Abstract
Background HIV cohort collaborations, which pool data from diverse patient cohorts, have provided key insights into outcomes of antiretroviral therapy (ART). However, the extent of, and reasons for, between-cohort heterogeneity in rates of AIDS and mortality are unclear.
Methods We obtained data on adult HIV-positive patients who started ART from 1998 without a previous AIDS diagnosis from 17 cohorts in North America and Europe. Patients were followed up from 1 month to 2 years after starting ART. We examined between-cohort heterogeneity in crude and adjusted (age, sex, HIV transmission risk, year, CD4 count and HIV-1 RNA at start of ART) rates of AIDS and mortality using random-effects meta-analysis and meta-regression.
Results During 61 520 person-years, 754/38 706 (1.9%) patients died and 1890 (4.9%) progressed to AIDS. Between-cohort variance in mortality rates was reduced from 0.84 to 0.24 (0.73 to 0.28 for AIDS rates) after adjustment for patient characteristics. Adjusted mortality rates were inversely associated with cohorts’ estimated completeness of death ascertainment [excellent: 96–100%, good: 90–95%, average: 75–89%; mortality rate ratio 0.66 (95% confidence interval 0.46–0.94) per category]. Mortality rate ratios comparing Europe with North America were 0.42 (0.31–0.57) before and 0.47 (0.30–0.73) after adjusting for completeness of ascertainment.
Conclusions Heterogeneity between settings in outcomes of HIV treatment has implications for collaborative analyses, policy and clinical care. Estimated mortality rates may require adjustment for completeness of ascertainment. Higher mortality rate in North American, compared with European, cohorts was not fully explained by completeness of ascertainment and may be because of the inclusion of more socially marginalized patients with higher mortality risk.
Keywords: HIV, AIDS, antiretroviral therapy, mortality, cohort, heterogeneity, prognostic model, socio-economic status
Introduction
Results from collaborations of HIV cohort studies have made important contributions to understanding the use and effects of antiretroviral therapy (ART), during the 16 years since it was introduced.1–8 Pooling data from a number of cohort studies leads to increased power to estimate associations of prognostic factors, such as CD4 cell count, HIV-1 RNA, age, sex and transmission risk group with clinical outcomes1 and to results that are generalizable to a range of treatment contexts. However, applicability of results will be limited if event rates vary greatly between cohorts.
Between-cohort heterogeneity in event rates may result from differences in patient characteristics and differential outcomes of health care or study characteristics, such as extent of loss to follow-up. However, such heterogeneity has rarely been a focus of research reports, which typically focus on overall trends. Here, we analyse data from a large collaboration of HIV cohort studies in Europe and North America to quantify between-cohort heterogeneity in rates of clinical events and death from initiation of ART. We investigate the extent to which such heterogeneity is explained by patient and cohort characteristics.
Methods
The ART Cohort Collaboration (ART-CC) is an international collaboration between the investigators of cohort studies of HIV-1–positive patients from Europe and North America. It was established in 2000 to estimate the prognosis of HIV-1 infected treatment-naïve patients initiating combination ART and has been described in detail elsewhere.1,9 Prospective cohort studies were eligible for participation if they had enrolled at least 100 HIV-1–positive patients aged ≥16 years, who had not previously received antiretroviral treatment, started ART with a combination of at least three antiretroviral drugs after 1996 and had a median duration of potential follow-up of at least 1 year after ART initiation. All cohorts provided anonymized data on a predefined set of demographic, laboratory and clinical variables, which were then pooled and analysed centrally. The data set analysed here was assembled during 2010 and included data from the following 17 cohorts: the AIDS Therapy Evaluation Project, Netherlands (ATHENA),10 the Agence Nationale de la Recherche sur le SIDA et les hépatites virales (ANRS) CO3 Aquitaine Cohort,11 the ANRS CO4 French Hospital Database on HIV (FHDH),12 the EuroSIDA Study Group,13 the Italian Cohort of Antiretroviral-Naïve Patients (ICONA),14 the Köln/Bonn Cohort (CBC), Germany,15 the Proyecto para la Informatización del Seguimiento Clínico-epidemiológico de la Infección por HIV y SIDA (PISCIS) Cohort,16 Cohorte de la Red de Investigación en Sida (CoRIS),17 the Royal Free Hospital Cohort, UK,18 the HAART Observational Medical Evaluation and Research (HOMER),19 British Columbia, Canada, the South Alberta Clinic Cohort, Canada,20 the Swiss HIV Cohort Study (SHCS),21 the 1917 Clinic Cohort from the University of Alabama (UAB),22 the Veterans Aging Cohort Study (VACS),23,24 the HIV Atlanta VA Cohort Study (HAVACS),25 Vanderbilt-Meharry Center for AIDS Research26 and the University of Washington HIV Cohort, USA.27
All cohorts completed a survey of cohort characteristics, including region covered, whether facilities were community-based or hospital clinics, coverage of local community, representativeness of ethnic groups, deprivation levels and methods of following up patients and ascertaining deaths. Cohorts also supplied information on estimated completeness of ascertainment of death, which was categorized as 1 = excellent: 96–100%, 2 = good: 90–95%, 3 = average: 75–89%. At all sites, institutional review boards had approved the collection of data.
Patients were eligible for inclusion in this analysis if they started ART between January 1, 1998 and July 1, 2009, had measurements of CD4 count and HIV-1 RNA measured within 3 months before start of ART, did not have an AIDS diagnosis before initiation of ART and were not thought to have been infected with HIV through injection drug use (IDU). IDU, who are known to have worse prognosis, were excluded because the proportion of IDU varied between cohorts, and our focus was on understanding between-cohort differences among similar patients. Patients were followed up for AIDS and all cause mortality between 31 days and 2 years after starting ART. Patients with AIDS diagnoses or who died within 30 days of starting ART were excluded, as early deaths and events after the start of ART are not well recorded in all cohorts. Patients not known to have died were considered lost to clinical follow-up at 3 months after their last CD4 measurement, except when the last measurement was within 6 months of the end of study date. Data on follow-up were available up to December 31, 2009.
Statistical methods
For each cohort, we used survival models based on the exponential distribution (Poisson regression models) to estimate crude and adjusted (for age, sex, HIV transmission risk, year started ART, CD4 count and HIV-1 RNA measured within 3 months before start of ART) average rates of AIDS and mortality between 31 days and 2 years after starting ART. Adjusted rates were calculated from a model that assumed similar covariate effects across cohorts and cohort-specific baseline hazards and are reported for the group of patients with the most frequently occurring risk factor categories [baseline CD4 count 100–199 cells/mm3, log HIV-1 RNA 4–4.99 log copies/ml, age 30–44 years, sex/risk group men who have sex with men (MSM), calendar period of starting ART (2000–03)]. We displayed heterogeneity using forest plots and used random-effects meta-analysis of log rate ratios to examine heterogeneity in crude and adjusted rates.28 The size of the squares in the forest plots represents the weight assigned to each study in the meta-analysis and is proportional to the variance. Residual heterogeneity was quantified as τ2, the between-cohort variance in the rate (estimated on the logarithmic scale using the method of DerSimonian and Laird)29 and as the estimated rate ratio comparing cohorts at the 10th and 90th percentile of the distribution of the outcome. We also calculated the I2 statistic,30 which is the percentage of variation across studies that is because of heterogeneity rather than chance. We used meta-regression to estimate associations with cohort characteristics [completeness of death ascertainment and region (Europe vs North America)] and the proportion of heterogeneity explained by these characteristics.
Sensitivity analyses
Two cohorts (HOMER and VACS) had a large proportion of patients with unknown risk transmission group, who may have been IDU. We, therefore, repeated analyses excluding ‘other’ risk group from all cohorts. A survey found that 30% of those lost to follow-up in FHDH had died.31 Therefore, we repeated analyses randomly assigning the outcome dead to 30% of those lost to follow-up in the whole study population. To see if non–AIDS-related deaths were driving the differences in mortality rate, we repeated analyses in those cohorts with cause of death available with AIDS-related death as the outcome.
All analyses were carried out using Stata version 11 (www.stata.com).
Results
Data were available on 38 706 patients enrolled in 17 HIV cohort studies. During 61 520 person-years of follow-up, 754 (1.9%) patients died and 1890 (4.9%) progressed to AIDS, of whom 245 died. Table 1 compares characteristics of the cohorts and their included patients. The number of patients contributing to this analysis varied from 225 to 19 054 between cohorts, and the proportion that died varied from <1% to >7%. The proportion of female patients ranged from just <40% in FHDH to <3% in the two cohorts of US veterans. The overall median age was 37 [inter-quartile range (IQR): 31–45] years, but both the cohorts of US veterans had a higher proportion of older patients: the median age in VACS was 46 (38–53) years, and ∼10% were aged >60 years, compared with <5% in the combined data set. After excluding the HOMER and VACS cohorts, which had substantial number of patients with unknown or ‘other’ transmission risk group, 39% of patients were MSM with a range across cohorts of 32–65%. The VACS cohort did not report details of risk transmission group, although those with known IDU or hepatitis C infection were identified and excluded from the data set considered here. Median CD4 cell count at start of ART was 250 cells/mm3, and varied between 200 and 292 cells/mm3; the proportion of patients with severe immunosuppression (<25 cells/mm3) at start of ART varied from <1% to ∼12%. Overall, 21, 38 and 41% of patients started ART during 1998–99, 2000–03 and 2004–08, respectively. Two cohorts contributed data only on patients who started treatment in more recent years: patients in UAB started ART after 2000 and in CoRIS after 2004.
Table 1.
Numbers of patients, outcomes, demographic characteristics, CD4 cell count and log-viral load at start of ART by cohort and overall
| ATHENA | Aquitaine | CBC | CoRIS | EuroSIDA | FHDH | ICONA | PISCIS | Royal Free | |
|---|---|---|---|---|---|---|---|---|---|
| The Netherlands | France | Germany | Spain | Europe | France | Italy | Spain | UK | |
| No. of patients | 4218 (10) | 860 (2.0) | 499 (1.2) | 602 (1.4) | 705 (1.7) | 19 054 (45) | 1479 (3.5) | 1617 (3.8) | 893 (2.1) |
| Observed deaths (%) | 56 (1.3) | 8 (0.9) | 9 (1.8) | 7 (1.2) | 7 (1) | 217 (1.1) | 3 (0.2) | 23 (1.4) | 6 (0.7) |
| AIDS events (%)a | 152 (3.6) | 23 (2.7) | 24 (4.8) | 20 (3.3) | 10 (1.4) | 757 (4) | 35 (2.4) | 51 (3.2) | 39 (4.4) |
| Female (%) | 27.6 | 24.8 | 25.5 | 28.6 | 26.1 | 37.5 | 33.2 | 26.6 | 26.5 |
| Age (years) | |||||||||
| 16–29 | 21.5 | 24.9 | 19.2 | 26.6 | 23.8 | 21.9 | 18.1 | 24.4 | 19.7 |
| 30–44 | 55.1 | 50.5 | 60.9 | 52.5 | 51.6 | 54.2 | 60.9 | 52.8 | 63.4 |
| 45–49 | 9.7 | 8.7 | 8.8 | 9.0 | 8.7 | 9.4 | 8.8 | 7.8 | 8.3 |
| 50–54 | 6 | 5.3 | 4.6 | 5.8 | 7.4 | 6.2 | 5.5 | 6.1 | 4.9 |
| 55–59 | 4.8 | 4.5 | 3.8 | 3.3 | 4.5 | 4 | 3.9 | 4.4 | 2.4 |
| 60–64 | 1.7 | 3.0 | 2.0 | 1.3 | 1.8 | 2.3 | 1.9 | 2.2 | 0.6 |
| ≥65 | 1.1 | 3.0 | 0.6 | 1.5 | 2.1 | 2.0 | 0.9 | 2.4 | 0.8 |
| Median age (years) | 37 (30, 44) | 36 (30, 44) | 36 (31, 42) | 35 (29, 43) | 37 (30, 44) | 36 (30, 44) | 36 (31, 43) | 35 (30, 43) | 35 (31, 41) |
| Risk | |||||||||
| MSM | 52.1 | 49.4 | 50.1 | 42.7 | 50.2 | 32 | 33.1 | 42.9 | 55.9 |
| Heterosexual | 41.3 | 42.2 | 21.8 | 53.7 | 41.4 | 56.9 | 58.0 | 50.1 | 42.4 |
| Other/unknown | 6.5 | 8.4 | 28.1 | 3.7 | 8.4 | 11.1 | 8.9 | 7.1 | 1.7 |
| CD4 cell count | |||||||||
| 0–24 | 5.2 | 1.9 | 2.4 | 4.5 | 3.4 | 3.4 | 3.2 | 4.2 | 3.5 |
| 25–49 | 3.1 | 2.7 | 3.8 | 3.0 | 3.3 | 3.2 | 2.8 | 3.6 | 3.4 |
| 50–99 | 8.5 | 6.5 | 6.2 | 10.8 | 7.7 | 6.4 | 5.6 | 9.4 | 10.3 |
| 100–199 | 25.7 | 16.6 | 23.4 | 22.4 | 23.0 | 19.7 | 16.4 | 18.7 | 25.2 |
| 200–349 | 37.2 | 36.0 | 36.7 | 44.5 | 37.3 | 36.1 | 36.5 | 35.3 | 36.7 |
| ≥350 | 20.3 | 36.3 | 27.5 | 14.8 | 25.4 | 31.2 | 35.4 | 28.8 | 20.9 |
| Median CD4 (cells/mm3) | 220 (130, 311) | 292 (192, 416) | 240 (150, 364) | 231 (127, 305) | 248 (151, 351) | 266 (168, 390) | 289 (185, 420) | 254 (144, 369) | 221 (134, 320) |
| HIV-1 RNA (log copies/ml) | |||||||||
| <4 | 22.4 | 18.5 | 23.4 | 16.9 | 12.3 | 32.4 | 14.7 | 15.6 | 10.5 |
| 4–4.99 | 39.8 | 42.2 | 38.1 | 38.7 | 42.4 | 36.5 | 46.2 | 39.5 | 36.8 |
| ≥5 | 37.8 | 39.3 | 38.5 | 44.4 | 45.2 | 31.1 | 39.0 | 44.9 | 52.6 |
| Median HIV-1 RNA | 4.8 (4.1, 5.3) | 4.8 (4.2, 5.3) | 4.7 (4.1, 5.3) | 4.9 (4.3, 5.3) | 4.9 (4.4, 5.3) | 4.6 (3.6, 5.1) | 4.8 (4.3, 5.3) | 4.9 (4.4, 5.4) | 5.0 (4.6, 5.5) |
| SHCS | S. Alberta | HOMER | HAVACS | UAB | VACS | Vanderbilt | WA | All | |
|---|---|---|---|---|---|---|---|---|---|
| Switzerland | Canada | Canada | USA | USA | USA | USA | USA | ||
| No. of patients | 2378 (5.6) | 277 (0.7) | 1374 (3.2) | 225 (0.5) | 238 (0.6) | 3195 (7.6) | 779 (1.8) | 313 (0.7) | 38 706 (100) |
| Observed deaths (%) | 41 (1.7) | 6 (2.2) | 97 (7.1) | 8 (3.6) | 6 (2.5) | 224 (7) | 29 (3.7) | 7 (2.2) | 754 (1.9) |
| AIDS events (%)a | 61 (2.6) | 14 (5.1) | 24 (1.7) | 11 (4.9) | 29 (12.2) | 557 (17.4) | 64 (8.2) | 19 (6.1) | 1890 (4.9) |
| Female (%) | 32.4 | 26.4 | 16.4 | 1.8 | 23.1 | 2.3 | 25.9 | 18.2 | 30.0 |
| Age (years) | |||||||||
| 16–29 | 21.6 | 25.6 | 13.8 | 13.3 | 22.7 | 5.9 | 21.6 | 23.0 | 20.3 |
| 30–44 | 53.7 | 56.0 | 53.3 | 46.7 | 57.6 | 40.4 | 55.5 | 58.1 | 53.5 |
| 45–49 | 8.9 | 7.9 | 14.1 | 11.1 | 9.2 | 18.0 | 12.7 | 12.5 | 10.2 |
| 50–54 | 6.2 | 4 | 8.8 | 11.6 | 3.8 | 16.2 | 4.9 | 3.5 | 6.9 |
| 55–59 | 4 | 3.6 | 4.9 | 11.6 | 3.8 | 9.8 | 3.1 | 1.6 | 4.6 |
| 60–64 | 3.3 | 1.1 | 2.4 | 3.6 | 1.3 | 5.0 | 1.3 | 0.3 | 2.4 |
| ≥65 | 2.2 | 1.8 | 2.6 | 2.2 | 1.7 | 4.7 | 1.0 | 1.0 | 2.1 |
| Median age (years) | 37 (30, 44) | 35 (29, 42) | 40 (33, 47) | 42 (34, 51) | 37 (30, 43) | 46 (38, 53) | 37 (30, 44) | 35 (30, 42) | 37 (31, 45) |
| Risk | |||||||||
| MSM | 41.7 | 50.2 | 16.2 | 59.1 | 42.9 | 0 | 42.9 | 64.5 | 34.6 |
| Heterosexual | 54.0 | 48.7 | 6.1 | 6.2 | 35.7 | 0.0 | 33.2 | 33.5 | 45.7 |
| Other/unknown | 4.4 | 1.1 | 77.7 | 34.7 | 21.4 | 100.0 | 23.9 | 1.9 | 19.7 |
| CD4 cell count | |||||||||
| 0–24 | 2.8 | 0.7 | 6.7 | 8.0 | 10.5 | 10.6 | 11.9 | 5.1 | 4.5 |
| 25–49 | 3.4 | 4.3 | 4.7 | 4.0 | 4.2 | 5.0 | 5.6 | 5.4 | 3.5 |
| 50–99 | 7.2 | 7.6 | 11.2 | 5.8 | 8.0 | 9.0 | 9.8 | 6.1 | 7.4 |
| 100–199 | 25.3 | 24.5 | 27.0 | 24.4 | 18.1 | 18.0 | 20.5 | 24.3 | 21.0 |
| 200–349 | 37.3 | 43.7 | 31.8 | 39.6 | 33.6 | 29.3 | 30.0 | 45.7 | 35.7 |
| ≥350 | 24.0 | 19.1 | 18.6 | 18.2 | 25.6 | 28.0 | 22.1 | 13.4 | 27.9 |
| Median CD4 (cells/mm3) | 233 (154, 339) | 243 (157, 313) | 200 (110, 300) | 220 (125, 307) | 237.5 (115, 350) | 234 (101, 367) | 209 (88, 330) | 226 (136, 293) | 250 (150, 368) |
| HIV-1 RNA (log copies/ml) | |||||||||
| <4 | 18.5 | 28.2 | 8.7 | 9.8 | 14.3 | 20.4 | 15.1 | 12.5 | 24.9 |
| 4–4.99 | 40.5 | 43.7 | 39.2 | 47.6 | 52.5 | 41.0 | 46.1 | 44.4 | 38.9 |
| ≥5 | 41.0 | 28.2 | 52.1 | 42.7 | 33.2 | 38.6 | 38.8 | 43.1 | 36.2 |
| Median HIV-1 RNA | 4.9 (4.3, 5.3) | 4.5 (3.8, 5.0) | 5.0 (4.6, 5.0) | 4.9 (4.4, 5.4) | 4.8 (4.4, 5.3) | 4.8 (4.2, 5.2) | 4.8 (4.3, 5.4) | 4.9 (4.4, 5.4) | 4.7 (4.0, 5.2) |
aObserved AIDS events and deaths are from 31 days to 2 years after starting ART.
Table 2 shows methods for ascertainment of deaths and of following up patients who miss appointments, based on the survey of cohort characteristics. Two cohorts are based only in community clinics, 13 are hospital-based and 2 recruit patients in hospitals and community treatment facilities. Of the 10 European cohorts, one is multinational, five are national (with coverage ranging from 35 to 100%), two are regional or provincial and two are based in city university hospital clinics. The two Canadian cohorts are provincial, with estimated 99% coverage in the areas that they serve. The US cohorts are also diverse: one regional, two associated with city university hospital clinics and two including only veterans who were previously in the US armed forces, one of which is a city-based hospital and the other a national database.
Table 2.
Method and completeness of death ascertainment and method of following up patients who miss appointments among 17 HIV cohort studies from Europe and North America
| Death ascertainment |
Lost to follow-up |
|||||
|---|---|---|---|---|---|---|
| Cohort | Linked to death registry (frequency in years) | Sources of information | Self-reported completeness of ascertainment (%) | % lost to clinical follow-up for AIDS | % lost to follow-up for death | Missed appointment follow-up |
| ATHENA, The Netherlands | No | Hospital, family/friends | 90–95 | 4 | 4 | Phone call to patient |
| Aquitaine, France | Yes (3) | Hospital, physician | 90–95 | 6 | 6 | Physician |
| Koln/Bonn cohort, Germany | No | Physician, local registry | 90–95 | 16 | 16 | Phone call to patient, physician |
| CoRIS, Spain | No | 90–95 | 9 | 9 | No | |
| EuroSIDA, Europe | Some | 90–95 | 5 | 5 | ||
| FHDH, France | No | Local registry, family/friends | 75–89 | 17 | 18 | Unknown, phone call or letter to patient |
| ICONA, Italy | No | Local registry/hospital | 90–95 | 12 | 12 | Phone call to patient |
| PISCIS, Spain | Yes (2) | Hospital, physician | 75–89 | 5 | 5 | Unknown |
| Royal Free, UK | Yes (1)a | Physician, registry | 75–89 | 7 | 8 | Phone call or letter to patient |
| SHCS, Switzerland | No | Local registry | 90–95 | 5 | 4 | Letter to patient |
| S. Alberta, Canada | Yes (1) | Hospital, coroner | 90–95 | 8 | 6 | No |
| HOMER, Canada | Yes (1/12) | Provincial registry, physician | 96–100 | 21 | 6 | Physician (questionnaire) |
| HAVACS, USA | Yes (0.25) | Registry | 96–100 | 3 | 3 | Phone call to patient |
| UAB, USA | Yes (0.5) | Hospital, registry, family/friends | 75–89 | 19 | 17 | Phone call to patient |
| VACS, USA | Yes (1) | Hospital, physician | 96–100 | 2 | 2 | |
| Vanderbilt, USA | Yes (0.5) | Hospital, registry, family/friends | 90–95 | 12 | 13 | Phone call to patient |
| WA, USA | Yes (0.25) | Hospital, registry | 96–100 | 12 | 12 | Phone call and letter to patient |
aLinkage to death registry was restricted to a subgroup of younger patients (aged <65 years).
In the Canadian cohorts, aboriginal people and immigrants from sub-Saharan Africa are over-represented compared with the general population in the cohort catchment area. In the US veterans cohorts, patients are mostly male, are on average older than patients in other HIV cohorts and have higher rates of alcohol and recreational drug use than in the wider population. In another US cohort, UAB, Black Americans are over-represented, whereas Latino and Hispanic people are under-represented, and the Washington cohort predominantly recruits White MSM. Four of seven North American cohorts reported substantial social deprivation (low income, unemployment and homelessness) among their patients. In the European cohorts, sub-Saharan Africans were reported to be over-represented in one cohort, but under-represented in another.
Excluding the multinational EuroSIDA cohort, 6 of the 16 cohorts claimed to be largely representative of all HIV patients in their country. Ten cohorts link to death registries, but the frequency of linkage varies from every month to every 3 years. Self-assessed completeness of death ascertainment was higher in cohorts from North America than from Europe. The proportion of patients lost to clinical follow-up for AIDS events by 2 years after the start of ART was 12%, but this varied across the cohorts from 2 to 21%.
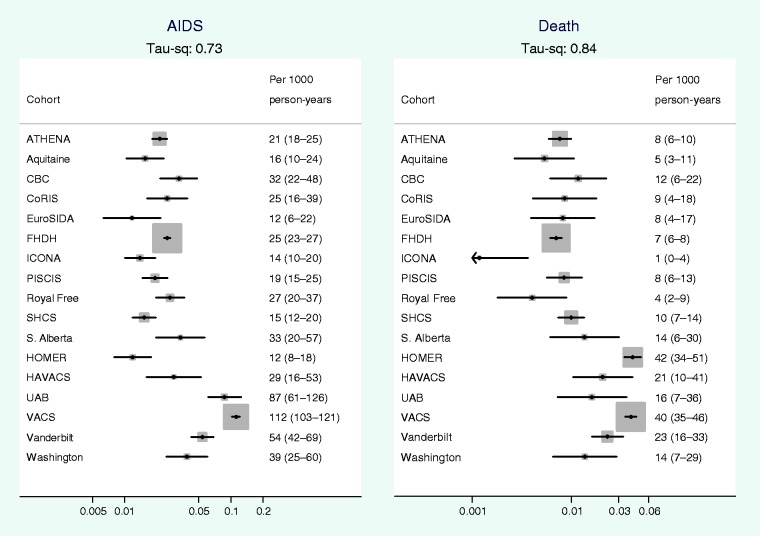
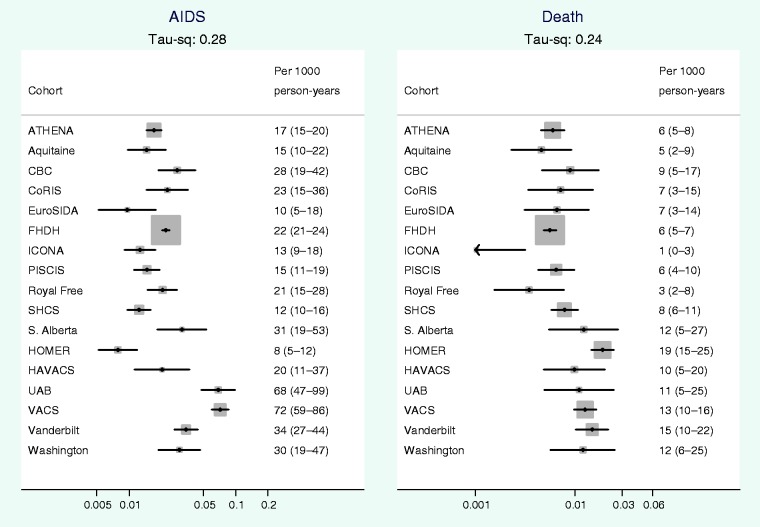
Figure 1 shows that there was considerable between-cohort heterogeneity in crude rates of AIDS and all cause mortality. Between-cohort heterogeneity is quantified in Table 3, which shows that the estimated magnitudes of between-cohort variance (τ2 = 0.73 for AIDS and 0.84 for death) correspond to 8.9-fold differences between crude rates of AIDS in cohorts at the 90th, compared with the 10th percentile and 10.5-fold differences in crude mortality rates. All P-values for heterogeneity were < 0.001. Figure 2 shows that on adjustment for patient characteristics between-cohort heterogeneity was substantially attenuated from 0.73 to 0.28 for rates of AIDS and from 0.84 to 0.24 for mortality.
Figure 1.
Crude rates (per 1000 person-years) of AIDS and all cause mortality by cohort, average rate estimated during the period from 31 days to 2 years after start of ART. The size of the squares represents the weight assigned to each study in the meta-analysis and is proportional to the number of events in that study (whiskers show 95% CI). τ2 is the between-cohort variance in the rate
Table 3.
Between-cohort heterogeneity in crude and adjusted rates of AIDS and mortality for (i) all patients; (ii) for MSM and heterosexual transmission risk groups only; and (iii) for all patients, assuming 30% of those lost to follow-up are dead
| Estimate | Between-cohort heterogeneity statistica τ2 | Rate ratio comparing cohorts at the 10th and 90th percentiles of the distribution of the outcome | Percentage of variation across studies because of heterogeneity, I2 | |
|---|---|---|---|---|
| All patients | ||||
| Rate of AIDS | ||||
| Crude | 0.73 | 8.93 | 98.5 | |
| Adjusted | 0.28 | 3.88 | 94.4 | |
| Mortality rate | ||||
| Crude | 0.84 | 10.48 | 97.0 | |
| Adjusted | 0.24 | 3.51 | 86.0 | |
| MSM and heterosexual onlyb | ||||
| Rate of AIDS | ||||
| Crude | 0.15 | 2.70 | 88.0 | |
| Adjusted | 0.12 | 2.43 | 84.6 | |
| Mortality rate | ||||
| Crude | 0.16 | 2.79 | 74.2 | |
| Adjusted | 0.13 | 2.52 | 67.6 | |
| All patients, adjusted for loss to follow-up | ||||
| Mortality rate | ||||
| Crude | 0.19 | 3.06 | 94.8 | |
| Adjusted | 0.15 | 2.70 | 92.2 | |
aτ2 is the estimated variance of the distribution of the rate of AIDS or rate of death among the cohorts.
bExcluding VACS.
Figure 2.
Adjusted rates (per 1000 person-years) of AIDS and all cause mortality by cohort, average rate estimated during the period from 30 days to 2 years after start of ART. Adjusted for CD4 cell count (100–199) cells/mm3, log HIV-1 RNA (4–4.99) log copies/ml, age (30–44 years), sex-risk group (MSM), year of starting ART (2000–03)
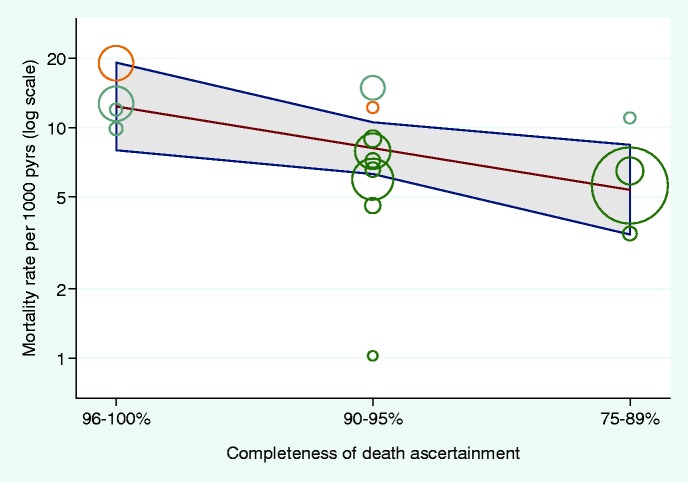
Adjusted cohort mortality rates were inversely associated with completeness of ascertainment of death (Figure 3): the ratio of mortality rates was 0.66 [95% confidence interval (CI) 0.46 to 0.94] per decreasing category of completeness. The between-cohort heterogeneity variance in adjusted mortality rates was further reduced from 0.28 to 0.11 (ratio comparing 90th to 10th percentile 2.34) after adjustment for completeness of death ascertainment. The adjusted mortality rate ratios comparing European with North American cohorts were 0.42 (95% CI 0.31–0.57) before and 0.47 (0.30–0.73) after adjusting for completeness of ascertainment.
Figure 3.
Association of cohort-specific death rate (adjusted for patient characteristics) with ascertainment of death category. Circle sizes represent the precision of each cohort-specific estimate (the inverse of within-cohort variance), yellow denotes Canadian, blue USA and green European cohorts. τ2 = 0.11 (from 0.84)
Results of sensitivity analyses
Risk group was ‘other’ in 20% of all patients, with a cohort range of 1–78% (HOMER) and 100% (VACS). The mortality hazard ratio for comparing ‘other’ with MSM varied by cohort, e.g. it was 1.38 (95% CI 0.91–2.09) in FHDH, but 6.16 (2.24–16.90) in HOMER. In analyses restricted to individuals with transmission risk group recorded either as MSM or heterosexual (16 cohorts n = 31 068 patients), the between-cohort variance in crude mortality rates was 0.16 (0.15 for AIDS rates), substantially lower than those in the main analyses (Table 3 and Supplementary Figure S1, available as Supplementary data at IJE online). After adjustment for patient characteristics at start of ART, these reduced further to 0.13 and 0.12 for mortality and for AIDS, respectively (Table 3 and Supplementary Figure S2, available as Supplementary data at IJE online). In the analysis that assumed 30% of those lost to follow-up were dead, between-cohort variance in crude mortality rates was 0.19 compared with 0.84 in the main analysis (Table 3 and Supplementary Figure S3, available as Supplementary data at IJE online). In analyses restricted to 14 cohorts with cause of death data available, between-cohort variance was 0.83 for AIDS-related deaths compared with 0.64 for all cause mortality.
Discussion
In analyses of data pooled from 17 cohorts of HIV-positive patients treated with ART from Europe and North America, substantial between-cohort heterogeneity in crude rates of AIDS and death from 1 month to 2 years after starting ART was reduced after adjustment for patient characteristics at baseline. However, even after adjustment, there were 3.88- and 3.51-fold differences for AIDS and death, respectively, comparing cohorts at the 90th and 10th percentiles of the distribution of rates. Cohort-specific mortality rates were inversely associated with self-assessed completeness of death ascertainment. Therefore, mortality rates estimated from different HIV cohort studies may require adjustment for completeness of death ascertainment especially, if loss to follow-up is high. Higher mortality rates in North American, compared with European, cohorts were not fully explained by completeness of ascertainment. However, much of the heterogeneity seemed to be attributable to higher death rates in patients with ‘unknown’ risk transmission group in the HOMER and VACS cohorts who are likely to be from more socially marginalized groups.
Strengths and limitations
Our study includes ∼40 000 patients enrolled in 17 diverse cohorts from two continents. None of the cohorts in ART-CC estimated self-assessed death ascertainment to be <75%; the effect of more extreme levels of under-ascertainment on estimated rates of AIDS and death is unpredictable. We excluded patients whose presumed transmission was through IDU or who had experienced AIDS before starting ART to reduce the influence of pre-existing conditions and co-morbidities in increasing between-cohort heterogeneity. Similarly, we focused on the first 2 years of treatment to reduce the impact of treatment switches or failure, and started follow-up after 1 month, because early events may not be completely recorded in some cohorts. Completeness of death ascertainment was self-assessed by cohorts. Although some cohorts have good empirical evidence to support such assessments,31 true completeness remains unknown. Some demographic and clinical variables that might contribute to between-cohort heterogeneity were not available to us, e.g. we did not have complete patient-level data on country of origin and race, nor did we have information on viral subtype, education or socio-economic status. Our analyses did not account for non-HIV biomarkers that have been shown to be associated with mortality rate in treated HIV-positive patients, such as haemoglobin,32 liver enzymes and creatinine,33 because these measures were not available from all cohorts. We were also unable to adjust for important lifestyle risk factors for all cause mortality, such as smoking, alcohol and drug use.
Results in context with other studies
A study that used a prognostic model fitted to data from the Collaborations in HIV Outcomes Research/US cohort to predict AIDS free survival in the Johns Hopkins HIV Clinical Cohort found considerable discrepancies between predicted and observed survival.34 This was only partially explained by measured risk factors and may have been partly because of substantial differences in loss to follow-up rates. There were clear differences in mortality rates during the first year of ART when data from this collaboration of cohorts from high-income countries were compared with those from a collaboration of ART treatment programmes in low-income countries.6 Such differences are likely to reflect the greater burden of background disease, poorer access to ART leading to lower CD4 counts at start of ART, sub-optimal ART regimens and lack of health care infrastructure in the low-income countries. That study also found estimated mortality rates to be substantially higher, and estimated rates of loss to follow-up correspondingly lower, in cohorts with active compared with passive follow-up.
Contributors to between-cohort heterogeneity
The differences in mortality rates between cohorts in our study may be attributable to under-ascertainment of mortality, loss to follow-up related to prognosis or to patients having higher mortality risk beyond that due to the characteristics accounted for in the analysis. We found evidence that mortality rates were related to completeness of death ascertainment, which varied by cohort and depended on factors such as linkage to registries, the existence of good subject identifiers, such as National Insurance numbers, and the proportion of migrants.35 Under-ascertainment of death was found in France when they conducted a national survey of mortality rate in HIV-positive individuals during 2000 using three source capture–recapture methods, which found that only 38% of deaths of persons in the FHDH had been recorded and that under-ascertainment was more common for non-AIDS compared with AIDS-related deaths.31 The mortality survey was repeated in 200536 ensuring more complete ascertainment of death in the data contributed to the present study. Frequent linkage with death registries is essential for the accurate estimation of mortality rate.37,38
We also found that rates of loss to follow-up varied by cohort from 2 to 18% and depend on the opportunities to access care outside the contributing centres, the ability of cohorts to track patient transfers and methods of chasing patients who miss appointments. Prognosis associated with loss to follow-up could bias estimates of mortality rates. In France, IDU and immigrants were more likely to be lost to follow-up and, compared with those remaining in care, CD4 count was lower and viral load higher,34 whereas in Switzerland those lost to follow-up had higher CD4 count.39 Patients lost to follow-up from cohort studies could remain in care in non-contributing institutions, such as primary care facilities, and also often return to care in the contributing institutions after a gap of over 1 year.38,40 Service delivery is a key factor that needs to be considered; among the population-based cohorts, several are solely responsible for providing antiretroviral care for these participants; therefore, they are less likely than clinic-based cohorts to lose participants because of transfer of care to other facilities or to under-reported mortality. For example, the high rates of loss to follow-up in the Royal Free cohort are partly because of patients attending multiple clinics in London.38
Methods of adjustment for under-ascertainment of deaths
Standard methods of survival analysis assume that censoring is uninformative, i.e. that study participants who are censored because they are lost to follow-up have similar mortality rates compared with those that remain in the study, conditional on their observed covariates. This is not the case in resource-limited settings; a systematic review of studies that traced patients lost to follow-up found that among those traced, 20–60% had died, a much higher mortality rate than that recorded for those remaining in care.41 Mortality rate in those lost to follow-up but later traced was inversely associated with the rate of loss to follow-up in the programme. Loss to follow-up may be treated as a missing data problem and missing survival time imputed accounting for covariate pattern and the excess mortality ratio in those lost to follow-up.42 Often, the cohort-specific excess mortality ratio is not known, but may be estimated from other studies or a range of plausible values used to explore sensitivity of overall mortality to this ratio.43 Future work will use similar methods to explore adjustments for under-estimation of mortality in European and North American cohorts.
Possible reasons for higher death rates in USA and Canada
We found that mortality rates were substantially higher in two North American cohorts (HOMER and VACS) and that this was largely attributable to patients with unknown transmission risk group, who are likely to be more socially marginalized. Based on discussion with representatives from the cohort, it is likely that in HOMER, some patients infected through IDU and likely to be co-infected with hepatitis C were classified as ‘other’ and should have been excluded from these analyses. Some variations in mortality patterns seen in our study can be attributed to the differences between population-based and clinic-based cohorts. Because of patient profiles and expertise, clinic-based cohorts may self-select for certain types оf participants, whereas population-based cohorts are more representative and, therefore, may include people who are more disadvantaged and with higher risk for death. Mortality rates may also be affected by staffing numbers, physician experience, active engagement of patients through social workers and active tracing of patients that miss appointments.
Rates of deaths unrelated to HIV may be greater in some North American cohorts, particularly those because of suicide, homicide, drug and alcohol overdose and accidents. Higher rates of co-infection with hepatitis B or C may also lead to worse mortality. Canadian and veterans cohorts in USA contributing to ART-CC have a high prevalence of drug use and alcohol misuse.44,45 Life expectancy varies between46 and within countries; compared with Whites, it is lower for Blacks in USA47 and for Aboriginal peoples in Canada44,48, and there are a sizable proportion of these groups in North American ART-CC cohorts. Despite the provision of free universal health care in Canada, neighbourhood measures of socio-economic status (percentage of residents below the poverty line) and lower educational attainment were associated with higher mortality on ART;49 migration (change of address) during ART was common and adversely affected adherence to ART50 and housing status predicted disease progression.51 Housing stability may vary considerably between cohorts in ART-CC but is not directly captured, although it is likely to contribute to variation in loss to follow-up rates.
Implications and conclusion
Our research suggests the need for linkage to death registries to reduce under-ascertainment of death. There is also a need for linkage of patient records between clinics to reduce loss to follow-up when care is transferred to another site. Comparative analyses of mortality in HIV-positive individuals from diverse geographical and socio-economic settings should include sensitivity analyses that adjust for under-ascertainment of death and loss to follow-up. However, methods of adjustment are not straightforward, and interpretation of results may be problematical. Prognostic models and estimates of life expectancy that use pooled data from collaborations of cohorts should account for the between-cohort variation in mortality rates. In the same way that the Framingham equation for cardiovascular disease has been recalibrated for populations with different mortality rates,52 prognostic models for HIV progression will also need to be calibrated for different populations of patients. An exploration of the drivers of the higher mortality in some of the HIV cohorts contributing data to ART-CC would require collection of detailed data on socio-economic variables and lifestyle risk factors and consideration of mortality in matched HIV-negative persons. As the proportion of deaths that are not AIDS-related increases, mortality patterns in HIV-positive individuals are increasingly likely to reflect social disadvantage, which will vary between and within cohorts. Targeting health resources to the most disadvantaged groups might decrease disparities in outcomes. We will be addressing these issues in future analyses that use data from ART-CC.
Supplementary Data
Supplementary Data are available at IJE online.
Funding
M.M. and S.I. were funded by UK Medical Research Council (grants G0700820 and MR/J002380/1). Further funding of contributing cohorts was provided by the Agence Nationale de Recherche contre le SIDA (ANRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), the French, Italian and Spanish Ministries of Health, the Swiss National Science Foundation (grant 33CS30_134277), the Stichting HIV Monitoring, the European Commission (EuroCoord grant 260694), the British Columbia and Alberta Governments, the National Institutes of Health (NIH), UW CFAR (NIH grant P30 AI027757) USA, the Michael Smith Foundation for Health Research, the Canadian Institutes of Health Research, the VHA Office of Research and Development and unrestricted grants from GlaxoSmithKline, Pfizer, Bristol Myers Squibb, Roche and Boehringer-Ingelheim, the UW CFAR (NIH grant P30 AI027757) and the Spanish Network for AIDS Research (RIS; ISCIII-RETIC RD06/006).
Supplementary Material
Acknowledgements
All authors contributed to the design of the study. M.M. and S.I. did the data cleaning and extraction. M.M. did the statistical analyses supervised by J.S. M.M. wrote the first draft of the article. All authors contributed to interpreting the results and revising the article. All authors approved the final manuscript. M.M. is guarantor for the validity of the article. A list of the study groups for each cohort is available online as supplementary data.
Conflict of interest: None declared.
KEY MESSAGES.
There were significant differences in rates of AIDS and death in cohorts of HIV patients treated with ART in Europe and North America.
The between-cohort variance in mortality rates was reduced by a substantial amount after adjustment for patient characteristics.
Some of the residual between-cohort heterogeneity can be explained by under-ascertainment of deaths.
Mortality rates estimated from different HIV cohort studies may require adjustment for completeness of death ascertainment, especially if loss to follow-up is high.
Higher mortality rates in North American, compared with European, cohorts may be because of the inclusion of more socially marginalized patients with higher mortality risk.
References
- 1.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospectivestudies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Vella S, Benfield TL, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–30. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 3.Babiker A, Darbyshire J, Pezzotti P, et al. Short-term CD4 cell response after highly active antiretroviral therapy initiated at different times from seroconversion in 1,500 seroconverters. J Acquir Immune Defic Syndr. 2003;32:303–10. doi: 10.1097/00126334-200303010-00010. [DOI] [PubMed] [Google Scholar]
- 4.Sabin CA, Smith CJ, D’Arminio MA, et al. Response to combination antiretroviral therapy: variation by age. AIDS. 2008;22:1463–73. doi: 10.1097/QAD.0b013e3282f88d02. [DOI] [PubMed] [Google Scholar]
- 5.Law M, Friis-Moller N, Weber R, et al. Modelling the 3-year risk of myocardial infarction among participants in the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) study. HIV Med. 2003;4:1–10. doi: 10.1046/j.1468-1293.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 6.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 7.Lodwick RK, Sabin CA, Porter K, et al. Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per microL in Europe and North America: a pooled cohort observational study. Lancet. 2010;376:340–45. doi: 10.1016/S0140-6736(10)60932-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May M, Sterne JA, Sabin C, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21:1185–97. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gras L, Kesselring AM, Griffin JT, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr. 2007;45:183–92. doi: 10.1097/QAI.0b013e31804d685b. [DOI] [PubMed] [Google Scholar]
- 11.Binquet C, Chene G, Jacqmin-Gadda H, et al. Modeling changes in CD4-positive T-lymphocyte counts after the start of highly active antiretroviral therapy and the relation with risk of opportunistic infections: the Aquitaine Cohort, 1996-1997. Am J Epidemiol. 2001;153:386–93. doi: 10.1093/aje/153.4.386. [DOI] [PubMed] [Google Scholar]
- 12.Grabar S, Pradier C, Le CE, et al. Factors associated with clinical and virological failure in patients receiving a triple therapy including a protease inhibitor. AIDS. 2000;14:141–49. doi: 10.1097/00002030-200001280-00009. [DOI] [PubMed] [Google Scholar]
- 13.Lundgren JD, Phillips AN, Vella S, et al. Regional differences in use of antiretroviral agents and primary prophylaxis in 3122 European HIV-infected patients. EuroSIDA Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:153–60. doi: 10.1097/00042560-199711010-00003. [DOI] [PubMed] [Google Scholar]
- 14.D’Arminio MA, Lepri AC, Rezza G, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. AIDS. 2000;14:499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 15.Fatkenheuer G, Theisen A, Rockstroh J, et al. Virological treatment failure of protease inhibitor therapy in an unselected cohort of HIV-infected patients. AIDS. 1997;11:F113–16. doi: 10.1097/00002030-199714000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Jaen A, Casabona J, Esteve A, et al. [Clinical-epidemiological characteristics and antiretroviral treatment trends in a cohort of HIV infected patients. The PISCIS Project] Med Clin (Barc) 2005;124:525–31. doi: 10.1157/13073938. [DOI] [PubMed] [Google Scholar]
- 17.Sobrino-Vegas P, Gutierrez F, Berenguer J, et al. La cohorte de la red española de investigación de VIH (CoRIS) y su asociado biobanco; cuestiones de organización, las principales conclusiones y pérdidas durante el seguimiento [The cohort of the Spanish HIV Research Network (coris) and its associated biobank; organizational issues, main findings and losses to follow-up] Enferm Infecc Microbiol Clin. 2011;29:645–53. doi: 10.1016/j.eimc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Lampe FC, Smith CJ, Madge S, et al. Success of clinical care for human immunodeficiency virus infection according to demographic group among sexually infected patients in a routine clinic population, 1999 to 2004. Arch Intern Med. 2007;167:692–700. doi: 10.1001/archinte.167.7.692. [DOI] [PubMed] [Google Scholar]
- 19.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 20.Mocroft A, Gill MJ, Davidson W, Phillips AN. Predictors of a viral response and subsequent virological treatment failure in patients with HIV starting a protease inhibitor. AIDS. 1998;12:2161–67. doi: 10.1097/00002030-199816000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Schoeni-Affolter F, Ledergerber B, Rickenbach M, et al. Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol. 2010;39:1179–89. doi: 10.1093/ije/dyp321. [DOI] [PubMed] [Google Scholar]
- 22.Chen RY, Accortt NA, Westfall AO, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42:1003–10. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 23.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 Suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 24.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006;44(8 Suppl 2):S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson KB, Guest JL, Rimland D. Hepatitis C virus coinfection increases mortality in HIV-infected patients in the highly active antiretroviral therapy era: data from the HIV Atlanta VA Cohort Study. Clin Infect Dis. 2004;39:1507–13. doi: 10.1086/425360. [DOI] [PubMed] [Google Scholar]
- 26.Lemly DC, Shepherd BE, Hulgan T, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis. 2009;199:991–98. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitahata MM, Dillingham PW, Chaiyakunapruk N, et al. Electronic human immunodeficiency virus (HIV) clinical reminder system improves adherence to practice guidelines among the University of Washington HIV Study Cohort. Clin Infect Dis. 2003;36:803–11. doi: 10.1086/368085. [DOI] [PubMed] [Google Scholar]
- 28.Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, Sterne JAC. metan: fixed- and random-effects meta-analysis. Stata J. 2008;8:3–28. [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical-trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Lewden C, Jougla E, Alioum A, et al. Number of deaths among HIV-infected adults in France in 2000, three-source capture-recapture estimation. Epidemiol Infect. 2006;134:1345–52. doi: 10.1017/S095026880600639X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris RJ, Sterne JA, Abgrall S, et al. Prognostic importance of anaemia in HIV type-1-infected patients starting antiretroviral therapy: collaborative analysis of prospective cohort studies. Antivir Ther. 2008;13:959–67. [PMC free article] [PubMed] [Google Scholar]
- 33.Justice AC, McGinnis KA, Skanderson M, et al. Towards a combined prognostic index for survival in HIV infection: the role of ‘non-HIV’ biomarkers. HIV Med. 2010;11:143–51. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shepherd BE, Sterling TR, Moore RD, Raffanti SP, Hulgan T. Cross-cohort heterogeneity encountered while validating a model for HIV disease progression among antiretroviral initiators. J Clin Epidemiol. 2009;62:729–37. doi: 10.1016/j.jclinepi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanoy E, Mary-Krause M, Tattevin P, et al. Predictors identified for losses to follow-up among HIV-seropositive patients. J Clin Epidemiol. 2006;59:829–35. doi: 10.1016/j.jclinepi.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Lewden C, May T, Rosenthal E, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: the “Mortalite 2000 and 2005” surveys (ANRS EN19 and Mortavic) J Acquir Immune Defic Syndr. 2008;48:590–98. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC) Electronic record linkage to identify deaths among persons with AIDS: district of Columbia 2000-2005. MMWR Morb Mortal Wkly Rep. 2008;57:631–34. [PubMed] [Google Scholar]
- 38.Hill T, Bansi L, Sabin C, et al. Data linkage reduces loss to follow-up in an observational HIV cohort study. J Clin Epidemiol. 2010;63:1101–09. doi: 10.1016/j.jclinepi.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Schoni-Affolter F, Keiser O, Mwango A, et al. Estimating loss to follow-up in HIV-infected patients on antiretroviral therapy: the effect of the competing risk of death in Zambia and Switzerland. PLoS One. 2011;6:e27919. doi: 10.1371/journal.pone.0027919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanoy E, Lewden C, Lievre L, et al. How does loss to follow-up influence cohort findings on HIV infection? A joint analysis of the French hospital database on HIV, Mortalite 2000 survey and death certificates. HIV Med. 2009;10:236–45. doi: 10.1111/j.1468-1293.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 41.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brinkhof MW, Spycher BD, Yiannoutsos C, et al. Adjusting mortality for loss to follow-up: analysis of five ART programmes in sub-Saharan Africa. PLoS One. 2010;5:e14149. doi: 10.1371/journal.pone.0014149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egger M, Spycher BD, Sidle J, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8:e1000390. doi: 10.1371/journal.pmed.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Public Health Agency of Canada. HIV/AIDS Epi Update 2010: Chapter 8 HIV/AIDS Among Aboriginal People in Canada. 2010. http://www.phac-aspc.gc.ca/aids-sida/publication/epi/2010/8-eng.php (10 August 2012, date last accessed)
- 45.Braithwaite RS, McGinnis KA, Conigliaro J, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–97. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Global Health Indicators: Part 2. http://www.who.int/whosis/whostat/EN_WHS08_Table4_HSR.pdf (10 August 2012, date last accessed)
- 47.Harrison KM, Song R, Zhang X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr. 2010;53:124–30. doi: 10.1097/QAI.0b013e3181b563e7. [DOI] [PubMed] [Google Scholar]
- 48.Wilkins R, Uppal S, Fines P, Senecal S, Guimond E, Dion R. Life expectancy in the Inuit-inhabited areas of Canada, 1989 to 2003. Health Rep. 2008;19:1–13. Statistics Canada. http://www.statcan.gc.ca/pub/82-003-x/2008001/article/10463-eng.pdf (10 August 2012, date last accessed) [PubMed] [Google Scholar]
- 49.Joy R, Druyts EF, Brandson EK, et al. Impact of neighborhood-level socioeconomic status on HIV disease progression in a universal health care setting. J Acquir Immune Defic Syndr. 2008;47:500–05. doi: 10.1097/QAI.0b013e3181648dfd. [DOI] [PubMed] [Google Scholar]
- 50.Lima V, Fernandes K, Rachlis B, Druyts E, Montaner J, Hogg R. Migration adversely affects antiretroviral adherence in a population-based cohort of HIV/AIDS patients. Soc Sci Med. 2009;68:1044–49. doi: 10.1016/j.socscimed.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 51.Palepu A, Milloy MJ, Kerr T, Zhang R, Wood E. Homelessness and adherence to antiretroviral therapy among a cohort of HIV-infected injection drug users. J Urban Health. 2011;88:545–55. doi: 10.1007/s11524-011-9562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marrugat J, D’Agostino R, Sullivan L, et al. An adaptation of the Framingham coronary heart disease risk function to European Mediterranean areas. J Epidemiol Community Health. 2003;57:634–38. doi: 10.1136/jech.57.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.