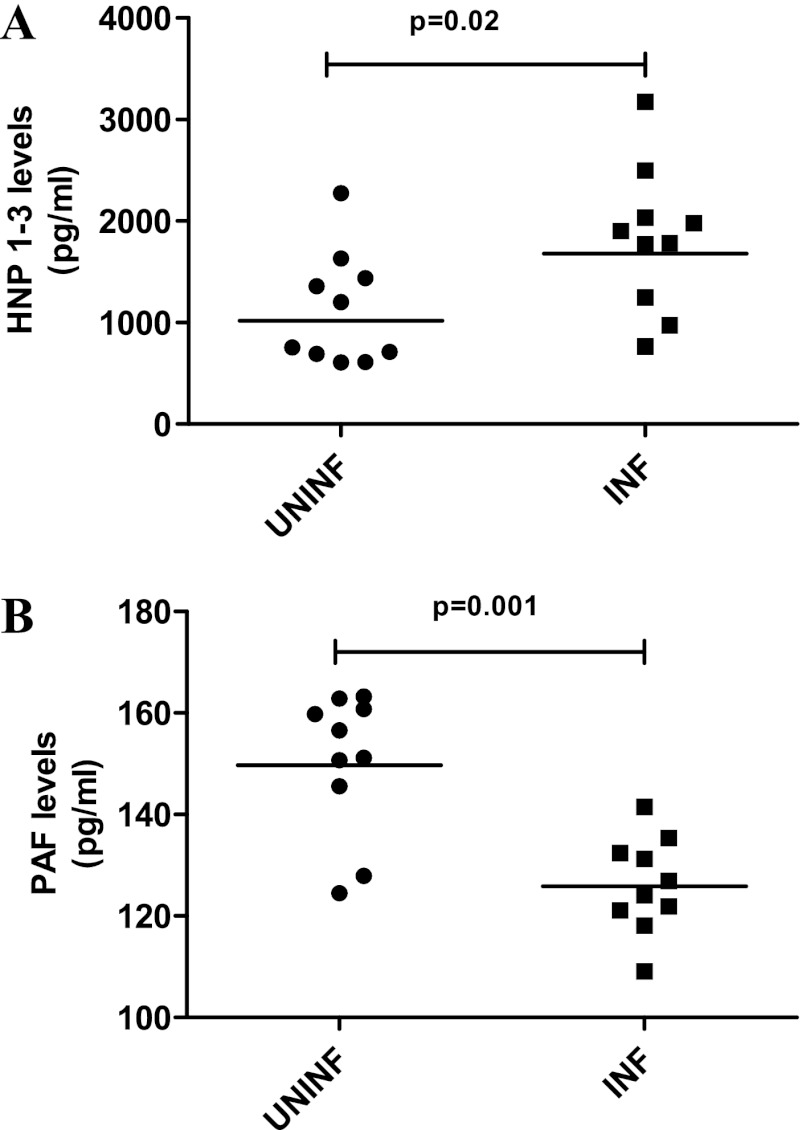
ICs from INF patients induce increased HNP1–3 and reduced PAF levels in whole blood.
Because granulocytes produce antimicrobial peptides and because ICs are known to induce production of these antimicrobial peptides (1), the levels of HNP1–3 upon whole-blood stimulation with ICs were examined. The levels of HNP1–3 were found to be significantly higher in the presence of ICs from INF subjects than in the presence of ICs from UNINF subjects (GM for INF subjects, 1,679 pg/ml; GM of UNINF subjects 1,019 pg/ml; P = 0.02) (Fig. 3A). Secretion of HNP1–3 by the negative control (unstimulated) and positive control (stimulated with fMLP) was also measured (GMs of 50 pg/ml for the negative control and 1,895 pg/ml for the positive control). PAF has been associated with immune complex-mediated diseases (2, 34). The levels of PAF in the supernatants of whole blood stimulated with ICs from INF subjects were significantly lower than those of ICs from UNINF subjects (GMs of 125.9 pg/ml for INF subjects and 149.7 pg/ml for UNINF subjects; P = 0.001) (Fig. 3B). Secretion of PAF by the negative control (unstimulated) and positive control (stimulated with fMLP) was also measured (GMs of 10 pg/ml for the negative control and 200 pg/ml for the positive control). On the other hand, there were no significant effects on ICs from either group on the release of NO from granulocytes (GMs of 164.0 pg/ml for INF subjects versus 164.8 pg/ml for UNINF subjects) (data not shown).
Fig 3.
PEG 6000-precipitated plasma ICs from patients with TB induce increased release of HNP1–3 and decreased release of PAF from granulocytes. Whole-blood samples from healthy volunteers (n = 10) were stimulated with PEG 6000 precipitates of plasma ICs from UNINF and INF subjects (10 subjects in each group) for 4 h, and the granular proteins (HNP1–3) (A) and PAF (B) in the culture supernatants were measured using ELISA. The P values were calculated using the Mann-Whitney U test. UNINF, uninfected individuals; INF, infected patients (patients with TB).
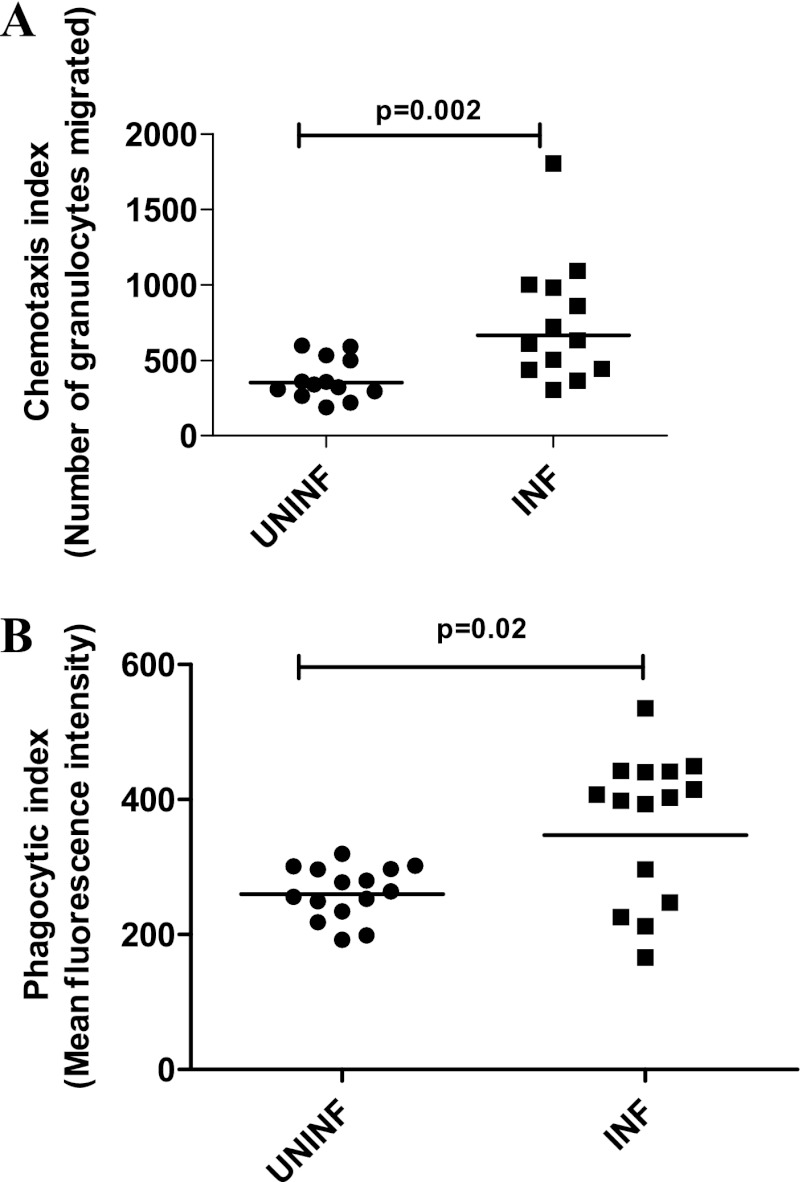
ICs from INF patients induce enhanced chemotaxis and phagocytosis of normal granulocytes.
As IC modulation of granulocytes could potentially involve changes in chemotactic and phagocytic activity, the effects of ICs on chemotaxis and the phagocytic function of granulocytes were examined. As can be seen in Fig. 4A, after stimulation with ICs from INF and UNINF subjects, the number of migrating granulocytes was significantly higher (P = 0.002) in those granulocytes stimulated with ICs from INF subjects (GM, 664.9) compared with those stimulated with ICs from UNINF subjects (GM, 352.0) (Fig. 4). Chemotaxis of negative (unstimulated) and positive (stimulated with fMLP alone) controls was also studied (GMs of 10.0 for the negative control and 1,005.5 for the positive control). Similarly, phagocytic ability was significantly increased (P = 0.02) in those granulocytes stimulated with ICs from INF subjects (GM, 347.3) compared with cells stimulated in the presence of ICs isolated from UNINF subjects (GM, 259.7) (Fig. 4B). Phagocytosis of negative control (cells kept on ice for arrest of phagocytosis) and positive control (cells stimulated with fMLP and incubated at 37°C) was also studied (GMs of 21.5 for the negative control and 500.0 for the positive control).
Fig 4.
PEG 6000-precipitated plasma ICs from patients with TB induce enhanced chemotaxis (A) and phagocytosis (B) of normal granulocytes. Whole-blood samples from healthy volunteers (n = 10) were stimulated with PEG 6000 precipitates of plasma ICs from UNINF and INF (10 subjects in each group) for 10 min, and chemotaxis and phagocytosis of granulocytes were measured using flow cytometry. The P values were calculated using the Mann-Whitney U test. UNINF, uninfected individuals; INF, infected patients (patients with TB).
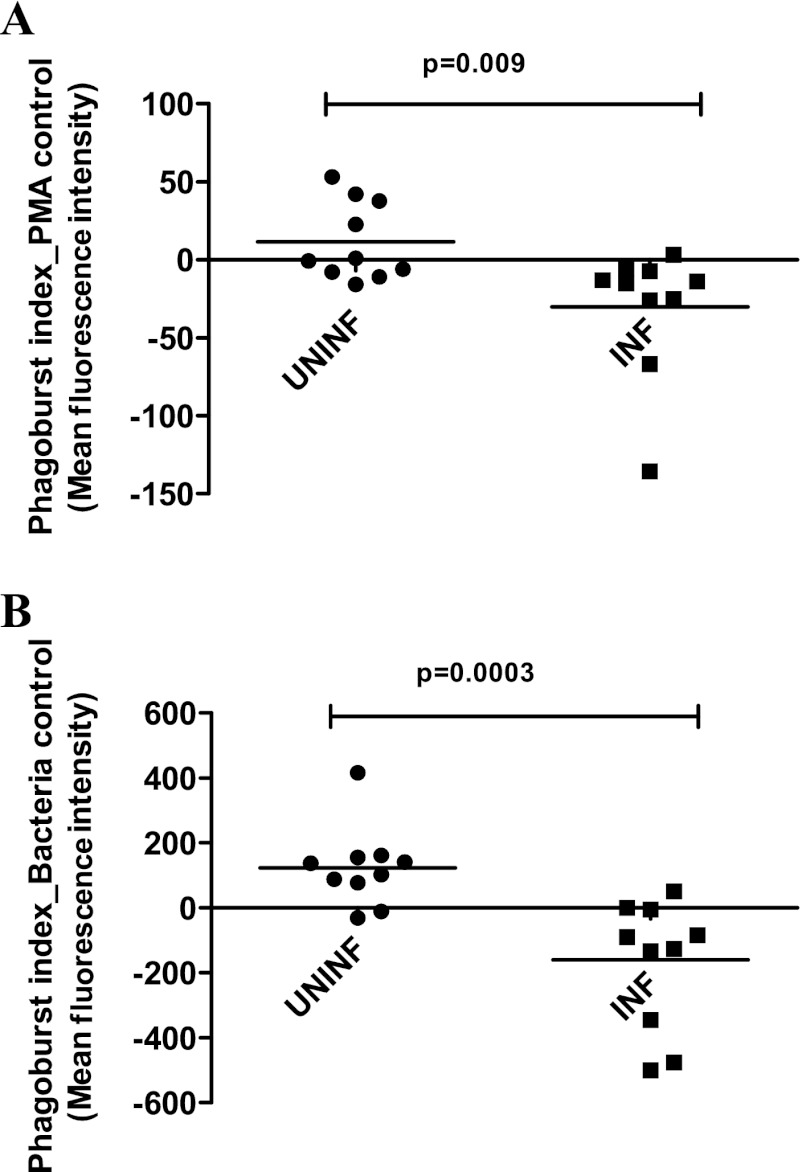
ICs from INF subjects induce reduced oxidative burst of normal granulocytes.
Because production of reactive oxygen species by an oxidative burst mechanism is an important function of granulocytes (11), the effect of ICs on the oxidative burst of granulocytes was studied. Granulocytes stimulated with ICs from INF subjects showed significantly reduced oxidative burst compared with those stimulated with ICs from UNINF subjects following phorbol myristate acetate (PMA) (P = 0.009) (Fig. 5A) or E. coli (P = 0.0003) (Fig. 5B) stimulation. Oxidative burst studies with negative control (mean fluorescence intensity [MFI], 1.5) using the wash solution alone and positive controls using PMA (MFI, 60) and bacteria (MFI, 200) alone in separate tubes were also studied.
Fig 5.
PEG 6000-precipitated plasma ICs from patients with TB induce decreased oxidative burst of granulocytes. Whole-blood samples from healthy volunteers (n = 10) were stimulated with PEG 6000 precipitates of plasma ICs from UNINF and INF subjects (10 subjects in each group) for 10 min, and oxidative burst of granulocytes was measured using flow cytometry in response to PMA (A) or E. coli (B). The P values were calculated using the Mann-Whitney U test. UNINF, uninfected individuals; INF, infected patients (patients with TB).
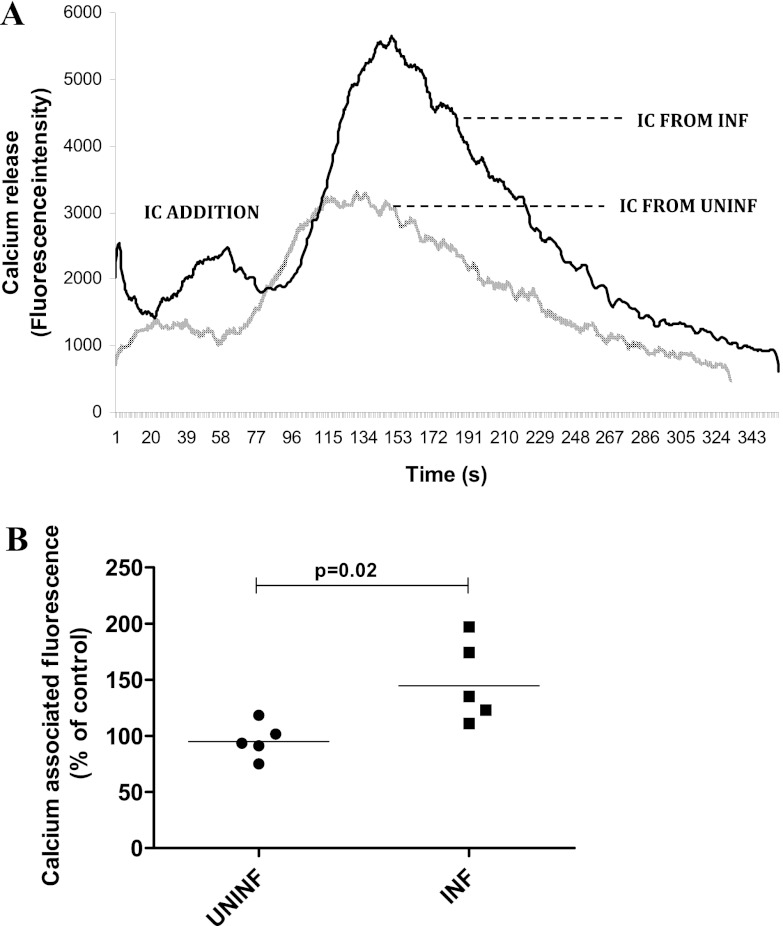
ICs from INF subjects induce increased calcium release by normal granulocytes.
As calcium release is an early indicator of granulocyte activation by ICs (21), the effect of ICs on calcium release from granulocytes was studied. The ICs from INF subjects showed an increased (P = 0.02) calcium release (GMs of 144.6% for INF versus 94.9% for UNINF subjects) (Fig. 6A and B). Nontreated granulocytes were used as controls, and data are presented as percentages of nontreated control for which calcium-associated fluorescence was measured in parallel cultures at the same time points.
Fig 6.
PEG 6000-precipitated plasma ICs from patients with TB induce increased release of calcium from granulocytes. Granulocytes from healthy volunteers (n = 5) were stimulated with PEG 6000-precipitates of plasma ICs from UNINF and INF subjects (five subjects per group), and calcium release was assessed using a fluorimeter. Data are presented as a representative histogram (A) showing calcium release in response to ICs from one INF individual and one UNINF individual and as percentages of nontreated control (B) for which calcium-associated fluorescence was measured in parallel cultures. The P values were calculated using the Mann-Whitney U test. UNINF, uninfected individuals; INF, infected patients (patients with TB).
DISCUSSION
Although many reports have highlighted the presence of increased CIC levels in TB patients, very few studies have demonstrated the biologic effects mediated by these complexes. In this study, we assessed the effect of ICs from either patients with TB disease or uninfected healthy controls on the functional activity of granulocytes. Granulocytes are used as responder cells to study the functional effects of the immune complexes, since they constitute the bulk of early recruited leukocytes that mediate and elicit responses to M. tuberculosis. Polymorphonuclear neutrophil activation by ICs is thought to play a significant role in the pathophysiology of a number of inflammatory diseases and in some vaculitides (9, 14). One important mechanism by which neutrophilic inflammation is generated and sustained is through IC formation and/or deposition in tissue (5, 13). Indeed, IC-induced, local generation of chemotactic factors and cytokines promotes neutrophil recruitment and an extracellular inflammatory milieu favoring cell activation (7, 12).
ICs isolated from patients with TB reduced the release of IL-4, IL-6, GM-CSF, and TNF-α from granulocytes compared to cells stimulated with ICs from healthy individuals.
IL-4 modulates the production of certain cytokines, induces class switching to IgE (6), and upregulates major histocompatibility complex class II (MHCII) production (10). IL-6 and GM-CSF are both prototypical proinflammatory cytokines. TNF-α is an important cytokine involved in systemic inflammation. Our study therefore reveals a global downregulation of cytokine production in normal granulocytes by ICs from patients with TB compared to uninfected individuals. Although previous reports (29) have demonstrated that ICs are potent stimulators of IL-10, we failed to observe any significant differential effect on IL-10 and other cytokines. Since proinflammatory cytokines are thought to mediate resistance to TB infection, our results on the decreased production of these cytokines might suggest that ICs might play an immunomodulatory role in TB and promote pathogenesis. This is further reinforced by our finding that the level of PAF (which was reported not to have protective immune response against tuberculosis) (37, 38) was lower and NO (which is readily demonstrated in macrophages from patients with inflammatory conditions such as tuberculosis) (23) levels were not significantly different upon stimulation with ICs from TB patients. On the other hand, ICs from TB patients do not globally impair granulocyte activation, since the levels of HNP1–3 (36), which have potent microbicidal, chemotactic, and cytotoxic activity were significantly higher when ICs from INF patients were used to stimulate polymorphonuclear neutrophils.
Efficient chemotaxis and phagocytosis, and the subsequent production of reactive oxygen intermediates (ROIs), are important for the intracellular killing of microorganisms by phagocytes, and defects in one or both of these functions may lead to deficient killing of intracellular microorganisms (34), thus predisposing the individual with pulmonary TB to further opportunistic infections. Therefore, the effects of ICs isolated from patients with TB on these granulocyte functions were examined in the present study. Neutrophils from individuals with active pulmonary TB have been shown to have an increased capacity for phagocytosis (28) and respiratory burst (20). Also it was shown in a previous study that granulocytes and monocytes from TB patients exhibited a significant reduction in their phagocytic capacity. We found that ICs increased the chemotactic and phagocytic ability of granulocytes but diminished the oxidative burst. However, since killing of M. tuberculosis by neutrophils occurs via nonoxidative means (17), the deficient respiratory burst activity in patients with TB may not necessarily have an adverse impact on the microbicidal activity of granulocytes. IC stimulation of circulating neutrophils is mediated by Fcγ receptors that cooperate in activating downstream signaling cascades, involving Src kinases, Syk, and phosphatidylinositol 3-kinase (PI3 kinase) and changes in cytosolic free calcium (4, 30) that, in human neutrophils, generate an oxidative burst and degranulation. Phagocytosis and chemotaxis are granulocyte effector functions that are dependent on early calcium release. Our present study indicates that early calcium release is a characteristic feature of granulocyte activation by ICs from TB patients.
Thus, the results from this study show that ICs can elicit immune responses through activation of granulocytes in PTB. IC-mediated modulation of granulocyte function can potentially account for a variety of IC-induced inflammatory events in TB. Future studies will examine the signal transduction pathways activated by these circulating ICs in PTB.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Financial assistance provided in the form of a Research Associate fellowship to P.S. by the Indian Council of Medical Research is gratefully acknowledged.
We thank S. Anbalagan for valuable technical assistance with flow cytometry and NIAID intramural editor B. R. Marshall for assistance.
We declare that we have no conflict of interest.
Footnotes
Published ahead of print 24 October 2012
REFERENCES
- 1.Bernardo J, Hartlaub H, Yu X, Long H, Simons ER. 2002. Immune complex stimulation of human neutrophils involves a novel Ca2+/H+ exchanger that participates in the regulation of cytoplasmic pH: flow cytometric analysis of Ca2+/pH responses by subpopulations. J. Leukoc. Biol. 72:1172–1179 [PubMed] [Google Scholar]
- 2.Bloch KJ, Ng BP, Bloch M. 1992. Blood levels of PAF are elevated during induction of immune complex mediated enteropathy in the rat. Mediators Inflamm. 1:341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Böyum A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 97:77–89 [PubMed] [Google Scholar]
- 4.Burgos RA, Conejeros I, Hidalgo MA, Werling D, Hermosilla C. 2011. Calcium influx, a new potential therapeutic target in the control of neutrophil-dependent inflammatory diseases in bovines. Vet. Immunol. Immunopathol. 143:1–10 [DOI] [PubMed] [Google Scholar]
- 5.Cochrane CG. 1977. Role of granulocytes in immune complex-induced tissue injuries. Inflammation 2:319–333 [DOI] [PubMed] [Google Scholar]
- 6.Coffman RL, et al. 1986. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J. Immunol. 136:4538–4541 [PubMed] [Google Scholar]
- 7.Dallegri F, Ottonello L. 1997. Tissue injury in neutrophilic inflammation. Inflamm. Res. 46:382–391 [DOI] [PubMed] [Google Scholar]
- 8.Daniel TM. 1986. Circulating immune complexes in tuberculosis. Am. Rev. Respir. Dis. 134:199–200 [DOI] [PubMed] [Google Scholar]
- 9.Fossati G, Bucknall RC, Edwards SW. 2002. Insoluble and soluble immune complexes activate neutrophils by distinct activation mechanisms: changes in functional responses induced by priming with cytokines. Ann. Rheum. Dis. 61:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon S, Martinez FO. 2010. Alternative activation of macrophages: mechanism and functions. Immunity 32:593–604 [DOI] [PubMed] [Google Scholar]
- 11.Graham DB, et al. 2007. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCgamma2 signaling axis in mice. J. Clin. Invest. 117:3445–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinzelmann M, Mercer-Jones MA, Passmore JC. 1999. Neutrophils and renal failure. Am. J. Kidney Dis. 34:384–399 [DOI] [PubMed] [Google Scholar]
- 13.Henson PM, Johnston RB., Jr 1987. Tissue injury in inflammation: oxidants, proteinases and cationic proteins. J. Clin. Invest. 79:669–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakus Z, Németh T, Verbeek JS, Mócsai A. 2008. Critical but overlapping role of FcgammaRIII and FcgammaRIV in activation of murine neutrophils by immobilized immune complexes. J. Immunol. 180:618–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarvis JN, et al. 1999. Immune complex size and complement regulate cytokine production by peripheral blood mononuclear cells. Clin. Immunol. 93:274–282 [DOI] [PubMed] [Google Scholar]
- 16.Johnson NM, McNicol MW, Burton-Kee EJ, Mowbray JF. 1981. Circulating immune complexes in tuberculosis. Thorax 36:610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones GS, Amirault HJ, Andersen BR. 1990. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J. Infect. Dis. 162:700–704 [DOI] [PubMed] [Google Scholar]
- 18.Kitamura H, et al. 2007. A case of Henoch-Schönlein purpura nephritis in pulmonary tuberculosis. Am. J. Med. Sci. 333:117–121 [DOI] [PubMed] [Google Scholar]
- 19.Maglione PJ, Xu J, Casadevall A, Chan J. 2008. Fc gamma receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J. Immunol. 180:3329–3338 [DOI] [PubMed] [Google Scholar]
- 20.Mandell GL, Fuller LF. 1972. Nitroblue tetrazolium dye test: a diagnostic aid in tuberculosis. Am. Rev. Respir. Dis. 106:123–125 [DOI] [PubMed] [Google Scholar]
- 21.Mandeville JT, Maxfield FR. 1996. Calcium and signal transduction in granulocytes. Curr. Opin. Hematol. 3:63–70 [DOI] [PubMed] [Google Scholar]
- 22.Neufert C, et al. 2001. Mycobacterium tuberculosis 19-kDa lipoprotein promotes neutrophil activation. J. Immunol. 167:1542–1549 [DOI] [PubMed] [Google Scholar]
- 23.Nicholson S, et al. 1996. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J. Exp. Med. 183:2293–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nimmerjahn F, Ravetch JV. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8:34–47 [DOI] [PubMed] [Google Scholar]
- 25.Ravetch JV, Bolland S. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290 [DOI] [PubMed] [Google Scholar]
- 26.Ridley MJ, Ridley DS, Willoughby DA. 1983. Extravascular immune complexes in experimental mycobacterial BCG granulomas. J. Pathol. 141:469–482 [DOI] [PubMed] [Google Scholar]
- 27.Ridley MJ, Marianayagam Y, Spector WG. 1982. Experimental granulomas induced by mycobacterial immune complexes in rats. J. Pathol. 136:59–72 [DOI] [PubMed] [Google Scholar]
- 28.Rieger M, Trnka L, Skvor J, Mison P. 1979. Immunoprofile studies in patients with pulmonary tuberculosis. III. Study of haemolytic complement in serum and phagocytic activity of blood neutrophils. Scand. J. Respir. Dis. 60:172–175 [PubMed] [Google Scholar]
- 29.Rönnelid J, Tejde A, Mathsson L, Nilsson-Ekdahl K, Nilsson B. 2003. Immune complexes from SLE sera induce IL10 production from normal peripheral blood mononuclear cells by an FcgammaRII dependent mechanism: implications for a possible vicious cycle maintaining B cell hyperactivity in SLE. Ann. Rheum. Dis. 62:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosales C, Brown EJ. 1992. Signal transduction by neutrophil immunoglobulin G Fc receptors. Dissociation of intracytoplasmic calcium concentration rise from inositol 1,4,5-trisphosphate. J. Biol. Chem. 267:5265–5271 [PubMed] [Google Scholar]
- 31.Saad AF, Virella G, Chassereau C, Boackle RJ, Lopes-Virella MF. 2006. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J. Lipid. Res. 47:1975–1983 [DOI] [PubMed] [Google Scholar]
- 32.Samuel AM, Ashtekar MD, Ganatra RD. 1984. Significance of circulating immune complexes in pulmonary tuberculosis. Clin. Exp. Immunol. 58:317–324 [PMC free article] [PubMed] [Google Scholar]
- 33.Scribner DJ, Fahrney D. 1976. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J. Immunol. 116:892–897 [PubMed] [Google Scholar]
- 34.Shalekoff S, Tiemessen CT, Gray CM, Martin DJ. 1998. Depressed phagocytosis and oxidative burst in polymorphonuclear leukocytes from individuals with pulmonary tuberculosis with or without human immunodeficiency virus type 1 infection. Clin. Diagn. Lab. Immunol. 5:41–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonney N, Bourrillon A, Lagrange PH. 2000. Analysis of circulating immune complexes (CICs) in childhood tuberculosis: levels of specific antibodies to glycolipid antigens and relationship with serum antibodies. Int. J. Tuberc. Lung. Dis. 4:152–160 [PubMed] [Google Scholar]
- 36.Spencer LT, et al. 2004. Role of human neutrophil peptides in lung inflammation associated with alpha1-antitrypsin deficiency. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L514–L520 [DOI] [PubMed] [Google Scholar]
- 37.Warren JS, Mandel DM, Johnson KJ, Ward PA. 1989. Evidence for the role of platelet-activating factor in immune complex vasculitis in the rat. J. Clin. Invest. 3:669–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weijer S, et al. 2003. Host response of platelet-activating factor receptor-deficient mice during pulmonary tuberculosis. Immunology 109:552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wipke BT, Allen PM. 2001. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 167:1601–1608 [DOI] [PubMed] [Google Scholar]
- 40.Xiao H, et al. 2005. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am. J. Pathol. 167:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]