Abstract
Changes in land use and management have been strongly affecting mountain grassland, however, their effects on the net ecosystem exchange of CO2 (NEE) and its components have not yet been well documented. We analysed chamber-based estimates of NEE, gross primary productivity (GPP), ecosystem respiration (R) and light use efficiency (LUE) of six mountain grasslands differing in land use and management, and thus site fertility, for the growing seasons of 2002 to 2008. The main findings of the study are that: (1) land use and management affected seasonal NEE, GPP and R, which all decreased from managed to unmanaged grasslands; (2) these changes were explained by differences in leaf area index (LAI), biomass and leaf-area-independent changes that were likely related to photosynthetic physiology; (3) diurnal variations of NEE were primarily controlled by photosynthetically active photon flux density and soil and air temperature; seasonal variations were associated with changes in LAI; (4) parameters of light response curves were generally closely related to each other, and the ratio of R at a reference temperature/ maximum GPP was nearly constant across the sites; (5) similarly to our study, maximum GPP and R for other grasslands on the globe decreased with decreasing land use intensity, while their ratio remained remarkably constant. We conclude that decreasing intensity of management and, in particular, abandonment of mountain grassland lead to a decrease in NEE and its component processes. While GPP and R are generally closely coupled during most of the growing season, GPP is more immediately and strongly affected by land management (mowing, grazing) and season. This suggests that management and growing season length, as well as their possible future changes, may play an important role for the annual C balance of mountain grassland.
1 Introduction
Due to the importance of the terrestrial carbon cycle for the global climate system there is considerable interest in understanding the factors that control the carbon balance of terrestrial ecosystems (Canadell et al., 2007; IPCC, 2007). In European mountain regions, grasslands are an important component of the landscape. Several studies have shown that temperate grasslands can act as both sinks and sources of CO2 (Gilmanov et al., 2007; Novick et al., 2004; Stoy et al., 2008; Wohlfahrt et al., 2008b). Differences and changes in land management can be expected to affect the carbon sequestration rate of these ecosystems (Cernusca et al., 2008), which in turn will feed back on atmospheric CO2 concentrations (Houghton, 1995, 1999; IPCC, 2007). Over the past decades, socio-economic changes have led to land-use changes in mountain grasslands (Cernusca et al., 1999; Tappeiner et al., 2003), which affect nitrogen availability (Robson et al., 2007; Zeller et al., 2000), species composition (Tasser et al., 1999), leaf and canopy gas exchange (e.g. Bahn et al., 1999; Tappeiner et al., 1999; Wohlfahrt et al., 2003) and soil and root respiration (Bahn et al., 2006, 2008).
While relationships between the net ecosystem CO2 exchange (NEE) of mountain grasslands and its abiotic and biotic drivers have been analysed in some detail (Fu et al., 2009; Gu et al., 2003; Hirota et al., 2009; Kato et al., 2004a, b; Wohlfahrt et al., 2008b, Yashiro et al., 2010), to our knowledge, there has so far been no study on whether and how land use change and land management might affect the diurnal, seasonal and interannual variation of NEE and its component processes gross primary productivity (GPP) and ecosystem respiration (R). This reflects a general scarcity of comparative studies of NEE on adjacent ecosystems (i.e. with comparable climate, geology and soil) differing in land-use (Don et al., 2009; Stoy et al., 2008).
Measurements of NEE are needed in order to determine the source-sink status of ecosystems, and to analyse how C exchange varies with seasonal and interannual variation in environmental conditions (Flanagan et al., 2002). Control of NEE is complex, often involving phenological variability, temporal variation in moisture availability, seasonal and interannual temperature variation, and canopy structure and variation in light intensity (e.g. Monson et al., 2002). Micrometeorological flux measurements are being made at more than 500 sites worldwide and form a global flux network (FLUXNET; Baldocchi et al., 2001; Baldocchi, 2008). The FLUXNET approach for assessing NEE is based on the eddy covariance (EC) method, which is most accurate when the atmospheric conditions are steady and the underlying surface is homogeneous (Baldocchi, 2003). These requirements currently limit this method to relatively homogeneous and flat terrain and greatly complicate the study of NEE in complex terrain (Schimel et al., 2002). As an alternative to the EC method, chamber-based measurements may be suitable for monitoring NEE from short-plant ecosystems located in complex terrain, such as mountain slopes (Li et al., 2008; Risch and Dougas, 2005). One of the strengths of the chamber approach is the possibility to control and manipulate environmental factors, which offers an opportunity to separate individual effects of environmental factors on NEE and to partition NEE into its key components, i.e. GPP and R while avoiding assumptions that commonly underlie the separation of the components as based on EC data (e.g. Wohlfahrt et al., 2005b).
In this paper, we present chamber-based measurements of NEE and its components for six mountain grasslands, including meadows, pastures and abandoned grasslands differing in site fertility, as obtained during seven consecutive growing seasons (2002–2008). Aim of this study was (1) to assess the importance of the major biotic and abiotic drivers determining the diurnal and seasonal variation of NEE in relation to land use and management, (2) to explore relationships between GPP, R and light use efficiency (LUE) across seasons and sites, and (3) compare the major components of NEE, i.e. GPP and R, for different types of grasslands around the globe. We tested the hypotheses that (1) differences in NEE and its component processes GPP and R between differently managed mountain grassland ecosystems are primarily driven by differences in the amount of photo-synthetically active leaf area and its CO2 assimilation potential, that (2) NEE and its component processes thus decrease as management intensity (in particular fertilisation) decreases (meadows>pastures>abandoned grasslands), and that (3) these changes occur in a similar manner for all component processes, which results in conservative ratios between CO2 uptake and release.
2 Material and methods
2.1 Study sites
The study sites are located in the Austrian Central Alps near the village Neustift (47°07′ N, 11°19′ E) in the Stubai Valley. Investigations were carried out during the growing seasons (May to November) on a meadow at the valley bottom (970 m a.s.l.) in the years 2002–2005, a mountain meadow (1750–1820 m a.s.l.) in the years 2002–2004 and 2005–2008, two pastures (1930 and 1950 m a.s.l.) in the years 2002–2004 and 2005–2008, a nutrient-rich abandoned grassland (1960 m a.s.l.) in the years 2002–2003, and a nutrient-poor abandoned grassland (2000 m a.s.l.) in the years 2003–2004 and 2005–2008.
Except for the valley bottom meadow all sites are located on slopes with inclinations of 19–29° (Table 1), which is typical for grasslands in mountain regions. The sites cover an annual average temperature range from 3.0 to 6.3 °C and an annual precipitation (values recorded at nearby weather stations) from 850 to 1097 mm (Table 1). The valley bottom meadow is cut three times per year and fertilised with manure annually (Wohlfahrt et al., 2008a), the mountain meadow is cut once a year and fertilised with manure every 2–3 years (Bahn et al., 2006). The pastures are grazed from May to mid-September. Cattle are moved around within the pastures by the farmer in order to ensure relatively even grazing within the entire area. As a consequence, the unfenced plots where NEE measurements were made had time to recover (and regrow) between grazing periods. Once grazing started again, an immediate response to grazing was thus observed. CO2-fluxes of the two pastures were very similar, consequently data from the two sites were pooled and are referred to as pastures. The two unmanaged grasslands were abandoned in 1983. The nutrient-rich abandoned was reforested and fertilised once in 1988. The trees were not included in the flux measurements. For soil type and vegetation compositions refer to Table 1.
Table 1.
General characterisation of the study sites.
| Grassland type | Meadows | Pastures | Abandoned | |||
|---|---|---|---|---|---|---|
| Altitude (m) | 970 | 1850 | 1950 | 1870 | 2000 | 1970 |
| Aspect | – | E-SE | SE | S-SE | S-SE | S-SE |
| Inclination (°) | 0 | 19 | 30 | 29 | 29 | 20 |
| MAT (°C) | 6.3 | 3 | 3 | 3 | 3 | 3 |
| MAP (mm) | 850 | 1097 | 1097 | 1097 | 1097 | 1097 |
| Land management | Organic fertilisation three cuts, grazed in autum |
Organic fertilisation one cut, grazed in late summer |
Grazed from May to Mid-September |
Grazed from May to Mid-September |
abandoned since 1983 fertilised 1989 |
abandoned since 1983 |
| Soil typea | Fluvisol | Dystric cambisol | Dystric cambisol | Dystric cambisol | Dystric cambisol | Dystric cambisol |
| Vegetation type | Pastinaco Arrhenteretum |
Trisetetum flavescentis |
Seslerio-Caricetum Sempervirentis |
Allchemillo Poetum supinae |
Vaccinio Callunetum |
Seslerio-Caricetum with dwarf shrubs |
| Dominant species |
Dactylis glomerata Poa pratensis Ranunculus acris Taraxacum officinale Trifolium pratense Trifolium repens Trifolium repens |
Alchemilla vulgaris Anthoxanthum odoratum Festuca rubra Leontodon hispidus Trifolium repens |
Alchemilla vulgaris Carex sempervirens Leontodon hispidus Lotus corniculatus Plantago lanceolata Plantago media Ranunculus montanus Sesleria albicans |
Alchemilla vulgaris Anthoxanthum odoratum Carex sempervirens Festuca rubra Leontodon hispidus Lotus corniculatus Sesleria albicans Trifolium repens |
Alchemilla vulgaris Avenella flexuosa Homogyne alpina Nardus stricta Vaccinium vitis-idaea |
Anthyllis vulneraria Calluna vulgaris Festuca rubra Vaccinium myrtillus Vaccinium vitis-idea |
| Study years | 2002–2005 | 2002–2004 | 2002–2004 | 2006–2008 | 2002–2003 | 2003–2008 |
| Above-ground biomass (g/m2)* |
2006–2008 | |||||
| spring | 311–607 | 190–313 | 157–334 | 198–374 | 291–376 | 106–215 |
| summer | 449–549 | 244–440 | 122–180 | 129–231 | 310–390 | 392–402 |
| autum | 266–517 | 224–261 | 119–138 | 91–148 | 105–240 | 217–386 |
FAO classification; MA, mean annual (values from two nearby weather stations, representing the situation of the valley bottom and the high mountain sites, respectively); T , temperature; P, precipitation.
Ranges refer to the minimum and maximum values in the given observation period.
2.2 Assessment of net ecosystem CO2 exchange
Measurements were conducted between 2002 and 2008 in episodic campaigns every three to four weeks. Throughout all campaigns the sites were sampled on the same day or within two to three consecutive days during stable weather conditions. During each campaign, chamber measurements of NEE were conducted over a diurnal course for each site. In order to document the diurnal course, from 2002 to 2005 the measurements began immediately before sunrise and ended about three hours after sunset. From 2006 to 2008 the measurements started in the night and ended at midday (mostly between 2:00 am and 12:00 am). All ecosystem respiration measurements reported in this paper were thus made during nighttime. We measured NEE with home-made manually operated temperature-stabilised closed transparent plexiglass chambers, as described by Wohlfahrt et al. (2005a). Chambers (0.4–0.7 m high) were placed on frames (0.6 m2 ground area) made of polyethylene that had been inserted 3 cm into the soil at the beginning of each season. Three frames were placed at each site and were alternately recorded once per hour with one chamber system. In total, 30 to 50 single measurements per day were taken. It was randomly chosen with which plot the sampling started. Measurements were usually carried out within 120 s during daytime and within 180 to not more than 300 s when flux rates were low (i.e. at levels of low or no light).
When placing the chamber on the frame a hole at the top of the chamber was kept open to avoid pressurization, in addition a vent connected to a tube at the lower part of the chamber prevented pressure differences to the atmosphere during measurements. On hot summer days air temperature inside the chamber was maintained within ± 2 °C relative to ambient by using cool packs, packed on a metal frame at the rear of the chamber. CO2 concentrations were measured with an infra-red gas analyser (LCA-2, ADC, Hoddesdon, UK) from 2002 to 2005 and CO2-Sensor (GMP343, Vaisala, Helsinki, Finnland) from 2006 to 2008. During all measurements the following parameters were recorded: air temperature outside and inside the chamber (temperature module 110, Voltcraft, Germany), air humidity (HM70, Vaisala, Helsinki, Finnland), soil temperature (HOBO data logger, Onset Computer, Bourne, MA, USA) and incident photosynthetically active photon flux density (PFD) (G1118 GaAsP, Ga Photodiode diffusion type, Hamamatsu Photonics GmbH, Germany).
NEE (μmol CO2 m−2 s−1) was calculated as
| (1) |
where dCO2/dt (μmol mol−1 s−1) is the change in CO2 mole fraction inside the chamber over time, P is the atmospheric pressure (kPa), Vm is the molar volume of CO2 (m3 mol−1), T is the chamber temperature (°C), P0 is the standard atmospheric pressure (kPa), V is the chamber volume (m3), and A is the chamber base area (0.6 m2). In this study, negative fluxes represent a net CO2 uptake by the ecosystem, positive ones the reverse.
Kutzbach et al. (2007) found that for their closed chamber system nonlinear regression models were more appropriate for estimating CO2 fluxes than linear ones. In contrast, for our chamber system we observed consistent and highly significant linear changes of CO2 concentrations over the comparatively short time of chamber closure, and therefore applied linear regressions in flux calculations.
An evaluation of the ecosystem chamber was made by a cross comparison with an eddy covariance system on flat terrain on the valley bottom meadow. The comparison was made using diurnal courses of NEE measured on 10 days between May and November 2002. The estimates of CO2 fluxes obtained by these two methods correspond well. NEE measured with chambers showed 12 and 23% higher flux rates during day- and nighttime, respectively, than estimated by the eddy covariance method. Hammerle et al. (2007) carried out a comparison at the mountain meadow and found an underestimation of 19% by the EC method. Similarly, a number of earlier studies observed higher flux rates obtained by chamber-based approaches as compared to the eddy covariance approach, which were in the range of 6–26% for daytime and 4–30% for nighttime fluxes (e.g. Angell et al., 2001; Dore et al., 2003; Dugas et al., 1999; Goulden et al., 1996; Lavigne et al., 1997; Wohlfahrt et al., 2005a; Zamolodchikov et al., 2003). Possible reasons for these discrepancies are differences in spatial sampling/footprint area (Lavigne et al., 1997), as well as an underestimation of EC-based respiration fluxes due to advection or insufficient turbulent mixing at night (Goulden at al., 1996; Lavigne et al., 1997). Chamber artefacts are mainly related to the influence of pressure on soil respiration (Davidson et al., 2002) and modifications of the microclimate, which were however minimised as described above.
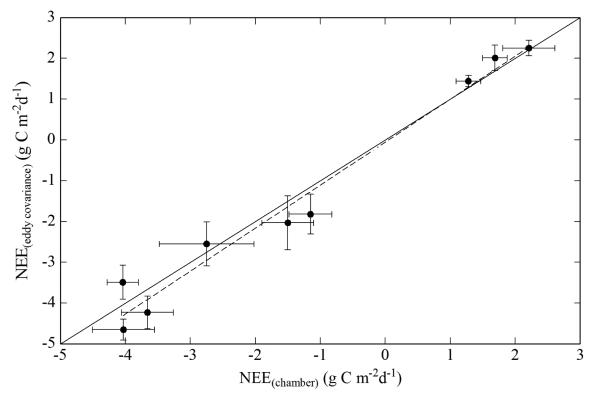
To take the comparison one step further, we tested how well daily NEE corresponded between eddy covariance-based and chamber-based data, thus comparing integrated daily values rather than individual data points. Daily average NEE based on chamber data was calculated for 7 day time periods using site specific environmental conditions and NEE light response curves measured during the course of a single day within this 7 day period. Daily average NEE based on the EC data was derived from gap-filled data as described in Wohlfahrt et al. (2008a). As depicted in the Fig. 1, the chamber method underestimated daily NEE measured by eddy covariance at the valley bottom meadow on average by only 5%.
Fig. 1.
Comparison between 7-day average daily integrated net ecosystem exchange of CO2 (NEE; g C m−2 s−1) derived from chamber and eddy covariance data at the valley bottom meadow. The solid line is the 1:1 line, the dotted line corresponds to a linear regression (NEE(eddy covariance) = 1.05 NEE(chamber) − 0.05; R2=0.97). Error bars represent standard errors and reflect temporal variability in gap-filled NEE (eddy covariance) and in meteorological input parameters of light response curve models (Eq. 2), which were used to simulate NEE based on chamber data.
2.3 Parameters of light response curves
To relate NEE to PFD we fitted the dataset obtained during each field campaign and site to the following rectangular hyperbolic model (Ruimy et al., 1995):
| (2) |
where NEE represents the net ecosystem exchange of CO2 (μmol m−2 s−1), α the apparent quantum yield (mol CO2 mol photons−1), PFD the photosynthetically active photon flux density (μmol m−2 s−1), GPPsat the asymptotic value of the gross primary production (GPP) at high irradiance (μmol m−2 s−1), and R denotes ecosystem respiration (μmol m−2 s−1).
For comparison across ecosystems we used Eq. (2) to calculate NEE and GPP at PFD of 2000 μmol m−2 s−1 (NEE2000, GPP2000). R was interpolated to a reference soil temperature of 10 °C (R10) by best fitting linear and exponential models. LUE was calculated by a linear regression fitted to the light response curves at low incident light intensities – the slope of the linear regression corresponds to the LUE.
Daily integrals of NEE were derived by applying Eq. (2) with input data (PFD) from 10 day periods around each measurement campaign. These 10-day periods were the same for all sites – the daily integrals of NEE are thus comparable and reflect differences between sites that were independent of the diurnal and day-to-day variability of PFD.
2.4 Measurement of biomass and leaf area index
Biomass and leaf area index were assessed at all sites by clipping of three square plots of 0.25 m2 at regular intervals throughout the vegetation periods from 2002 to 2008. The harvested plant material was separated into leaves, stems, reproductive organs, dead plant matter and cryptogams (mainly mosses). Silhouette area was determined with an area meter (LI-3100, Li-Cor, Lincoln, USA). Dry biomass was determined after drying at least for 24 h at 60 °C.
3 Results
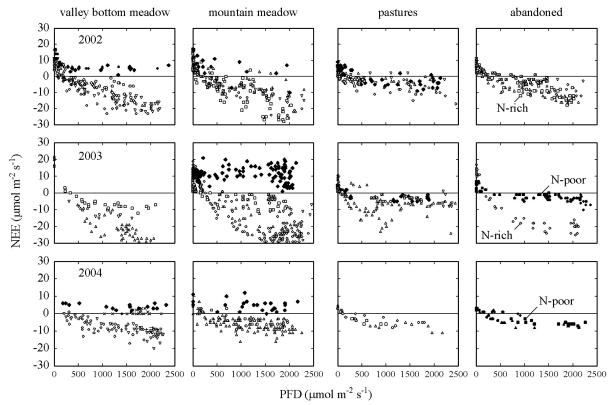
Minimum and maximum rates of net ecosystem CO2 exchange (NEE) as measured during all campaigns were highest for the meadows and fertilised abandoned grassland (Fig. 2), which all exhibited a high above-ground biomass (Table 1). Lowest peak net fluxes of CO2 were measured for the pastures and the unfertilised abandoned that relatively low abandoned grassland. grassland, supported above-ground biomass and leaf area index (LAI). For all study years, highest rates of net CO2 uptake coincided with values of peak biomass, mountain meadow, mountain meadow, occurring between June (valley bottom meadow) and August (abandoned sites), maximum NEE ranging from −16.5 μmol m−2 s−1 (late part of July 2004) to −30 μmol m−2 s−1 (June 2003). There were marked differences in the light response curves of NEE between the two abandoned sites in July and August (Fig. 2). In the year when both abandoned sites were studied simultaneously, the nutrient-rich abandoned grassland showed much higher values of NEE at any given temperature and light intensity, as compared to the nutrient-poor abandoned grassland.
Fig. 2.
NEE in relation to photon flux density (PFD) for two meadows, two pastures and two abandoned grasslands during the growing seasons 2002–2004. Negative values denote net CO2 uptake by the canopy, positive values a net loss of CO2 to the atmosphere. Each data point shows a single set of chamber measurements. The measurements were taken during clear days. The following symbols are used: × March, + April,  May,
May,  June, Δ June,
June, Δ June,  August,
August,  September,
September,  October,
October,  November; closed symbols indicate NEE after mowing and grazing on the meadows at the valley bottom (1000 m) and the mountain slope (1850m), as well as the pastures. For the abandoned sites open and closed symbols indicate values for the nutrient-rich and the nutrient-poor abandoned grassland, respectively.
November; closed symbols indicate NEE after mowing and grazing on the meadows at the valley bottom (1000 m) and the mountain slope (1850m), as well as the pastures. For the abandoned sites open and closed symbols indicate values for the nutrient-rich and the nutrient-poor abandoned grassland, respectively.
Across sites, ecosystem respiration (R) ranged from 0.6 (unfertilised abandoned grassland in October 2003 at Tair = −3 °C / Tsoil = 2.9 °C) to 22.1 μmol m−2 s−1 (valley bottom meadow in July 2003 at Tair = 13.5 °C / Tsoil = 13.7 °C). Across seasons and sites, ecosystem respiration generally increased linearly and exponentially with soil (R2 = 0.28–0.78, p < 0.01 – 0.06) and air (R2 = 0.25–0.66, p < 0.01–0.06) temperature (data not shown).
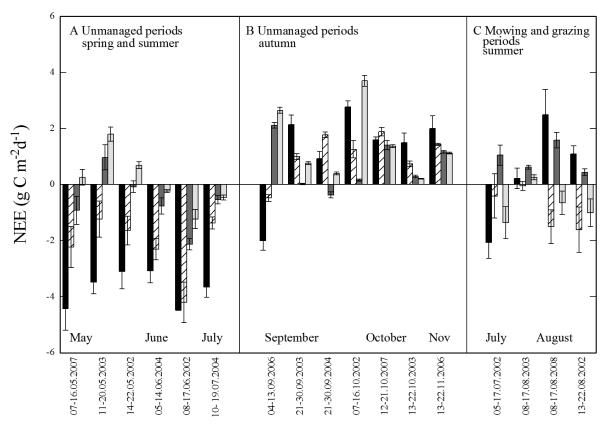
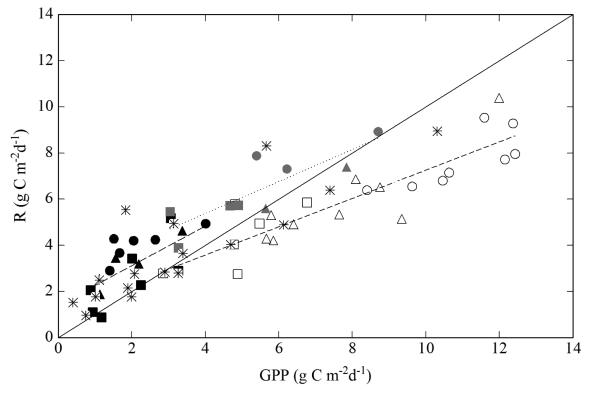
Daily average NEE, as compared for the same time periods across sites, mostly decreased from valley bottom meadow to mountain meadow to pastures and abandoned grasslands (Fig. 3a, b). For unmanaged periods, at all study sites, daily NEE followed a marked seasonal trend varying from −4.47 (valley bottom meadow), −4.20 (mountain meadow), −2.13 (pastures) and −1.36 g C m−2 d−1 (abandoned) in spring and summer to 2.77, 1.89, 2.11 and 3.70 g C m−2 d−1, respectively, in autumn (Fig. 3a, b). Correspondingly, average daily integrated gross primary productivity (GPP) exceeded R for unmanaged periods in spring and summer on the meadows and pastures, while the reverse was generally true in autumn, when GPP was more strongly reduced relative to the summer months than R (Fig. 4). Daily average GPP and R of the abandoned sites generally clustered around the 1:1 line of Fig. 4. Mowing and grazing resulted in a transient net release of CO2 from the meadows and the pastures (Fig. 3c), which lasted for approximately 6–10 days (not shown).
Fig. 3.
Daily integrated average net ecosystem exchange of CO2 (NEE; g C m−2 s−1) of (A, B) unmanaged, and (C) mowing and grazing periods from 2002 to 2008. Groups of bars refer to the same 10-day time periods for all sites as explained in the text. NEE was calculated using Eq. (2) based on 10-day periods during which at least one complete day of chamber measurements was available, and using site-specific microclimatic conditions as input. Sites are indicated by black bars (valley bottom meadow), criss-cross bars (mountain meadow), dark grey bars (pastures) and light grey bars (abandoned). Error bars represent standard errors and reflect the temporal variability in meteorological input parameters to Eq. (2).
Fig. 4.
Relationship of daily integrated average gross primary productivity (GPP) and ecosystem respiration (R) at all sites for unmanaged and mowing and grazing periods from 2002 to 2008, as based on the approach for Fig. 3. Sites and management periods are indicated by symbols: circles (valley bottom meadow), triangles (mountain meadow), quadrats (pastures) and stars (abandoned); open symbols (unmanaged periods, spring and summer), closed symbols (un-managed periods, autumn) and grey symbols (mowing and grazing periods, summer). The solid line is the 1:1 line, the dotted regression lines indicate linear relationships, as described by R = a× GPP + b. a, b and R2 of the linear regressions are as follows: 0.61, 1.13 and 0.80 for unmanaged periods in spring and summer, 0.86, 1.41 and 0.44 for unmanaged periods in autumn, and 0.69, 2.60, 0.71, respectively, for mowing and grazing periods (during summer).
Pooling all NEE data across the season, their variation was associated with variations in photosynthetically active photon flux density (PFD), temperature, above-ground biomass, plant area index (PAI) and LAI. A stepwise multiple regression across all sites and years explained 68% of NEE, 75% of gross primary productivity (GPP) and 60% of R for these grasslands by the factors PFD, air and soil temperature, above-ground biomass, LAI, type of grassland, measurement year and the time of the season. PFD was the most important factor determining NEE and GPP, explaining 61% (p < 0.001) and 67% (p < 0.001) of the respective variability. Soil temperature was the most prominent parameter determining R (0.33, p < 0.001). While during the heat wave 2003 Western parts of Europe suffered from drought, the Austrian Alps remained largely unaffected and NEE was not moisture limited (Foelsche, 2004; Wohlfahrt et al., 2008b). At the sites studied here biomass was high in 2003 and peak values of NEE and R exceeded those from 2002 to 2008.
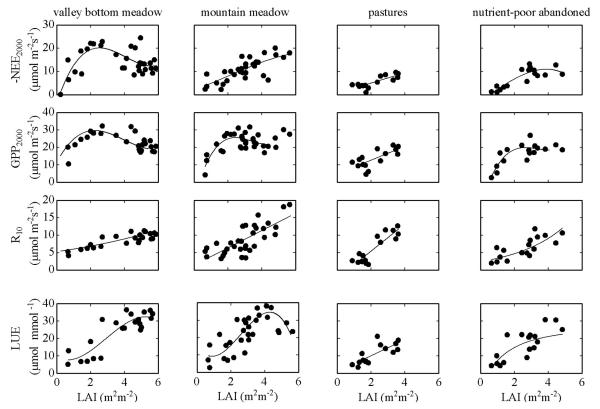
NEE, GPP, R and light use efficiency (LUE) were significantly related to LAI (Fig. 5) (for regression statistics cf. Table 2). At all sites, except the pastures, optimum values for LAI ranged from 2.7 to 4.8 m2 m−2.
Fig. 5.
Relationships between the net ecosystem CO2 exchange at a photon flux density (PFD) of 2000 μmol m−2 s−1 (NEE2000), the gross primary productivity at a PFD of 2000 μmol m−2 s−1 (GPP2000), the ecosystem respiration at a reference temperature of 10 °C (R10) and the light use efficiency (LUE) in response to leaf area index (LAI) at the study sites. Regressions are based on all available data during the growing seasons from 2002 to 2008. For regression statistics see Table 2.
Table 2.
Regression statistics of the relationships between the net ecosystem CO2 exchange at a PFD of 2000 μmol m−2 s−1 (NEE2000), the gross primary productivity at a PFD of 2000 μmol m−2 s−1 (GPP2000), the ecosystem respiration at a reference temperature of 10 °C (R10) and the light use efficiency (LUE) in response to leaf area index (LAI). The following functions were used: NEE2000 vs. LAI: cubic and linear, GPP2000 vs. LAI: cubic and linear, R10 vs. LAI exponential- and linear, LUE vs. LAI: cubic and linear.
| Site | NEE2000vs. LAI | GPP2000 vs. LAI | R10 vs. LAI | LUE vs. LAI | ||||
|---|---|---|---|---|---|---|---|---|
| R 2 | p value | R 2 | p value | R 2 | p value | R 2 | p value | |
| Valley bottom meadow | 0.50 | < 0.001 | 0.55 | < 0.001 | 0.72 | < 0.001 | 0.71 | < 0.001 |
| Mountain meadow | 0.55 | < 0.001 | 0.54 | < 0.001 | 0.56 | < 0.001 | 0.57 | < 0.001 |
| Pastures | 0.63 | < 0.001 | 0.50 | 0.002 | 0.78 | < 0.001 | 0.56 | < 0.001 |
| Abandoned | 0.71 | < 0.001 | 0.63 | 0.001 | 0.56 | < 0.001 | 0.63 | < 0.001 |
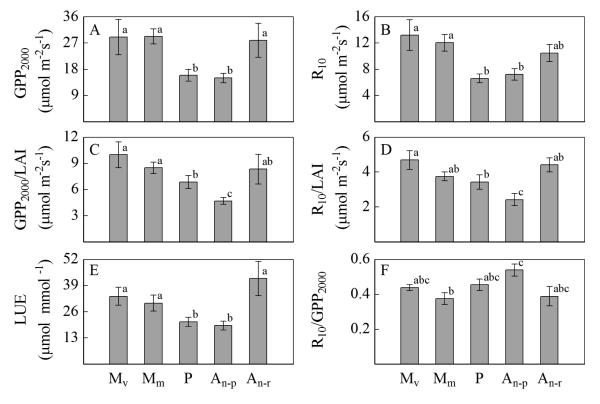
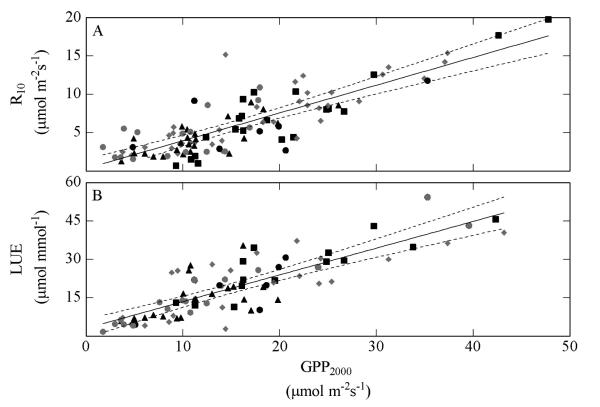
Across all years seasonal peak values of GPP, R and light use efficiency (LUE), measured at optimum LAI, decreased significantly from the meadows to the pastures and the N-poor abandoned grassland, whereas these parameters increased on the fertilized abandoned grassland (N-rich) (Fig. 6a, b, e). When normalized for LAI, GPP also decreased significantly from the meadows to the pastures and the N-poor abandoned grassland (Fig. 6c). Normalized R tended to decrease in the same manner, and was significantly lower on the N-poor abandoned grassland as compared to the other sites (Fig. 6d). The ratio R/GPP was not significantly different between sites, except between the mountain meadow and the nutrient poor abandoned grassland, the highest ratio occurring on the N-poor abandoned grassland (Fig. 6f). When pooling all data across seasons and years, we observed a similar trend as for peak season values: GPP, R and LUE decreased from the meadows to the pastures and the N-poor abandoned grassland (data not shown). Across study sites and years there were positive correlations between GPP, R and LUE (Fig. 7).
Fig. 6.
Mean peak season values of (A) gross primary productivity per unit ground area at a photon flux density of 2000 μmol m−2 s−1 (GPP2000), (B) ecosystem respiration at a reference temperature of 10 °C (R10), (C) GPP2000 per unit leaf area, (D) R10 per unit leaf area, (E) light use efficiency (LUE), (F) R10/GPP2000 at optimum LAI based on all field campaigns across all study years. Sites are indicated as: Mv (valley bottom meadow), Mm (mountain meadow), P (pastures), An–r (nutrient-rich abandoned grassland), An–p (nutrient-poor abandoned grassland). Significantly different means are indicated by different letters (oneway ANOVA). Error bars represent standard errors, Mv: n=4 (May 2002 to 2005), Mm: n=7 (August 2002 to 2004 and 2006 to 2008), P: n=7 (August 2002 to 2004 and 2006 to 2008), An–p: n=6 (August 2003 to 2004 and 2006 to 2008), An–r: n=2 (March 2002 to 2003).
Fig. 7.
Relationship of the gross primary productivity at a photon flux density of 2000 μmol m−2 s−1 (GPP2000) and (A) the ecosystem respiration at a reference temperature of 10 °C (R10) and (B) the light use efficiency (LUE). The equations for the fitted lines for all sites and years are: R10 = 0.36 GPPmax + 0.32 (R2 = 0.69; p < 0.01) and LUE = 0.88 GPP2000 + 2.86 (R2 = 0.66; p < 0.01). Dotted lines represent 95% confidence intervals. Sites are indicated by following symbols: ■ valley bottom meadow,  mountain meadow, ▲ pastures, ● nutrient-rich abandoned grassland,
mountain meadow, ▲ pastures, ● nutrient-rich abandoned grassland,  nutrient-poor abandoned grassland.
nutrient-poor abandoned grassland.
4 Discussion
A number of previous studies have shown that NEE is affected by interactions of microclimate, canopy structure and the photosynthetic and respiratory physiology of plants (for grasslands e.g. Flanagan and Johnson, 2005; Gu et al., 2003; Tappeiner and Cernusca, 1998; Wohlfahrt et al., 2003). In most ecosystems light is the prime driver and explains most variability of NEE (Flanagan et al., 2002; Li et al., 2005, 2008; Ruimy et al., 1995; Wilsey et al., 2002; Zhao et al., 2006). PFD, air and soil temperature, above-ground biomass, LAI, type of grassland, measurement year and the time of the season (see results section) explained 68% of NEE, 75% of GPP and 60% of R in our study. The diurnal variation of NEE was mostly determined by PFD during daytime and by soil and air temperature during nighttime. The seasonal and inter-annual variation was also affected by above-ground biomass and LAI. The unexplained variability in the stepwise multiple regression indicates that NEE was related to a number of factors, including the spatial variability in nutrient availability and species composition and related effects on above- (Bahn et al., 1999) and belowground processes (Bahn et al., 2006). Contrary to results from studies in water limited systems (Wilsey et al., 2002), it appears that soil water availability did not induce seasonal variation in NEE at our sites in years with typical amount of precipitation. The extreme summer of 2003 was characterised in many parts of Europe by hot and dry days (Ciais et al., 2005; Foelsche 2004), which at our sites led to a high production of above-ground biomass and LAI and particularly high values of NEE, GPP and R (Fig. 2). This finding indicates that unusually warm years need not generally cause a reduction of GPP (Ciais et al., 2005; Reichstein et al., 2006), but can potentially also enhance site productivity at sites with sufficient water supply (Jaksic et al., 2006; Mirzae et al., 2008).
LAI is a unique biophysical factor accounting for differences in phenological development, assimilation and biomass growth in plant canopies. Leaf area exerts a major influence on canopy photosynthesis (Saigusa et al., 1998; Tappeiner and Cernusca, 1996), which also provides assimilates for the respiration of roots and soil microorganisms (Bahn et al., 2008, 2009; Reichstein et al., 2003). Both, mowing and grazing, cause a substantial reduction of leaf area and thus GPP (Fig. 4), turning the meadows and the pastures from sinks to short-term sources of CO2 (Fig. 3). For the valley bottom meadow (three cuts per year) of our study it took on average 16 days after the first cut to become (on a daily basis) a net sink for CO2 again (Wohlfahrt et al., 2008a). This pattern repeated itself after the second and third cut, whereas daily average rates of net CO2 uptake and loss before and after cutting, respectively, decreased from the first to the third cut. Other studies in warm temperate grasslands showed that between 6 to 11 days are required before net carbon gain (on a daily basis) is resumed (Dugas et al., 1999; Novick et al., 2004).
Our study showed that land use and management were responsible for the differences in NEE between sites (Fig. 3). Generally, NEE, GPP and R increased with management intensity (Figs. 3 and 4). The fertilised meadows showed the highest, the unmanaged (unfertilised) abandoned grassland the lowest values of NEE. For unmanaged periods in spring and summer NEE was more strongly determined by CO2-uptake than release (Fig. 4). In contrast, during unmanaged periods in autumn, NEE was more affected by CO2-release.
At all sites, both NEE and GPP at a PFD of 2000 μmol m−2 s−1 were closely related to LAI, the relationships saturating at LAI of 2.7–4.8 m2 m−2. This is typical for productive grasslands, for which maximum GPP was observed at LAI of 2–5 m2 m−2. (Gilmanov et al., 2007; Veenendaal et al., 2007; Wohlfahrt et al., 2008b). At the pastures studied here, grazing maintained LAI below a critical value of 4, which avoided trade-offs between increasing assimilatory area and associated self-shading effects.
The key parameters of light response curves of NEE include GPP at maximum incident light (GPP2000), R at a reference temperature (R10) and the initial slope of the curve (LUE), which were all affected by land management and land-use change. In our study, across all years seasonal mean as well as seasonal peak values of GPP2000, R10 and LUE decreased significantly from managed to unmanaged sites (Fig. 3a, b, e). Such a decrease could potentially be caused by differences in leaf area, its spatial arrangement, as well as the photosynthetic physiology of the dominant species. Accounting for effects of leaf area by normalizing GPP to LAI, we observed a reduction of GPP2000 per unit leaf area with decreasing intensity of land management, in particular N supply. This finding is in agreement with ecophysiological studies documenting a reduction of leaf photosynthetic capacities with decreasing intensity of grassland management (Bahn et al., 1999), which are related to changes in species composition and nutrient availability.
Also normalized R10 tended to decrease from the meadows and the N-rich abandoned grassland to the pastures and the N-poor abandoned grassland (Fig. 6d). Ecosystem respiration is the sum of respiration of aboveground biomass and soil respiration. Soil respiration is considered to be the main contributor to R in most ecosystems, particularly grasslands (Wohlfahrt et al., 2005a). Grassland soil respiration has been shown to be closely related to LAI and GPP (Bahn et al., 2008, 2009). Likewise, our study demonstrates a close link between GPP and R across sites and study years (Fig. 7), which confirms earlier observations for a range of grasslands (Gilmanov et al., 2007; Li et al., 2005; Wohlfahrt et al., 2008b). Our study indicates that the ratio of R10 / GPP2000 is generally not affected by land management, however, may be somewhat higher on nutrient poor sites (Fig. 6f).
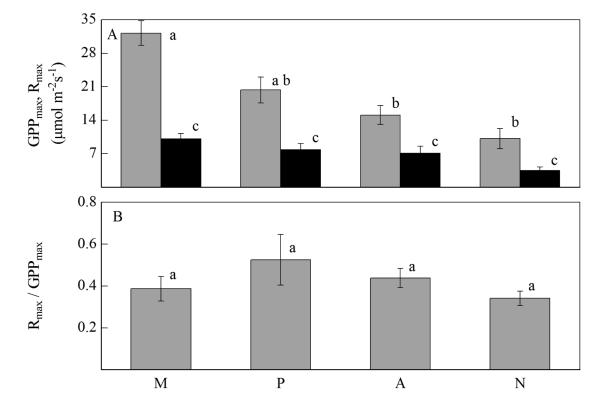
To test the generality of our findings, we compared maximum GPP and R for ecosystems differing in land use from around the globe (Fig. 8). Similar to our study, GPPmax and Rmax decreased from meadows to pastures and abandoned and natural grasslands (Fig. 8a). We assume that the main reason for lower fluxes at unmanaged systems is the lower soil fertility in comparison to the managed systems, which are usually fertilised leading to higher flux rates of GPP and R. We found no significant relationship between GPPmax or Rmax and the mean annual temperature and/ or precipitation at the sites, suggesting that climatic effects did not confound the observed land-management and land-use effects. The ratio Rmax / GPPmax (Fig. 8b) was relatively constant across all types of ecosystems, which indicates a close coupling of maximum R to potential site productivity and vice versa. This global comparison confirms the trend observed in our study and thus the notion that the intensity of land use exerts a major influence on the components of NEE, while leaving their ratio largely unaffected.
Fig. 8.
(A) Maximum gross primary productivity (GPPmax) (grey bars) and maximum ecosystem respiration (Rmax) (black bars), (B) ratio of Rmax and GPPmax for different ecosystems across the globe. Ecosystems are indicated as: M (meadows), P (pastures), A (abandoned), N (natural grasslands, including steppes and prairies, tundras and savannas). Significantly different means are indicated by different letters (oneway ANOVA). Error bars represent standard errors, meadows n=28 (References: Barcza et al., 2003; Byrne et al., 2005; Falge et al., 2002; Gilmanov et al., 2007; Lawton et al., 2006; Li et al., 2003; Maljanen et al., 2001; Otieno et al., 2009; Rogiers et al., 2004; Wilsey et al., 2002; Wohlfahrt 2004; Zhao et al., 2006), pastures n=19 (References: Byrne et al., 2005; Don et al., 2009; Falge et al., 2002; Gilmanov et al., 2007; Grace et al., 1998; Hunt et al., 2004; Jaksic et al., 2006; Nieveen et al., 2005; Priante-Filho et al., 2004; Rogiers et al., 2004; Santos et al., 2004; Susiluoto et al., 2008; Wilsey et al., 2002), abandoned n=13 (Reference: Don et al., 2009; Flanagan et al., 2002; Flanagen et al., 2005; Gilmanov et al., 2007; Hanan et al., 2003; Miranda et al., 1997; Otieno et al., 2009; Ripley and Redmann, 1978; Soegaard and Nordstroem, 1999; Suyker et al., 2001; Zamolodchikov et al., 2003), natural grasslands n=18 (References: Angell et al., 2001; Baldocchi et al., 2006; Corradi et al., 2005; Gilmanov et al., 2005; Obrist et al., 2003; Susiluoto et al., 2008; Zhao et al., 2006).
5 Conclusions
From our study we conclude that in mountain grassland, and most likely also in grassland in general, land use and management may importantly affect the net ecosystem CO2 exchange (NEE) and its component fluxes gross primary productivity (GPP) and ecosystem respiration (R). In our study, these effects were mediated by differences in leaf area index, biomass and leaf-area-independent changes that were likely related to photosynthetic physiology. NEE and its component fluxes decreased with decreasing intensity of management and thus site fertility. The ratio of R at a reference temperature/ maximum GPP remained remarkably constant across gradients of land-use intensity both within the present study of adjacent sites and for a global compilation of grasslands. During short periods following mowing or grazing, the studied grasslands turned from carbon sinks to sources. As such changes in the source-sink relations also occur as part of the seasonal dynamics of NEE, future studies should address possible trade-offs between effects of land-use changes and those related to phenology and growing season length in a climate change context.
Acknowledgements
This study was funded by the EU FP 5 project CarboMont (EVK2-CT2001-00125), the Austrian Science Fund (FWF) (P18756-B16) and the Tiroler Wissenschaftsfonds (GZ UNI-0404/402). We thank Matthias Drösler and Erich Tasser for advice on the chamber method and statistical analyses, respectively, Florian Miczka, Margit Knapp, Mathias Gort and Sebastian Waldhuber for assistance with the field measurements, Karin Bianchi, Miriam Bahn and Veronika Saxl for helping with the harvesting and analysis of plant biomass, and the editor and two anonymous referess for valuable comments on earlier versions of the manuscript.
References
- Angell RF, Svejcar T, Bates J, Saliendrab NZ, Douglas A, Johnson DA. Bowen ratio and closed chamber dioxide flux measurements over sagebrush steppe vegetation. Agr. Forest Meteorol. 2001;108:153–161. [Google Scholar]
- Bahn M, Wohlfahrt G, Haubner E, Horak I, Michaeler W, Rottmar K, Tappeiner U, Cernusca A. Land-use changes in European mountain ecosystems, Ecomont – Concept and Results. Blackwell; Berlin: 1999. Leaf photosynthesis, nitrogen contents and specific leaf area of 30 grassland species in differently managed mountain ecosystems in the Eastern Alps; pp. 247–255. [Google Scholar]
- Bahn M, Knapp M, Garajova Z, Pfahringer N, Cernusca A. Root respiration in temperate mountain grasslands differing in land use. Glob. Change Biol. 2006;12:995–1006. [Google Scholar]
- Bahn M, Rodeghiero R, Anderson-Dunn M, Dore S, Gimeno C, Drösler M, Williams M, Ammann C, Berninger F, Flechard C, Jones S, Balzarolo M, Kumar S, Newesely C, Priwitzer T, Raschi A, Siegwolf R, Susiluoto S, Tenhunen J, Wohlfahrt G, Cernusca A. Soil respiration in European grasslands in relation to climate and assimilate supply. Ecosystems. 2008;11:1352–1367. doi: 10.1007/s10021-008-9198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn M, Schmitt M, Siegwolf R, Richter A, Brüggemann N. Does photosynthesis affect grassland soil-respired CO2 and its carbon isotope composition on a diurnal timescale? New Phytol. 2009;182:451–460. doi: 10.1111/j.1469-8137.2008.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldocchi DD, Falge E, Gu L, Olson R, Hollinger D, Running S, Anthoni P, Bernhofer Ch., Davis K, Fuentes J, Goldstein A, Katul G, Law B, Lee X, Malhi Y, Meyers T, Munger JW, Oechel W, Pilegaard K, Schmid HP, Valentini R, Verma S, Vesala T, Wilson K, Wofsy S. FLUXNET: A new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapour and energy flux densities. B. Am. Meteorol. Soc. 2001;82:2415–2435. [Google Scholar]
- Baldocchi DD. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: past, present and future. Glob. Change Biol. 2003;9:479–492. [Google Scholar]
- Baldocchi DD, Tang J, Xu L. How Switches and Lags in Biophysical Regulators Affect Spatio-Temporal Variation of Soil Respiration in an Oak-Grass Savanna. J. Geophys. Res. – Biogeosciences. 2006;111:G02008. doi:10.1029/2005JG000063. [Google Scholar]
- Baldocchi DD. “Breathing” of the Terrestrial Biosphere: Lessons Learned from a Global Network of Carbon Dioxide Flux Measurement Systems. Aust. J. Bot. 2008;56:1–26. [Google Scholar]
- Barcza Z, Haszpra L, Kondo H, Saigusa N, Yamamoto S, Bartholy J. Carbon exchange of grass in Hungary. Tellus B. 2003;55:187–196. [Google Scholar]
- Byrne KA, Kiely G, Leahy P. CO2 fluxes in adjacent new and permanent temperate grasslands. Agr. Forest Meteorol. 2005;135:82–92. [Google Scholar]
- Canadell JG, Le Quéré C, Raupach MR, Field CB, Buitenhuis ET, Ciais P, Conway TJ, Gillett NP, Houghton RA, Marland G. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. P. Natl. Acad. Sci. USA. 2007;104:18866–18870. doi: 10.1073/pnas.0702737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernusca A, Tappeiner U, Bayfield N. Land-use changes in European Mountain ecosystems, Ecomont – concepts and results. Blackwell; Berlin: 1999. [Google Scholar]
- Cernusca A, Bahn M, Berninger F, Tappeiner U, Wohlfahrt G. Effects of land-use changes on sources, sinks and fluxes of carbon in European mountain grasslands. Ecosystems. 2008;11:1335–1337. [Google Scholar]
- Ciais P, Reichstein M, Viovy N, Granier A, Ogée J, Allard V, Aubinet M, Buchmann N, Bernhofer C, Carrara A, Chevallier F, De Noblet N, Friend AD, Friedlingstein P, Grünwald T, Heinesch B, Keronen P, Knohl A, Krinner G, Loustau D, Manca G, Matteucci G, Miglietta FJ, Ourcival M, Papale D, Pilegaard K, Rambal S, Seufert G, Soussana JF, Sanz MJ, Schulze ED, Vesala T, Valentini R. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature. 2005;437:529–533. doi: 10.1038/nature03972. [DOI] [PubMed] [Google Scholar]
- Corradi C, Kolle O, Walter K, Zimov SA, Schulze ED. Carbon dioxide and methane exchange of a north-east Siberian tussock tundra. Glob. Change Biol. 2005;11:1–16. [Google Scholar]
- Davidson EA, Savage K, Verchot LV, Navarro R. Minimizing artefacts and biases in chamber-based measurements of soil respiration. Agr. Forest Meteorol. 2002;113:21–37. [Google Scholar]
- Don A, Rebmann C, Koll EO, Scherrer-Lorenzen M, Schulze ED. Impact of afforestation-associated management changes on the carbon balance of grassland. Glob. Change Biol. 2009;15:1990–2002. [Google Scholar]
- Dore S, Hymus GJ, Johnson DP, Hinkle CR, Valentini R, Drake BG. Cross validation of open-top chamber and eddy covariance measurements of ecosystem CO2 exchange in a Florida scrub-oak ecosystem. Glob. Change Biol. 2003;9:84–95. [Google Scholar]
- Dugas WA, Heuer ML, Mayeux HS. Carbon dioxide fluxes over bermudagrass, native prairie, and sorghum. Agr. Forest Meteorol. 1999;93:121–139. [Google Scholar]
- Falge E, Baldocchi D, Tenhunen J, Aubinet M, Bakwin P, Berbigier P, Bernhofer Ch., Burba G, Clement R, Davis KJ, Elbers JA, Goldstein AH, Grelle A, Granier A, Guðmundsson J, Hollinger D, Kowalski A, Katul G, Law B, Malhi Y, Meyers T, Monson R, Munger JW, Oechel W, Paw UKT, Pilegaard K, Rannik U, Rebmann C, Suyker A, Valentini R, Wilson K, Wofsy S. Seasonality of ecosystem respiration and gross primary production as derived from Fluxnet measurements. Agr. Forest Meteorol. 2002;113:53–74. [Google Scholar]
- Flanagan LB, Wever LA, Carlson PJ. Seasonal and interannual variation in carbon dioxide exchange and carbon balance in a northern temperate grassland. Glob. Change Biol. 2002;8:599–615. [Google Scholar]
- Flanagan LB, Johnson BG. Interacting effects of temperature, soil moisture and plant biomass production on ecosystem respiration in a northern temperate grassland. Agr. Forest Mete-orol. 2005;130:237–253. [Google Scholar]
- Foelsche U. Regionale Entwicklung und Auswirkungen extremer Wetterereignisse am Beispiel Österreich. Springer; Berlin-Heidelberg: 2004. pp. 25–29. [Google Scholar]
- Fu Y, Zheng Z, Yu G, Hu Z, Sun X, Shi P, Wang Y, Zhao X. Environmental influences on carbon dioxide fluxes over three grassland ecosystems in China. Biogeosciences. 2009;6:2879–2893. http://www.biogeosciences.net/6/2879/2009/ [Google Scholar]
- Gilmanov TG, Tieszen LL, Wylie BK, Flanagan LB, Frank AB, Haferkamp MR, Meyers TP, Morgan JA. Integration of CO2 flux and remotely-sensed data for primary production and ecosystem respiration analyses in the Northern Great Plains: Potential for quantitative spatial extrapolation. Global Ecol. Biogeogr. 2005;14:271–292. [Google Scholar]
- Gilmanov TG, Soussanae JF, Airesa L, Allarde V, Ammanni C, Balzarolop M, Barczaq Z, Bernhofer C, Campbell CL, Cernusca A, Cescatti A, Clifton-Brown J, Dirks BOM, Dore S, Eugster W, Fuhrer J, Gimeno C, Gruenwald T, Haszpra L, Hensen A, Ibrom A, Jacobs AFG, Jones MB, Lanigan G, Laurila T, Lohila A, Manca G, Marcolla B, Nagy Z, Pilegaardm K, Pinter K, Pio C, Raschi A, Rogiers N, Sanz MJ, Stefani P, Sutton M, Tuba Z, Valentini R, Williams ML, Wohlfahrt G. Partitioning European grassland net ecosystem CO2 exchange into gross primary productivity and ecosystem respiration using light response function analysis. Agr. Ecosyst. Environ. 2007;121:93–120. [Google Scholar]
- Goulden M, Munger J, Fan S-M, Daube B, Wofsy S. Measurement of carbon sequestration by long-term eddy covariance: methods and a critical evaluation of accuracy. Glob. Change Biol. 1996;2:169–182. [Google Scholar]
- Grace J, Lloyd J, Miranda AC, Miranda H, Gash JHC. Fluxes of carbon dioxide and water vapour over a C4 pasture in south-western Amazonia (Brazil) Aust. J. Plant Physiol. 1998;25:519–530. [Google Scholar]
- Gu S, Tang YH, Du MY, Kato T, Li YN, Cui XY, Zhao XA. Short-term variation of CO2 flux in relation to environmental controls in an alpine meadow on the Qinghai-Tibetan Plateau. J. Geophys. Res. 2003;108:4670–4678. doi:10.1029/2003JD003584. [Google Scholar]
- Hammerle A, Bahn M, Cernusca A, Haselwanter A, Schmitt M, Tappeiner U, Wohlfahrt G. Eddy covariance measurements of carbon dioxide, latent and sensible energy fluxes above a meadow on a mountain slope. Bound-Lay. Meteorol. 2007;122:397–416. doi: 10.1007/s10546-006-9109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanan NP, Kabat P, Dolman JA, Elbers JA. Photosynthesis and carbon balance of a Sahelian fallow savanna. Glob. Change Biol. 2003;4:523–238. [Google Scholar]
- Hirota M, Zhang P, Gu S, Du M, Shimono A, Shen H, Li Y, Tang Y. Altitudinal variation of ecosystem CO2 fluxes in an alpine grassland from 3600 to 4200 m. J. Plant Ecol. 2009;2:197–205. [Google Scholar]
- Houghton RA. Land-use change and the carbon cycle. Glob. Change Biol. 1995;1:275–287. [Google Scholar]
- Houghton RA. The annual net flux of carbon to the atmosphere from changes in land use 1850–1990. Tellus B. 1999;51:298–313. [Google Scholar]
- Hunt JH, Kelliher FM, Mc Seveny TM, Ross DJ, Whitehead D. Long-term carbon exchange in a sparse, seasonally dry tussock grassland. Glob. Change Biol. 2004;10:1785–1800. [Google Scholar]
- Intergovernmental Panel on Climate Change: The Physical Science Basis . In: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, Qin D, Manning M, et al., editors. Cambridge Univ. Press; Cambridge, U. K.: 2007. p. 996. [Google Scholar]
- Jaksic V, Kiely G, Albertson J, Oren R, Katul G, Leahy P, Byrne KA. Net ecosystem exchange of grassland in contrasting wet and dry years. Agr. Forest Meteorol. 2006;139:323–334. [Google Scholar]
- Kato T, Tang YH, Gu S, Cui X, Hirota M, Du M, Li Y, Zhao X, Oikawa T. Carbon dioxide exchange between the atmosphere and an alpine meadow ecosystem on the Qinghai-Tibetan Plateau, China. Agr. Forest Meteorol. 2004a;124:121–134. [Google Scholar]
- Kato T, Tang YH, Gu S, Hirota M, Cui XY, Du MY, Li YN, Zhao XQ, Oikawa T. Seasonal patterns of gross primary production and ecosystem respiration in an alpine meadow ecosystem on the Qinghai-Tibetan Plateau. J. Geophys. Res. 2004b;109:D12109. doi:10.1029/2003JD003951. [Google Scholar]
- Kutzbach L, Schneider J, Sachs T, Giebels M, Nykänen H, Shurpali NJ, Martikainen PJ, Alm J, Wilmking M. CO2 flux determination by closed-chamber methods can be seriously biased by inappropriate application of linear regression. Biogeosciences. 2007;4:1005–1025. doi:10.5194/bg-4-1005–2007. [Google Scholar]
- Lavigne MB, Ryan MG, Anderson DE, Baldocchi DD, Crill PM, Fitzjarrald DR, Goulden ML, Gower ST, Massheder JM, Mc Caughey JH, Rayment M, Striel RG. Comparing nocturnal eddy covariance measurements to estimates of ecosystem respiration made by scaling chamber measurements at six coniferous boreal sites. J. Geophys. Res. 1997;102:28977–28985. [Google Scholar]
- Lawton D, Leahy P, Kiely G, Byrne AK, Calanca P. Modeling of net ecosystem exchange and its components for a humid grassland ecosystem. J. Geophys. Res. 2006;111:G04013. doi:10.1029/2006JG000160. [Google Scholar]
- Li SG, Lai CT, Yokohama T, Oikawa T. Carbon dioxide and water vapor exchange over a Miscanthus-type grassland: Effects of development of the canopy. Ecol. Res. 2003;18:661–675. [Google Scholar]
- Li SG, Asanuma J, Eugster W, Kotani A, Liu JJ, Urano T, Oikawa T, Davaa G, Oyunbaatar D, Sugitar M. Net ecosystem carbon dioxide exchange over grazed steppe in central Mongolia. Glob. Change Biol. 2005;11:1941–1955. [Google Scholar]
- Li Y-L, Tenhunen J, Owen K, Schmitt M, Bahn M, Droesler M, Otieno D, Schmidt M, Gruenwald Th., Hussain MZ, Mirzae H, Bernhofer Ch. Patterns in CO2 gas exchange capacity of grassland ecosystems in the Alps. Agr. Forest Meteorol. 2008;148:51–68. [Google Scholar]
- Maljanen M, Martikainen PJ, Silvola J, Walden J. CO2 exchange in an organic field growing barley or grass in eastern Finland. Glob. Change Biol. 2001;7:679–692. [Google Scholar]
- Miranda AC, Miranda HS, Lloyd J, Grace J, Francey RJ, Mc Intyre JA, Meir P, Riggan P, Lockwood R, Brass J. Fluxes of carbon, water and energy over Brazilian cerrado: an analysis using eddy covariance and stable isotopes. Plant Cell Environ. 1997;20:315–328. [Google Scholar]
- Mirzaei H, Kreyling J, Hussain MZ, Li Y, Tenhunen J, Beierkuhnlein C, Jentsch A. A single drought event of 100-year recurrence enhances subsequent carbon uptake and changes carbon allocation in experimental grassland communities. J. Plant Nutr. Soil Sc. 2008;171:681–689. [Google Scholar]
- Monson RK, Turnipseed AA, Sparks JP, Harley PC, Scott-Denton LE, Sparks K, Huxman TE. Carbon sequestration in a high-elevation, subalpine forest. Glob. Change Biol. 2002;8:459–478. [Google Scholar]
- Nieveen JP, Campbell DI, Schipper LA, Blair IJ. Carbon exchange of grazed pasture on a drained peat soil. Glob. Change Biol. 2005;11:607–618. [Google Scholar]
- Novick KA, Stoy PC, Katul GG, Ellsworth DS, Siqueira MBS, Juang J, Oren R. Carbon dioxide and water vapor exchange in a warm temperate grassland. Oecologia. 2004;138:259–274. doi: 10.1007/s00442-003-1388-z. [DOI] [PubMed] [Google Scholar]
- Obrist D, De Lucia EH, Arnone JA., III Consequences of wildfire on ecosystem CO2 and water vapour fluxes in the Great Basin. Glob. Change Biol. 2003;9:563–574. [Google Scholar]
- Otieno DO, Wartinger M, Nishiwaki A, Hussain MZ, Muhr J, Borken W, Lischeid G. Responses of CO2 Exchange and Primary Production of the Environmental Changes in a Mountain Peatland. Ecosystems. 2009;12:590–603. [Google Scholar]
- Priante-Filho N, Vourtilis GL, Hayayhi MMS, Nogueira JS, Campelo-Junior JH, Nunes PC, Sanches L, Couto EG, Hoeger W, Raiter F, Trienweiler JL, Miranda EJ, Priante PC, Fritzen CL, Lacerda M, Pereira LC, Biudes MS, Suli GS, Shiraiwa S, Paulo SR, Silveira M. Comparison of the mass and energy exchange of a pasture and a mature transitional tropical forest of the southern Amazon Basin during a seasonal transition. Glob. Change Biol. 2004;10:863–876. [Google Scholar]
- Reichstein M, Rey A, Freibauer A, Tenhungen J, Valentini R, Banza J, Casals P, Cheng Y, Grünzweig JM, Irvine J, Joffre R, Law BE, Loustau D, Miglietta F, Oechel W, Ourcival JM, Pereira JS, Peressotti A, Ponti F, Qi Y, Rambal S, Rayment M, Romanya J, Rossi F, Tedeschi V, Tirone G, Xu M, Yakir D. Modeling temporal and large-scale spatial variability of soil respiration from soil water availability, temperature and vegetation productivity indices. Global Biogeochem. Cy. 2003;17(4):1104. doi:10.1029/2003GB002035. [Google Scholar]
- Reichstein M, Ciai P, Papale D, Valentini R, Running S, Viovi N, Cramer W, Granier A, Ogée J, Allard V, Aubinet M, Bernhofer C, Buchmann N, Carrara A, Grünwald T, Heimann M, Heinesch B, Knohl A, Kutsch W, Loustau D, Manca G, Matteucci G, Miglietta F, Ourcival JM, Pillegaard K, Pumpanen J, Rambal S, Schaphoff S, Seufert G, Soussana JF, Sanz MJ, Vesala T, Zhao M. Reduction of ecosystem productivity and respiration during the European summer 2003 climate anomaly: a joint flux tower, remote sensing and modelling analysis. Glob. Change Biol. 2006;12:1–18. [Google Scholar]
- Ripley EA, Redmann RE. Seasonal dynamics of carbon dioxide exchange in a mixed grassland ecosystem. Can. J. Botany. 1978;56:1999–2005. [Google Scholar]
- Risch AC, Dougas F. Carbon dioxide fluxes in a spatially and temporally heterogeneous temperate grassland. Oecologia. 2005;147:291–302. doi: 10.1007/s00442-005-0261-7. [DOI] [PubMed] [Google Scholar]
- Robson MT, Lavorela S, Clementa JC, Le Roux X. Neglect of mowing and manuring leads to slower nitrogen cycling in subalpine grasslands. Soil Biol. and Biochem. 2007;39:930–941. [Google Scholar]
- Rogiers N, Eugster W, Furger M, Siegwolf R. Effect of land management on ecosystem carbon fluxes at a subalpine grassland site in the Swiss Alps. Theor. Appl. Climatol. 2004:1–17. [Google Scholar]
- Ruimy A, Jarvis PG, Baldocchi DD, Saugier B. CO2 fluxes over plant canopies and solar radiation: a review. Adv. Ecol. Res. 1995;26:1–68. [Google Scholar]
- Saigusa N, Oikawa T, Liu S. Seasonal variations in the exchange of CO2 and H2O between a grassland and the atmosphere: an experimental study. Agr. Forest Meteorol. 1998;89:131–139. [Google Scholar]
- Santos AJB, Quesada CA, Da Silva GT, Maia JF, Miranda HS, Miranda AC, Lloyd J. High rates of net ecosystem carbon assimilation by Brachiara pasture in the Brazilian Cerrado. Glob. Change Biol. 2004;10(5):877–885. [Google Scholar]
- Schimel DS, Kittel TGF, Running SW, Monson R, Turnipseed A, Anderson D. Carbon sequestration studies in Western US Mountains. EOS, Transactions, American Geophysical Union. 2002;83:445–449. [Google Scholar]
- Soegaard H, Nordstroem C. Carbon dioxide exchange in a high-arctic fen estimated by eddy covariance measurements and modelling. Glob. Change Biol. 1999;5:547–562. [Google Scholar]
- Suyker AE, Verma SB. Year-round observations of the net ecosystem exchange of carbon dioxide in a native tallgrass prairie. Glob. Change Biol. 2001;7:279–289. [Google Scholar]
- Susiluoto S, Rasilo T, Pumpanen J, Berninger F. Effects of Grazing on the Vegetation Structure and Carbon Dioxide Exchange of a Fennoscandian Fell Ecosystem. Arct. Antarct. Alp. Res. 2008;40(2):422–431. [Google Scholar]
- Stoy PC, Katul GG, Siqueira MBS, Juang JY, Novick KA, Mc Carthy HR, Oishi AC, Oren R. Role of vegetation in determining carbon sequestration along ecological succession in the southeastern United States. Glob. Change Biol. 2008;14:1–19. [Google Scholar]
- Tappeiner U, Cernusca A. Microclimate and fluxes of water vapour, sensible heat and carbon dioxide in structurally differing subalpine plant communities in the Central Caucasus. Plant Cell Environ. 1996;19:403–417. [Google Scholar]
- Tappeiner U, Cernusca A. Model simulation of spatial distribution of photosynthesis in structurally differing plant communities in the Central Caucasus. Ecol. Model. 1998;113:201–223. [Google Scholar]
- Tappeiner U, Sapinsky S. Land-use Changes in European Mountain Ecosystems. Ecomont - Concepts and Results. Blackwell; Berlin: 1999. Canopy structure, primary production and litter decomposition; pp. 127–129. [Google Scholar]
- Tappeiner U, Tappeiner G, Hilbert A, Mattanovich E. Evaluation of the Alpine Region. Blackwell; Berlin: 2003. The EU Agricultural Policy and the Environment; p. 274. [Google Scholar]
- Tasser E, Prock S, Mulser J. Land-use changes in European mountain ecosystems. ECOMONT – Concept and Results. Blackwell; Berlin: 1999. Impact of land-use on the vegetation along an Eastern Alpine transect; pp. 235–246. [Google Scholar]
- Veenendaal EM, Kolle O, Leffelaar PA, Schrier-Uijl AP, Van Huissteden J, Van Walsem J, Möller F, Berendse F. CO2 exchange and carbon balance in two grassland sites on eutrophic drained peat soils. Biogeosciences. 2007;4:1027–1040. doi:10.5194/bg-4-1027–2007. [Google Scholar]
- Wilsey BJ, Parent G, Roulet NT, Moore TR, Potvin C. Tropical pasture carbon cycling: relationships between C source/sink strength, above-ground biomass and grazing. Ecol. Lett. 2002;5:367–376. [Google Scholar]
- Wohlfahrt G, Bahn M, Newesely C, Sapinsky S, Tappeiner U, Cernusca A. Canopy structure versus physiology effects on net photosynthesis of mountain grasslands differing in land use. Ecol. Model. 2003;170:407–426. [Google Scholar]
- Wohlfahrt G. Modelling fluxes and scalar concentrations of CO2, H2O and sensible heat within and above a mountain meadow canopy: a comparison of three Lagrangian models and three parameterisation options for the Lagrangian time scale. Bound-Lay. Meteorol. 2004;113:43–80. [Google Scholar]
- Wohlfahrt G, Anfang C, Bahn M, Haslwanter A, Newesely C, Schmitt M, Drösler M, Pfadenhauer J, Cernusca A. Quantifying nighttime ecosystem respiration of a meadow using eddy covariance, chambers and modelling. Agr. Forest Meteorol. 2005a;128:141–162. [Google Scholar]
- Wohlfahrt G, Bahn M, Haslwanter A, Newesely C, Cernusca A. Estimation of daytime ecosystem respiration to determine gross primary production of a mountain meadow. Agr. Forest Meteorol. 2005b;130:13–25. [Google Scholar]
- Wohlfahrt G, Hammerle A, Haslwanter A, Bahn M, Tappeiner U, Cernusca A. Seasonal and inter-annual variability of the net ecosystem CO2 exchange of a temperate mountain grassland: effects of weather and management. J. Geophys. Res. 2008a;113:D08110. doi: 10.1029/2007jd009286. doi:10.1029/2007JD009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfahrt G, Anderson-Dunn M, Bahn M, Balzarolo M, Berninger F, Campbell C, Carrara A, Cescatti A, Christensen T, Dore S, Eugster W, Friborg T, Furger M, Gianelle D, Gimeno C, Hargreaves K, Hari P, Haslwanter A, Johansson T, Marcolla B, Milford C, Nagy Z, Nemitz E, Rogiers N, Sanz MJ, Siegwolf RTW, Susiluoto S, Sutton M, Tuba Z, Ugolini F, Valentini R, Zorer R, Cernusca A. Biotic, abiotic and management controls on the net ecosystem CO2 exchange of European mountain grasslands. Ecosystems. 2008b;11:1338–1351. [Google Scholar]
- Yashiro Y, Shizu Y, Hirota M, Shimono A, Ohtsuka T. The role of shrub (Potentilla fruticosa) on ecosystem CO2 fluxes in an alpine shrub meadow. J. Plant Ecol. 2010;3(2):89–97. [Google Scholar]
- Zamolodchikov DG, Karelin DV, Ivaschenko AI, Oechel WC, Hastings SJ. CO2 flux measurements in Russian Far East tundra using eddy covariance and closed chamber techniques. Tellus B. 2003;55:879–892. [Google Scholar]
- Zeller V, Bahn M, Aichner M, Tappeiner U. Impact of land-use change on nitrogen mineralization in subalpine grasslands in the Southern Alps. Biol. Fert. Soils. 2000;31:441–448. [Google Scholar]
- Zhao L, Li Y, Xu S, Zhou H, Gu S, Yu G, Zhao X. Diurnal, seasonal and annual variation in net ecosystem CO2 exchange of an alpine shrubland on Qinghai-Tibetan plateau. Glob. Change Biol. 2006;12:1940–1953. [Google Scholar]