Abstract
Malaria is a deadly infectious disease in many tropical and subtropical countries. Previous efforts to eradicate malaria have failed, largely due to the emergence of drug-resistant parasites, insecticide-resistant mosquitoes and, in particular, the lack of drugs or vaccines to block parasite transmission. ATP-binding cassette (ABC) transporters are known to play a role in drug transport, metabolism, and resistance in many organisms, including malaria parasites. To investigate whether a Plasmodium falciparum ABC transporter (Pf14_0244 or PfABCG2) modulates parasite susceptibility to chemical compounds or plays a role in drug resistance, we disrupted the gene encoding PfABCG2, screened the recombinant and the wild-type 3D7 parasites against a library containing 2,816 drugs approved for human or animal use, and identified an antihistamine (ketotifen) that became less active against the PfABCG2-disrupted parasite in culture. In addition to some activity against asexual stages and gametocytes, ketotifen was highly potent in blocking oocyst development of P. falciparum and the rodent parasite Plasmodium yoelii in mosquitoes. Tests of structurally related tricyclic compounds identified additional compounds with similar activities in inhibiting transmission. Additionally, ketotifen appeared to have some activity against relapse of Plasmodium cynomolgi infection in rhesus monkeys. Further clinical evaluation of ketotifen and related compounds, including synthetic new derivatives, in blocking malaria transmission may provide new weapons for the current effort of malaria eradication.
INTRODUCTION
Human Plasmodium falciparum malaria is a devastating disease that kills nearly a million individuals each year, mainly due to the lack of an effective vaccine and to widespread drug resistance (1, 2). Deployment of artemisinin (ART) and its derivatives against these parasites has proven effective, and ART combination therapy (ACT) is currently the recommendation for treating malaria in most regions where malaria is endemic (2, 3). Indeed, promising results from ACT, long-lasting insecticide-treated bed nets, and indoor residual spraying—along with unprecedented financial support from governments and philanthropic foundations—have generated enthusiasm for malaria eradication within malaria-affected communities (4). Eradication of malaria, however, requires interruption of parasite transmission, which has become one of the priorities in malaria research.
A major limitation of current antimalarial drugs is their negligible efficacy against the transmissible mature gametocytes and/or mosquito stages. Although the disease symptoms are largely caused by the replicating asexual stages, the mature gametocyte is the stage responsible for transmission from human to mosquito and is naturally resistant to most antimalarial drugs (5). The lack of drugs that can kill gametocytes or mosquito stages hampers malaria elimination efforts, because residual mature gametocytes can persist in a patient's blood for 2 to 4 weeks after cure of malaria symptoms. Currently, primaquine (PQ) is the only drug that can be used to kill gametocytes and hepatic stages in humans, and promising results of mass drug administration using ACT and PQ have been reported recently (6, 7). In one study, an ACT of artemisinin-piperaquine and a low dose of PQ (9 mg for adults, at 10-day intervals for 6 months) have been used to eliminate malaria infections in 17 villages in Cambodia (7). The percentage of children infected with P. falciparum was reduced from 37% to 1.4%, reaching 0% in 8 of 17 villages 3 years after a mass treatment. The results suggested that the combination of ACT with a drug that can block transmission and/or prevent relapse may be a good strategy for eliminating malaria in some regions where malaria is endemic; however, PQ has not been used widely to treat malaria because of its side effects, particularly regarding glucose-6-phosphate dehydrogenase (G6PD) deficiency (8), low cure rate, and drug resistance (5, 9, 10). Although there are many leads in the pipeline of antimalarial drug development, few appear to target the sexual or mosquito stages (11). To identify drugs that can block transmission of malaria parasites, various strategies can be considered. A straightforward method is to screen small-molecule libraries against gametocytes or stages in mosquitoes; however, it is generally difficult to produce large numbers of gametocytes in vitro for high-throughput drug screening, and obtaining mosquito stages for drug screening is still not possible at present. Only a few low- to medium-throughput methods for screening compounds against P. falciparum gametocytes have been developed thus far (12–14).
In our efforts to screen small-molecule libraries for antimalarial drugs and to study genes that are crucial for gametocyte development, we identified a class of compounds that have activities against asexual stages and gametocytes and, particularly, are highly potent in blocking oocyst formation. Our approach began with a hypothesis that many ATP-binding cassettes (ABCs) may play a role in drug transport and metabolism in malaria parasites, and disruption of a gene encoding an ABC transporter may affect the parasite response to antimalarial drugs or chemical compounds. ABC transporters are grouped into several subclasses, and each subclass contains many members (15). Several P. falciparum ABC transporters, including a homolog of the human P-glycoprotein (PfPgh-1, or PfMDR1) (16–19), PfMDR2 (PF14_0455) (20), and a member of the multidrug resistance-associated protein (PFA0590w, or PfMRP) (21, 22), have been shown to play a role in response to many drugs or heavy metals. The genome of P. falciparum carries 11 putative ABC transporters (23), including a putative ABC transporter belonging to subclass G number 2 (PfABCG2, or Pf14_0244) that might play a role in the parasite's response to drugs. PfABCG2 is a homolog of the breast cancer resistance protein (BCRP), ATP-binding cassette placenta (ABCP) protein, and mitoxantrone resistance (MXR) protein (24, 25). Members of this family are mostly expressed on the plasma membrane and have been shown to confer drug resistance in cancer cells and can protect the cells from exogenous and endogenous toxins (25). Here, we disrupted the gene encoding PfABCG2 and characterized the effects of the gene disruption on the parasite response to antimalarial drugs, parasite growth, and gametocytogenesis in culture. We also tested the wild-type (WT) 3D7 and pfabcg2 knockout (KO) lines against 2,816 drugs (26), and we identified a tricyclic antihistamine (ketotifen) that was less active against the pfabcg2 KO lines. Because the gene is expressed at a higher level in gametocytes than in asexual stages (27, 28) and gene disruption greatly affected gametocytogenesis, we also evaluated the activity of ketotifen on gametocyte and oocyst formation in mosquitoes. We showed that, in addition to activities against asexual stages and gametocytes, ketotifen and other tricyclic analogs were highly effective in blocking oocyst development of both Plasmodium yoelii and P. falciparum. The discovery of these drugs may have a significant impact on malaria control and eradication.
MATERIALS AND METHODS
Parasite culture and gametocyte production.
Procedures for in vitro parasite culture have been previously described (29). The parasite clone 3D7 in our hands is sensitive to chloroquine (CQ) and is capable of producing gametocytes. For gametocyte cultures, trophozoites diluted to 0.1 to 0.5% parasitemia were cultured with daily medium changes until mature gametocytes were obtained (14, 30). Cultures were treated with N-acetylglucosamine at a final concentration of 50 mM to eliminate asexual-stage parasites.
Generation of PfABCG2 null parasite lines.
PfABCG2-disrupted lines from 3D7 were generated using a negative selection system as described previously (30, 31). Two DNA fragments from chromosome 14 (bp 1,027,515 to 1,028,251 for the 5′ segment and bp 1,028,909 to 1,029,630 for the 3′ segment) were amplified from 3D7 genomic DNA by using primers Pf14_0244DI Fwd/Rev and Pf14_0244DII Fwd/Rev, respectively, and ligated into the multiple-cloning sites (SpeI/BglII and ClaI/NcoI) flanking the human dhfr expression cassette of the plasmid pHTK (31) (see Fig. S1A and Table S1 in the supplemental material). After parasites were established in culture approximately 4 weeks after addition of 2 nM WR99210, ganciclovir was added to a 4 μM final concentration for 9 days. After growing in drug-free medium for 14 days, the parasites were again grown under 2 nM WR99210. Selected parasites were cloned using limiting dilution in 96-well plates at a density of 0.2 infected red blood cells (RBCs) per well. Integration of the insert into the chromosome and disruption of the PfABCG2 gene were confirmed by using an allele-specific PCR with one primer from the gene encoding human DHFR (hDHFR) and another from the sequence flanking the PfABCG2 coding region (see Fig. S1B). Lack of WT parasites was also confirmed by the lack of PCR amplification of the WT allele (2.3 kb) and the presence of a recombinant allele (3.9 kb) (see Fig. S1C). We also performed Southern blotting to further confirm the disruption of pfabcg2 (see Fig. S1D). Ten micrograms of genomic DNA from 3D7 and the C11 and B2 clones was digested with HincII, electrophoresed, transferred onto a nylon membrane, denatured, and hybridized with a digoxygenin-labeled PCR product amplified using Pf14_0244 promoter Fwd 2 and Pf14_0244 promoter Rev primers (see Fig. S1A and Table S1) following the manufacturer's recommendations (Roche).
To verify that changes in the parasite response to drugs was due to disruption of pfabcg2, we constructed plasmid vectors with pfabcg2 (position bp 1,027,908 to 1,029,890) from the 3D7 parasite fused to green fluorescent protein (GFP) by using the Pf14_0244 Fwd and Rev primers (see Table S1 in the supplemental material). This PCR fragment was inserted into the AvrII/BglII cloning site on pDC2-AttP-BSD 1,600-bp crt promoter-XX-GFP-hsp86 3′ UTR plasmid previously described (32). In addition to the construct driven by the 1,600-bp 5′-untranslated region (UTR) of the CQ resistance transporter (pfcrt; crt 5′), approximately 1,000 bp of the native Pf14_0244 promoter was also amplified (Pf14_0244 promoter Fwd and Rev primers) (see Table S1) and cloned into the ApaI/AvrII site of the expression plasmid. As a transfection control plasmid, the luciferase gene was digested from pHLH1 (33) using ClaI and cloned into the AvrII/BglII site of the pDC2 plasmid after blunting the digestion product ends, with expression driven by the crt 5′ UTR. Parasite C11 was transfected independently in duplicate with the crt 5′-UTR construct, the 1,000-bp native promoter construct, and the luciferase plasmid control construct. Two days after transfection, 4 μM blasticidin S (BSD; Invitrogen) was applied to select for parasites carrying the transfected plasmid. We were unable to establish parasites transfected with the Pf14_0244-GFP fusion construct driven by the native promoter; however, both the crt 5′-UTR-driven construct and luciferase control transfectants were stable under BSD pressure, as verified by PCR using a crt 5′-UTR-specific primer (P5) and either hsp86 3′-UTR (P6) or luciferase antisense primer (P7) (see Table S1 and Fig. S1E). Expression of the Pf14_0244-GFP fusion was confirmed by Western blotting with a rabbit anti-GFP primary antibody (1:2,500; Abcam) and a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000; Millipore).
In vitro drug activity assay.
Parasite responses to drugs were tested using a SYBR green growth inhibition assay as described previously (22, 34). All drugs were dissolved in the diluent dimethyl sulfoxide (DMSO) except for CQ, which was dissolved in water, and stored at −80°C until use. Briefly, parasites were diluted to 0.5% parasitemia at a 2% hematocrit, and diluted parasites (100 μl) were added to wells in duplicate in a 96-well plate containing 100 μl of 2-fold-serially diluted drugs. The parasites were incubated with the drugs at 37°C for 72 h. After incubation, DNA was released and stained with lysis buffer (Tris, 20 mM [pH 7.5]; EDTA, 5 mM; 0.008% [wt/vol] saponin; 0.08% [vol/vol] Triton X-100) containing SYBR green dye. The plate was kept in darkness for 30 min and read in a FLUOstar Optima microplate reader (BMG Labtech). Parasite susceptibility to each drug was tested independently at least three times.
Screening of bioactive compounds.
P. falciparum-infected erythrocytes were screened against a collection of 2,816 compounds approved for human or animal use (26) (see Table S2 in the supplemental material) in a quantitative high-throughput SYBR green viability assay as described previously (19, 35, 36).
Gametocyte killing assay.
P. falciparum cultures were diluted to 0.2% parasitemia and induced for gametocyte production as described previously (30). Drugs were added 8 days after setting up gametocyte cultures at final concentrations of 0.5, 1, 3, and 5 μM and were washed away after a 24-hour incubation. The cultures were maintained with a daily change of medium until day 14, when the gametocytes became mature. The numbers of gametocytes at day 14 were counted by microscopic examination of Giemsa-stained thin blood smears.
Mosquito feeding assays.
For testing transmission-blocking activity in vivo, female CD-1 mice, aged 6 to 8 weeks old, were injected intraperitoneally with 5 × 106 Plasmodium yoelii nigeriensis N67. To compensate for variation in production of gametocytes between animals, three mice per group were used for mosquito feeding. In a preliminary experiment, the animals were administered a single dose of test compounds or pharmaceutical-grade phosphate-buffered saline (PBS; pH 7.4) 3 days postinfection. Starting 1 h after the administration of a compound, the animals were anesthetized and brought into contact with a colony of Anopheles stephensi mosquitoes. The mosquitoes were allowed to feed on the mice for 30 min. Unfed or partially engorged mosquitoes were removed from the cages. The mosquitoes were maintained at 24 ± 1°C and 80 to 90% relative humidity for 7 to 10 days before their midguts were dissected to determine oocyst counts. In subsequent experiments, the activity of ketotifen at different concentrations (0.1, 0.5, 1, 5, 10, 30, or 60 mg/kg of body weight) in inhibiting oocyst formation was evaluated similarly. PQ, CQ, and ART were used as reference drugs. Two doses of 0.1 or 0.5 mg/kg ketotifen each were given at 4-hour intervals, and responses 6 or 24 h after the final drug administration were also tested. All the experimental procedures were conducted in accordance with the NIH-approved animal study protocol LMVR-11E.
For the P. falciparum (NF54) feeding assay, gametocyte cultures were monitored daily beginning on day 14 after initiation of gametocyte culture for the presence of stage V parasites by microscopic examination of Giemsa-stained thin blood smears. Cultures with mature gametocytes were distributed onto 6-well plates (4 ml of culture per well). Ketotifen and/or ART was added to individual wells to a final concentration of 0.5 μM (equivalent to ∼0.1 mg/kg) or higher doses in 0.02% DMSO and incubated for 24 h. The same amount of DMSO or PBS was added to the cultures and served as the controls. For the exflagellation assay, 15 μl of culture with mature gametocytes was placed onto a microscope glass slide and incubated at 19°C for 20 min. Mobile exflagellation centers were counted under a light microscope at 400× magnification. Total numbers of exflagellation centers from 10 microscopic fields with similar RBC numbers were recorded.
Membrane feeding assays were conducted as follows. Gametocyte cultures were centrifuged for 3 min at 530 × g and resuspended in fresh O+ RBC and O+ human serum to give a final blood meal with a 38.5% hematocrit. A blood meal of 260 μl was then deposited on a parafilm membrane feeder prewarmed to 40°C to feed batches of 50 to 60 A. stephensi mosquitoes, aged 4 to 6 days old, for 20 min. Mosquitoes that had taken a blood meal (determined by the presence of eggs) were dissected 7 days after feeding. Midguts were stained with 0.1% mercuro-bromo fluorescein in 1× PBS, and the number of oocysts was counted under a light microscope.
Inhibition of relapse in a Plasmodium cynomolgi/rhesus monkey model.
A donor rhesus monkey was infected with P. cynomolgi strain B by intravenous (i.v.) injection of 1 × 106 frozen infected RBCs thawed from frozen stock. A colony of Anopheles stephensi mosquitoes was allowed to feed on the monkey when parasitemia reached 0.1 to 2%. When mature sporozoites reached the salivary glands at day 10 to 12, the mosquitoes were allowed to feed on 9 malaria-naive monkeys for 30 min, with 35 to 50 mosquitoes per monkey. Approximately 7 to 8 days after feeding, parasites were detected in peripheral blood, and the animals were treated with quinine (32 mg/kg) twice daily by intramuscular (i.m.) injection for 5 consecutive days to clear parasites in circulation. Nine monkeys were divided into three groups the next day after the completion of quinine therapy: one group was treated with a daily oral dose of 15 mg/kg ketotifen for 4 days (or 8 days in repeated experiments); the second group was treated with a daily i.v. dose of 8 mg/kg artesunate for 4 days; the third group received no additional treatment after quinine therapy and served as a control. Parasitemia was monitored daily for 60 days to observe relapse patterns. A second quinine treatment (i.m., 32 mg/kg twice daily for 7 days) was applied to all groups when the first relapse was observed in the control group. At the end of the study, all monkeys were treated with a combination of CQ (oral daily dose of 20 mg/kg for 3 days) and PQ (oral daily dose of 0.5 mg/kg for 14 days), and a final blood smear was taken at day 90 to make sure the animals were clear of parasites.
RESULTS
Disruption of the gene encoding PfABCG2.
To investigate whether PfABCG2 plays a role in the parasite response to antimalarial drugs, we genetically disrupted the gene encoding PfABCG2 in the CQ-sensitive 3D7 line by using a double-crossover construct (see Fig. S1A in the supplemental material). After cloning the parasites by limiting dilution, we obtained five clones with the integrated plasmid, two of which—B2 and C11—were randomly selected for further phenotype characterization. Integration of the plasmid construct into the expected chromosome locus was verified using a PCR that detected the integration event (see Fig. S1B and C in the supplemental material) and Southern blotting (see Fig. S1D). A PCR product was produced only from parasites with the gene encoding the human dihydrofolate reductase (hdhfr) integrated into the specific chromosome locus, because one primer in each reaction was specific for the hdhfr gene (p2 and p3 in Fig. S1A and B of the supplemental material). Integration of the plasmid construct also introduced a diagnostic HincII site, which generated a restriction fragment of reduced size (∼3.5 kb) as detected by Southern blotting (see Fig. S1D).
Phenotypes of pfabcg2 null parasites.
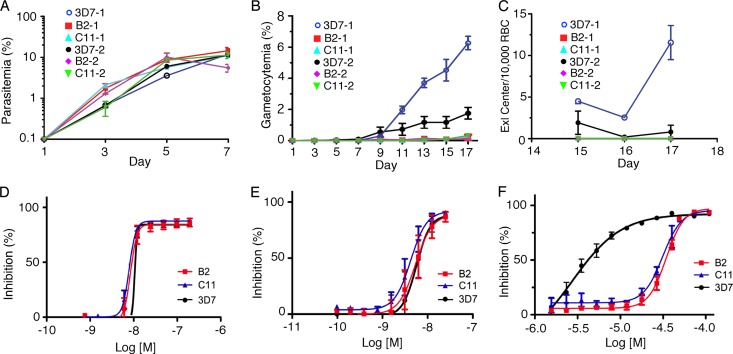
Disruption of the pfabcg2 gene did not affect asexual parasite growth in in vitro culture (Fig. 1A), but the production of gametocytes in the two KO clones was greatly reduced (Fig. 1B). Consequently, mobile exflagellation centers of male gametes were undetectable after triggering the exflagellation process in the gametocyte culture (Fig. 1C). Disruption of pfabcg2 did not change the parasite response to drugs targeting asexual stages, such as CQ and DHA (Fig. 1D and E), suggesting that the gene does not contribute to the response to these two drugs. The results were consistent with previous reports of higher RNA transcript levels of pfabcg2 in gametocytes (27, 28) and suggest that this gene may play a more significant role in nonerythrocytic stages.
Fig 1.
Comparison of growth and drug response phenotypes between Plasmodium falciparum 3D7 and the two pfabcg2 null lines (B2 and C11 were obtained from a single transformation experiment and may be derived from a common progenitor). (A) Parasitemia of asexual stages. (B) Gametocytemia of all gametocyte stages from day 1 (setup day) to day 17. (C) Exflagellation center counts from days 15 to 17. (D) Inhibition curves in response to CQ. (E) Inhibition curves in response to DHA. (F) Inhibition curves in response to ketotifen (KET). The results are consistent with those from the initial high-throughput screening; the ketotifen IC50 for 3D7 was 6.3 μM (log IC50, 5.2), and it was >30 μM for both B2 and C11 (or log IC50 ≥4.5). For the experiments shown in panels A to C, two independent repeats, designated 1 and 2, in duplicates were plotted separately due to a large variation in 3D7 gametocytemia; for panels D to F, the results of four independent experiments were averaged and plotted. Standard errors of means were estimated from the repeats/duplicates accordingly.
Screening of WT and pfabcg2 KO parasites against a library of approved drugs.
To investigate whether disruption of the gene affects the parasite response to other compounds, we screened 2,816 drugs approved for human and animal use (26) against the 3D7 parasites and the two pfabcg2 null parasites in a viability assay as described previously (19). Except for some antifolate compounds, only two drugs—ambenomium and ketotifen—had 50% inhibitory concentrations (IC50s) with at least 5-fold differences between the WT (ambenomium IC50, 3.2 μM; ketotifen IC50, 6.3 μM) and the two pfabcg2 null parasites (amebnomium IC50, 0.50 μM for B2 and 0.63 μM for C11; ketotifen IC50, >30 μM for both B2 and C11) in the initial screening (see Table S2 in the supplemental material). In confirmation assays using 96-well plates, only ketotifen, an H1 antihistamine (37), consistently showed a large differential potency (Fig. 1F), with pfabcg2-disrupted parasites becoming more resistant to ketotifen (unpaired t test on IC50s, P = 0.001 for 3D7 versus C11 or B2). Discrepancy between the IC50s of ambenomium for the two assays might have been due to technical variations of the assays and relatively small differences in IC50s of the drugs between the 3D7 and the KO parasites. Parasite responses to several antifolate compounds were also different (see Table S2), but these differences likely arose from the hdhfr gene in the introduced plasmid, which mediates antifolate resistance as a selection marker.
Partial restoration of the drug response phenotype after episomal expression of PfABCG2.
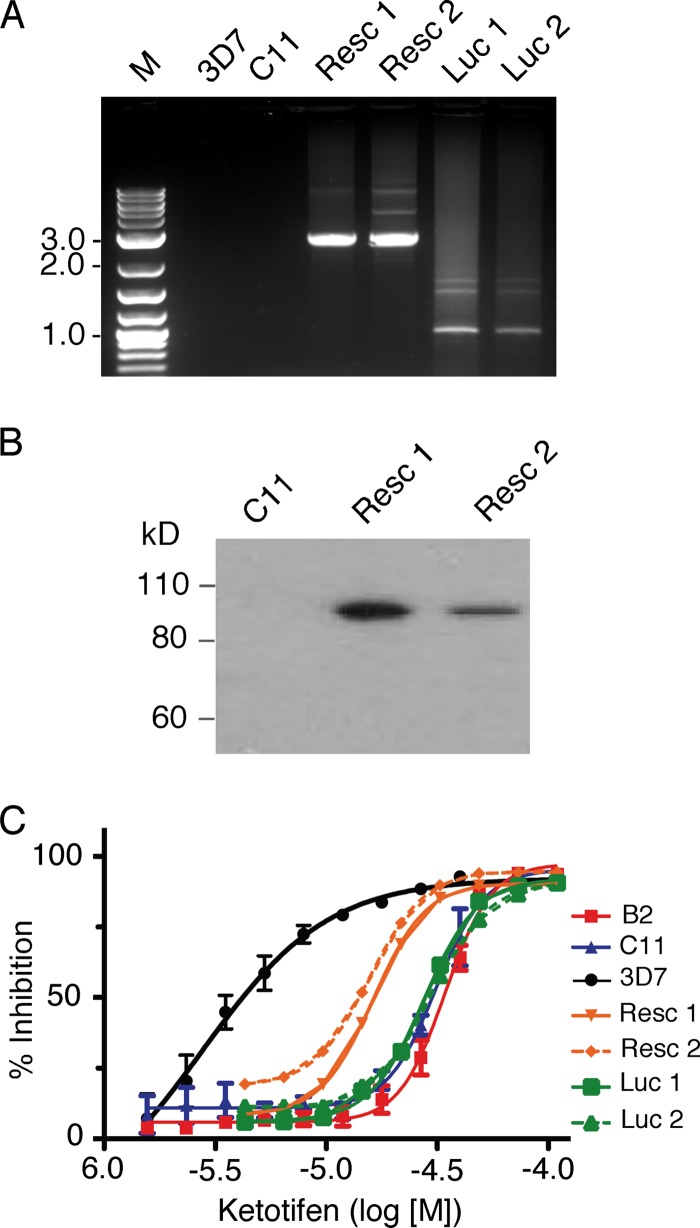
The change in IC50s in the KO parasites suggested that PfABCG2 might play a role in the response to ketotifen. To further verify that the changes in parasite response to ketotifen were caused by disruption of PfABCG2 and not by other unexpected changes in the parasite genome during the transfection process, we introduced a construct containing the pfabcg2 gene fused with the gene encoding GFP or a control plasmid containing the luciferase gene (see Fig. S1E in the supplemental material). Gene expression was controlled by the promoter region of pfcrt, which is expressed in asexual stages (a construct was also made using the native pfabcg2 promoter, but viable transgenic parasites were not recovered after several attempts). The presence of the plasmid (Fig. 2A) and expression of the GFP fusion protein (Fig. 2B) in the transfected parasites—but not in nontransfected 3D7 or C11 paraites—were detected using PCR and Western blotting with antibodies against GFP. Complementation of the disrupted pfabcg2 gene partially restored the sensitivity of the parasite to ketotifen in two independently transfected C11 parasites but not in the parasites transfected with the control luciferase plasmid, confirming that the altered ketotifen susceptibility observed in the pfabcg2 null parasites was at least partially mediated by PfABCG2 (Fig. 2C). The results provide additional evidence that PfABCG2 may play a role in the parasite response to ketotifen, although further characterization is necessary to reveal the molecular mechanism of the parasite response to ketotifen.
Fig 2.
Complementation of the pfabcg2 KO C11 parasite with a pfabcg2 transgene partially restored parasite sensitivity to ketotifen. (A) PCR products with expected sizes amplified from DNA samples extracted from parasites with or without the plasmids to introduce the genes encoding PfABCG2 or luciferase. Resc-1 and Resc-2 were derived from C11 lines transfected with the pfabcg2 expression plasmid shown in Fig. S1E (top) in the supplemental material. Luc-1 and Luc-2 were derived from C11 lines transfected with the control luciferase expression plasmid (see Fig. S1E, bottom). (B) Immunoblot showing expression of the PfABCG2-GFP fusion protein in the transfected parasites, as detected by antibodies against GFP. (C) Changes in parasite response to ketotifen in the presence or absence of the pfabcg2 transgene. The parasites were the same as those shown in panel A. Standard errors of means were estimated from 3 to 4 repeat experiments. The IC50s of Resc-1 and Resc-2 were not significantly different from that of 3D7 (unpaired t tests of IC50s; P = 0.62 for 3D7 versus Resc-1 and P = 0.64 for 3D7 versus Resc-2), the IC50s of controls (Luc-1 and Luc-2) remained significantly different from that of 3D7 or close to significance (unpaired t tests of IC50s; P = 0.03 for 3D7 versus Luc-1 and P = 0.08 for 3D7 versus Luc-2), and the differences between the IC50s of the complemented lines and the parental knockout clone C11 were also significant (unpaired t test, P = 0.04 for C11 versus Resc-1and P = 0.03 for C11 versus Resc-2).
Effects of ketotifen on P. falciparum gametocytes in vitro and oocysts in vivo.
Because PfABCG2 is expressed at a higher level in sexual stages than in asexual stages and disruption of PfABCG2 greatly affected gametocyte development, we investigated the in vitro gametocytocidal activities of ketotifen and cyproheptadine, a closely related analog. Addition of 0.5 to 5 μM PQ (a known gametocytocidal drug used as a positive control), ketotifen, or cyproheptadine to day 8 gametocyte cultures (mostly stage III gametocytes) for 24 h inhibited P. falciparum gametocyte development in vitro, with a significant reduction in gametocytemia at 5 μM, although ketotifen and cyproheptadine were not as effective as PQ in killing P. falciparum gametocytes in vitro (Table 1).
Table 1.
In vitro inhibition of Plasmodium falciparum gametocytes by primaquine, cyproheptadine, or ketotifena
| Drug (expt no.) | Gametocytemia (mean ± SD) at drug concn |
||||
|---|---|---|---|---|---|
| No drug | 0.5 μM | 1 μM | 3 μM | 5 μM | |
| PQ (1) | 2.1 ± 0.3 | 1.3 ± 0.4 | 0.6 ± 0.05** | 0.1 ± 0.1** | 0.01 ± 0.01** |
| PQ (2) | 3.2 ± 0.5 | 1.6 ± 0.6 | 0.7 ± 0.20** | 0.2 ± 0.1** | 0.0 ± 0.0** |
| CYP (1) | 2.4 ± 0.6 | 2.6 ± 0.2 | 1.4 ± 0.20 | 0.6 ± 0.1* | 0.2 ± 0.1* |
| CYP (2) | 2.8 ± 0.3 | 2.3 ± 0.4 | 1.6 ± 0.10* | 0.9 ± 0.1** | 0.3 ± 0.1** |
| KET (1) | 2.4 ± 0.2 | 2.7 ± 0.1 | 2.6 ± 0.40 | 1.7 ± 0.1 | 1.0 ± 0.3** |
| KET (2) | 3.2 ± 0.2 | 2.7 ± 0.1 | 2.3 ± 0.10 | 2.2 ± 0.5* | 1.2 ± 0.3** |
Drugs were added to day-8 gametocytes for 24 h before washing off, and gametocytes were counted at day 14. Each experiment was performed in triplicate (no-drug controls) or duplicate.
, P < 0.05;
, P < 0.01, based on paired t test (compared with the no-drug group). CYP, cyproheptadine. SD, standard deviation.
To further investigate how ketotifen affected fertilization and oocyst development of P. falciparum parasites, we produced mature gametocytes in vitro and treated the gametocyte cultures with ketotifen, ART, or a combination of ketotifen and ART for 24 h before feeding the cultures to Anopheles mosquitoes. Control groups of mosquitoes fed with PBS or DMSO harbored an average of 44.9 and 43.0 oocysts per mosquito, respectively, whereas mosquitoes treated with ketotifen (5 μM) had an average of 22.2 oocysts per mosquito (∼50% reduction). The group treated with ART (5 μM) had an average of 0.6 oocysts per mosquito, and those treated with ART plus ketotifen combination (5 μM each) had no oocysts (Table 2). We repeated the experiments, except that the culture media were replaced with fresh media containing the same dosages of drugs 4 h after the initial treatment. Mosquitoes fed either with the PBS or DMSO harbored an average of 8.2 and 9.2 oocysts per mosquito, respectively, whereas no oocysts were found in the ketotifen, ART, or ART plus ketotifen groups. In the third experiment, we treated gametocyte cultures with higher doses of ketotifen (25 μM and 50 μM) and obtained almost complete inhibition of oocyst development (Table 2). Interestingly, male gamete exflagellation center counts were also greatly reduced in the drug-treated groups. These results clearly show that ketotifen is quite effective in inhibiting P. falciparum oocyst formation at a relatively low dose.
Table 2.
Inhibition of Plasmodium falciparum oocyst development by KET or a combination of KET and ART
| Expt no. and treatmenta | % GV | % adjusted GV | Exflagellation center count/field | No. of oocysts/midgut (SD) | No. of mosquitoes dissected | % of mosquitoes with oocysts |
|---|---|---|---|---|---|---|
| Expt 1 | ||||||
| PBS | 1.9 | 0.3 | 2.7 | 44.9 (21.3) | 23 | 100 |
| DMSO | 2.1 | 0.3 | 2.0 | 43.0 (24.4) | 28 | 96.4 |
| ART | 2.3 | 0.3 | 0.2 | 0.6 (1.2)*** | 29 | 27.6 |
| KET | 2.0 | 0.3 | 0.7 | 22.2 (15.5)*** | 25 | 96 |
| KET + ART | 2.0 | 0.3 | 0 | 0*** | 26 | 0 |
| Expt 2 | ||||||
| PBS | 0.7 | 0.2 | 3.7 | 8.2 (4.2) | 20 | 90.0 |
| DMSO | 0.8 | 0.2 | 3.5 | 9.3 (6.1) | 22 | 95.5 |
| ART | 0.5 | 0.2 | 0.7 | 0*** | 19 | 0 |
| KET | 0.8 | 0.2 | 0.8 | 0*** | 20 | 0 |
| KET + ART | 0.6 | 0.2 | 0 | 0*** | 18 | 0 |
| Expt 3 | ||||||
| DMSO | 0.8 | 0.2 | 3.5 | 7.4 (4.5) | 25 | 92.0 |
| 5 μM KET | 0.8 | 0.2 | 2.0 | 3.5 (4.0)** | 25 | 64.0 |
| 25 μM KET | 0.8 | 0.2 | 0.8 | 0.6 (0.9)*** | 25 | 36.0 |
| 50 μM KET | 0.9 | 0.2 | 0.5 | 0.1 (0.3)*** | 25 | 8.0 |
In vitro-cultured gametocytes were fed to mosquitoes by using a membrane feeder, and mosquitoes were dissected 7 days after feeding. GV (reported as a percentage), stage V gametocytemia. The adjusted GV is the percentage of stage V gametocytemia, adjusted prior to feeding experiments. Exflagellation center counts per field were made after adjustment for gametocytemia. A concentration of 5 μM KET, ART, or the combination of KET and ART each was used in the treatments, except for the KET concentrations indicated for experiment 3. Oocyst counts often varied between feedings, depending on the quality of gametocyte cultures and unknown factors. We considered an average of 4.0 oocysts per mosquito or higher in the DMSO control group as a successful feeding in our assays. Experiment 3 was conducted to test the effects of different amounts of ketotifen on oocyst development.
, P < 0.01;
, P < 0.001 (compared with the DMSO group; unpaired t test).
Effects of ketotifen on P. yoelii oocyst formation after in vivo treatments.
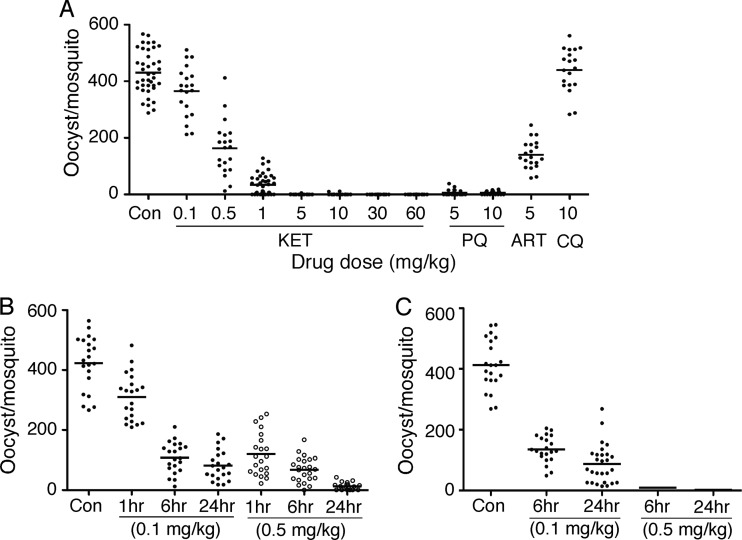
We also infected CD-1 mice with P. yoelii nigeriensis N67 and treated the mice with different amounts of ketotifen before exposing them to mosquitoes to investigate the effects of ketotifen on sexual development in vivo. Remarkably, ketotifen dramatically reduced the number of oocysts from an average of 460 to 34 oocysts (93% reduction) per mosquito after 1-hour treatment of the mice with a single drug dose of 1 mg/kg and almost completely blocked oocyst development with a 5-mg/kg dose (Fig. 3A). Oocyst counts from the 5-mg/kg ketotifen treatment group (averaging 0.13 oocysts per mosquito; 2 of 44 mosquitoes had oocysts) were also lower than those of the 5-mg/kg PQ treatment group (averaging 6 oocysts per mosquito; 7 of 21 mosquitoes had oocysts). A significant reduction in oocyst counts was also obtained after injecting a single dose of 0.5 or 0.1 mg/kg of ketotifen into mice for 6 or 24 h prior to mosquito feeding (averaging 12 and 82 oocysts per mosquito or 97% and 82% reduction, respectively) (Fig. 3B). Two doses of 0.5 mg/kg or 0.1 mg/kg ketotifen administered 4 h apart were similarly tested (Fig. 3C). Whereas the oocyst counts in the two-dose treatment of 0.1 mg/kg with 6- or 24-hour treatment intervals were similar to those of the single-dose treatment, two-dose treatment with 0.5 mg/kg for 6 or 24 h almost completely eliminated oocysts in mosquitoes (averaging 2 oocysts per mosquito; 4 of 23 mosquitoes had oocysts in the 24-hour treatment group). The results showed that ketotifen is highly active in blocking oocyst formation in vivo and, therefore, parasite transmission. ART (5 mg/kg) also had some effect on blocking oocyst formation in vivo, but no inhibition was observed with a CQ dose of 10 mg/kg (Fig. 3A).
Fig 3.
Inhibition of oocyst development by ketotifen (KET), PQ, ART, and CQ. Mice (three per group) infected with Plasmodium yoelii nigeriensis N67 were treated with KET at different concentrations for various time periods and were exposed to mosquitoes. Oocysts were counted from dissected midguts 9 days after feeding. (A) Numbers of oocysts from mosquitoes fed on mice treated with different concentrations of KET for 1 h before feeding were compared with those treated with PQ (5 and 10 mg/kg), ART (5 mg/kg), or CQ (10 mg/kg). (B) Numbers of oocysts from mosquitoes fed on mice treated with a single dose of 0.1 mg/kg (filled dots) or 0.5 mg/kg (open circles) of KET for 1, 6, or 24 h before feeding to mosquitoes. (C) Number of oocysts from mosquitoes fed on mice treated with two doses (4-hour interval) of 0.1 mg/kg (filled dots) or 0.5 mg/kg KET for 6 or 24 h. Con, no-drug control. The short horizontal bars are mean oocyst counts.
Inhibition of oocyst formation by additional TCA drugs.
To determine whether other compounds with structures similar to ketotifen (a tricyclic antihistamine and antidepressant [TCA]) also have activity against mosquito stages, we tested additional TCAs and analogs for activity in blocking oocyst formation in mosquitoes (Table 3). Groups of five mice infected with P. yoelii nigeriensis N67 were injected with the compounds 1 h before they were exposed to mosquitoes, and oocysts in the mosquito midgut were dissected and counted 9 days postfeeding. The results showed that although pizotifen (10 mg/kg), doxipin (30 mg/kg), and MLS000556883-02 (10 mg/kg) had little or no effect on oocyst counts, MLS000708402-02 (10 mg/kg), protryptyline (10 mg/kg), and cyproheptadine (10 mg/kg) reduced oocyst numbers by 91 to 99%. Imipramine (100 mg/kg), chlorpromazine (30 mg/kg), trimipramine (10 mg/kg), and MLS000556884-02 (10 mg/kg) also showed some activity in reducing oocyst counts (35 to 69% reduction). These results suggest that there are common targets or transport mechanisms in the parasite for the tricyclic compounds, possibly including PfABCG2. Testing of additional existing or newly synthesized TCA derivatives may lead to discovery of additional drugs that are potent in blocking parasite transmission.
Table 3.
Tricyclic compounds that inhibit Plasmodium yoelii nigeriensis N67 oocyst formation in the mosquito midgut
| Compounda | Action | Dose (mg/kg) | No. of mosquitoes dissected | Mean (SD) no. of oocysts | % reduction | % mosquitoes with oocysts |
|---|---|---|---|---|---|---|
| No-drug control 1 | 14 | 457.5 (55.6) | 100 | |||
| No-drug control 2b | 22 | 101.2 (65.3) | 90.9 | |||
| Primaquineb | Antimalarial | 10 | 30 | 0*** | 100 | 0 |
| Ketotifen | Tricyclic antihistamine | 10 | 18 | 0*** | 100 | 0 |
| MLS000708402-02b | Tricyclic compound | 10 | 24 | 1.1 ( 2.6)*** | 98.9 | 33.3 |
| MLS000708402-02b | Tricyclic compound | 10 | 17 | 2.2 ( 4.2)*** | 99.5 | 23.5 |
| Cyproheptadineb | Psychiatric | 10 | 27 | 9.6 (33.7)*** | 90.5 | 55.6 |
| Protryptyline | Antidepressant | 10 | 16 | 20.9 (25.5)*** | 95.4 | 93.8 |
| Desloratadineb | Tricyclic antihistamine | 10 | 18 | 18.8 (33.7)*** | 81.4 | 70.6 |
| Chlorpromazine | Antipsychotic | 30 | 14 | 143.0 (48.7)*** | 68.7 | 100 |
| Imipramine | Antidepressant | 100 | 15 | 201.2 (86.5)*** | 56 | 100 |
| MLS000556884-02b | Tricyclic compound | 10 | 22 | 48.2 (41.6)** | 52.4 | 100 |
| Trimipramineb | Tricyclic antidepressant | 10 | 29 | 65.5 (46.0)* | 35.3 | 89.7 |
| Doxipin | Antidepressant | 30 | 13 | 420.6 (73.5) | 8.1 | 100 |
| Pizotifenb | Serotonin antagonist | 10 | 21 | 109.0 (65.8) | −1 | 95.2 |
| MLS000556883-02b | Tricyclic compound | 10 | 26 | 116.8 (78.4) | −1.15 | 96.2 |
Mice infected with the parasites were injected with the compounds 1 h before allowing mosquitoes to feed, and the mosquitoes were dissected 8 days after feeding. *, P < 0.5; **, P < 0.01; ***, P < 0.001 (unpaired t test).
Data from second feeding; note that the oocyst count of the control was different from that for the first feeding.
Oral administration of ketotifen and cyproheptadine.
Oral administration of antimalarial drugs is often the preferred method of dosing patients. To determine whether oral administration of the drugs was also effective in blocking oocyst formation, we fed mosquitoes on P. yoelii nigeriensis N67-infected mice 6 h after oral administration of ketotifen, cyproheptadine, PQ, or combinations of ketotifen and ART (Table 4). A single dose of 10 mg/kg cyproheptadine or two doses of a combination of 5 mg/kg ART and 0.5 mg/kg ketotifen completely blocked oocyst formation, while control mosquitoes (no drug) had an average of ∼200 oocysts per mosquito. Two doses of 0.5 mg/kg ketotifen given 4 h apart also greatly reduced the oocyst count (99.9% reduction).
Table 4.
Plasmodium yoelii nigeriensis N67 oocyst counts from mosquitoes fed on mice treated with one or two oral doses of cyproheptadine, primaquine, ketotifen, or artemisinin plus ketotifen
| Compound | Dose (mg/kg) | No. of mosquitoes dissected | Mean (SD) no. of oocysts | % reduction | % mosquitoes with oocysts |
|---|---|---|---|---|---|
| DMSO | 0.0 | 26 | 201.0 (121.3) | 96.2 | |
| CYP | 10 | 35 | 0 (0)*** | 100 | 0 |
| PQ | 10 | 36 | 0.2 (0.5)*** | 99.9 | 16.7 |
| KET | 0.5 | 27 | 40.7 (39.7)*** | 79.8 | 96.3 |
| KET | 0.5 × 2 | 30 | 0.2 (0.6)*** | 99.9 | 13.3 |
| ART + KET | 5 + 0.5 | 31 | 13.4 (16.4)*** | 93.3 | 90 |
| ART + KET | (5 + 0.5) × 2 | 32 | 0.0 (0.0)*** | 100.0 | 0 |
CYP, cyproheptadine.
, P < 0.001 (unpaired t test).
Inhibition of the first relapse of Plasmodium cynomolgi by ketotifen.
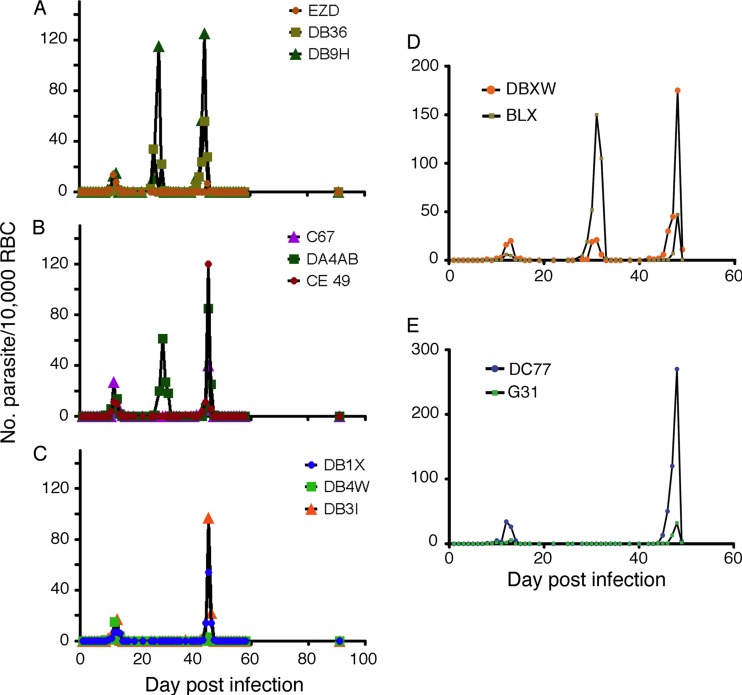
To evaluate whether ketotifen has any activity against liver stages or can prevent malaria relapse, we infected nine rhesus monkeys with P. cynomolgi through mosquito bites and cleared the initial asexual parasites from the circulation with twice-daily quinine doses (32 mg/kg each) for 5 days. Three monkeys were then treated with an additional oral dose of 15 mg/kg ketotifen per day for 4 days after quinine treatment. Two control groups of three monkeys were treated with either quinine alone or with artesunate (8 mg/kg) for 4 days after quinine treatment. Two of the control monkeys (quinine alone) had a first relapse with parasitemia peak at day 26 or 28 and a second peak at day 44 (Fig. 4A). In the group treated with artesunate, in addition to the peak at day 45, one monkey also had a first relapse peak on day 29 (Fig. 4B). Two of the three monkeys treated with ketotifen relapsed only at day 45 (the third did not have a relapse) (Fig. 4C). To confirm these observations, we repeated the experiments in four more monkeys and using similar treatment regimens (two received ketotifen treatment and two had no additional treatment), except we extended the ketotifen treatment to 8 days, considering that a 14-day PQ treatment is generally used for treating relapse. Again, a relapse parasitemia peak at day 29/30 was observed in the control monkeys (Fig. 4D) but not in the two monkeys treated with ketotifen (Fig. 4E). The results suggest that ketotifen had some effect on preventing the first relapse, perhaps by killing “preactive” or “active” forms (trophozoites or schizonts) in the liver.
Fig 4.
Relapse patterns in Plasmodium cynomolgi-infected rhesus monkeys after 5 days of quinine treatment to clear the initial parasitemia. (A) Control monkeys, treated with quinine only (32 mg/kg twice daily for 5 consecutive days); (B) monkeys treated with 8 mg/kg artesunate for 4 days after quinine treatment; (C) monkeys treated with 15 mg/kg ketotifen for 4 days after quinine treatment; (D) no-drug controls, treated as for panel A (repeat); (E) monkeys treated as for panel C, except ketotifen treatment was for 8 days. EZD, DB36, DB9H, C67, DA4AB, CE49, DB1X, DB4W, DB31, DBXW, BLX, DC77, and G31 are designations for individual monkeys.
DISCUSSION
This study identified and evaluated the malaria transmission-blocking activities of several tricyclic compounds after screening a drug library against parasites with a disrupted gene encoding a putative ABC transporter. Although our initial goal was to investigate whether PfABCG2 played a role in drug transport and resistance in gametocytes of P. falciparum parasites, identification of this group of transmission-blocking drugs may have a significant impact on malaria control and eradication if results from clinical field trials confirm our laboratory observations. The sexual stages inhibited by ketotifen do not contribute to the increase of parasite numbers in the human host; with combinations including drugs against asexual stages, the risk of selecting parasites resistant to ketotifen can be minimized. Although we have good evidence showing that PfABCG2 plays a role in the parasite response to ketotifen, identification of the drug target(s) and the mechanism of action in blocking oocyst development in mosquitoes require additional investigation.
In addition to ketotifen, our study also identified several other promising tricyclic compounds, including cyproheptadine, protryptyline, and MLS000708402-02, which were highly active in blocking P. yoelii nigeriensis N67 oocyst formation in the mosquito midgut. MLS000708402-02 has not been tested in humans but may represent a new lead for a potential therapeutic agent. Ketotifen, cyproheptadine, and protryptyline, on the other hand, all have been approved for use in humans. Ketotifen and cyproheptadine are antihistamines that act as a 5-HT2 receptor antagonist in humans (38, 39), and protryptyline is an antidepressant. Ketotifen is used to relieve irritation associated with seasonal allergies and to prevent asthma attacks (38). It can be administered orally or topically with generally mild side effects. Clinical results have shown no serious signs or symptoms after ingestion of up to 20 mg of ketotifen fumarate (38). Cyproheptadine is also used to relieve mild allergy symptoms as well as to treat posttraumatic stress disorder (39) and shows few side effects, although the dosage is limited to 12 mg per day for children and 32 mg per day for adults. The efficacy of these related tricyclic compounds may provide structural activity data for designing additional and better transmission-blocking drugs.
Because ketotifen has been approved for human use, its pharmacokinetics (PK) and pharmacodynamics (PD) have been extensively studied. Ketotifen has been extensively tested in animals for safety and toxicity (38, 40). Large doses have been tested in rats and rabbits with minimum side effects, including during pregnancy. Ketotifen is well absorbed after oral administration, with peak plasma drug concentrations within 2 to 4 h. Peak plasma drug concentrations after multiple oral doses of 1 mg twice daily were 1.92 mg/liter in adults and 3.25 mg/liter in children (41). The drug is 75% protein bound and is metabolized in the liver to the inactive form, ketotifen-N-glucoronide, and active norketotifen. The terminal elimination half-life is 2 to 27 h, with a mean half-life of 12 h. Approximately 60 to 70% of a dose of ketotifen is eliminated in the urine within 48 h, with the remainder excreted in the feces; ∼50% is recovered as ketotifen-N-glucuronide and ∼10% as norketotifen (38). In a recent study evaluating antimalarial activity, both ketotifen and its metabolite norketotifen were shown to be active against schizonts and liver-stage Plasmodium berghei parasites, and the plasma drug concentration was maintained above the IC50 for approximately 8 h for ketotifen and over 24 h for norketotifen (38, 40). The form of ketotifen metabolite(s) that is active against the sexual stages of the malaria parasite is still unknown.
Ketotifen, cyproheptadine, and other antihistamines have been shown to be effective in reversing CQ resistance in P. falciparum (42–47) and in P. yoelii nigeriensis (48). These compounds have also been tested for reducing gametocytemia, although no obvious effect on patient gametocytemia was observed (49, 50). It was not clear, however, whether the gametocytes in some of the previous studies were still capable of infecting mosquitoes or if the oral dose used (0.0125 to 0.25 mg/kg) was sufficient to eliminate gametocytes from the blood circulation. Our observations of significant reductions in gametocyte counts against in vitro-cultured P. falciparum gametocytes and more than 50% reductions in oocyst counts in mosquitoes after treatment with 5 μM ketotifen suggested that doses as low as 0.1 mg/kg body weight should have some effect on gametocyte and/or oocyst formation in vivo. Indeed, treatment of P. yoelii parasites with a single dose of 0.1 mg/kg reduced oocyst count from 27% (1 h) to 81% (24 h) (Fig. 3). Ketotifen showed slightly higher potency in blocking oocyst formation than PQ at 5 mg/kg and 10 mg/kg in mice (Fig. 3), which suggests that ketotifen may act more effectively on mosquito stages or the process of fertilization. In addition, ketotifen appeared to affect gamete production; treatment of P. falciparum gametocytes with ketotifen greatly reduced the numbers of exflagellation centers (Table 2). Because the 3D7 line is sensitive to CQ, it is difficult to test whether disruption of pfabcg2 will affect the ability of ketotifen and other antihistamines in reversing CQ resistance. Future studies using a CQ-resistant parasite with disrupted pfabcg2 may provide insights on how these compounds reverse CQ resistance.
Interestingly, ART was more active than ketotifen in inhibiting oocyst formation after in vitro treatment of P. falciparum gametocytes, whereas ketotifen appeared to be more potent against P. yoelii in vivo (Fig. 3; Table 2). The discrepancies could have been due to species differences in responses to the drugs or, more likely, to a higher rate of ART removal from the body (half-life, ∼1 h) and the loss of activity against gametocytes shortly after administration to the animal. Thus, the ART exposure time was likely longer in vitro, enhancing the effectiveness of ART. In one study, artesunate was found to reduce gametocyte infectivity of patient blood dramatically, but it could not abolish the infectivity completely (51). More recently, ART and derivatives were shown to have some activities against exflagellation and to reduce the number of oocysts (52). These observations suggest that even if ART has some activity against gametocytes, addition of another drug that can kill gametocytes and/or mosquito stages to ACT may be necessary to completely block malaria transmission. Our preliminary results showed that a combination of low-dose ketotifen and ART was more effective in blocking oocyst formation in mosquitoes than either drug alone (Tables 2 and 3).
Ketotifen appeared to have some effect on relapse of P. cynomolgi in rhesus monkeys, although the results were not conclusive. The first relapse peak seen in the control monkeys did not appear in the monkeys treated with ketotifen, but the second peak at day 45 appeared in all the groups, which was duplicated in a second independent experiment. There are several potential explanations for the disappearance of the first relapse peak in the ketotifen-treated group. First, the first relapse peak in the control group could have come from residual parasites that were not totally cleared by quinine treatment, which was unlikely, and additional treatment with ketotifen killed those residual parasites in the blood because ketotifen also has some activity against asexual stages. Second, assuming all the blood forms were killed by quinine treatment, ketotifen might be active against more mature or “active” stages (late trophozoites and schizonts) but not the stage still dormant in the liver (53), which might prevent the appearance of the first relapse peak but not the second peak. It would be interesting to investigate whether a second ketotifen treatment at day 35 or a longer ketotifen treatment period prevented the second relapse peak at day 45. The standard regimen of PQ for treating Plasmodium vivax relapse in humans is a daily dose of 15 mg/day for 2 weeks. In a study of mass treatment to eliminate malaria in Cambodian villages, a dose of 9 mg per adult was given at 10-day intervals for up to 6 months (7). Ketotifen may prevent further relapses if a similar treatment regimen is given. Indeed, ketotifen and its metabolite, norketotifen, have recently been shown to be active against the liver stage of P. berghei; no evidence of live parasites was detected after treatment with both drugs at 160 mg/kg/day for 3 days (40).
The mechanism of how ketotifen, cyproheptadine, and other TCA drugs block P. falciparum oocyst formation in mosquitoes requires further investigation. Many ABC transporters, including ABCG2, have been shown to play a role in drug transport as well as other transport functions (54, 55). Genetic modification of genes encoding a drug target or a drug transporter has widely been employed to demonstrate the involvement of a gene in modulating drug susceptibility of malaria parasites (18, 56–58). In addition to showing that disruption of pfabcg2 changed the parasite response to ketotifen in asexual stages, we also showed that reintroduction of pfabcg2 could partially reverse this differential effect. The partial restoration of sensitivity could be due to differences in gene expression level and/or protein localization of the endogenous gene and reintroduced episomal copies. Indeed, the green fluorescent protein signal was present throughout the transfected parasites (data not shown), instead of the expected membrane localization. In addition, the rescue construct also encoded a C-terminal GFP tag fused to the protein, which may have perturbed its transporter function. The same reasons could also be used to explain our failure to restore gametocyte production in the pfabcg2-disrupted parasite C11 (four attempts [data not shown]), although we could not rule out the possibility that the loss of gametocyte production was due to a changes in another gene(s) in the genome during parasite transformation and selection. Interaction of ketotifen with an ABC transporter has been reported; ketotifen was found to restore the sensitivity of P-glycoprotein-overexpressing multidrug-resistant MCF-7/adr cells to doxorubicin, mitoxantrone, VP-16, and vinblastine (59). In theory, resistance to this drug may develop due to mutations in pfabcg2; however, since the gene appears to play almost no role in asexual stages, the pressure for selecting resistant mutations will be minimal. Gametocytes, gametes, and ookinetes do not replicate (except that the male gametocyte that produces eight gametes), and the chance of selecting drug-resistant parasites from sexual stages, the intended target of the drug, should be greatly reduced. We cannot rule out the possibility of a related cellular process that is rendered essential by the drug treatment, as seen in screening yeast either (60). Although the normal asexual stage of growth of the parasites with a disrupted pfabcg2 gene suggests that the gene is not essential during blood-stage development, the change in the IC50 to ketotifen does not prove that PfABCG2 transports the drug, and more studies are necessary to answer this question. Overexpression of an ABC transporter generally makes a cell more resistant to a drug; however, the opposite has also been reported (61). ABCG2 has also been shown to contribute to cell survival in an oxygen-poor environment by reducing the accumulation of toxic heme metabolites (55). A large amount of heme is produced during gametocyte maturation, and a potential biologic function of PfABCG2 could be in extruding heme that accumulates during gametocyte development. Although more studies are necessary to elucidate the functional roles of PfABCG2 in parasite development and drug responses, the identification and testing of this group of urgently needed drugs represent significant contributions in malaria research and disease control.
Various high-throughput methods have been developed for screening antimalarial drugs against asexual stages (19, 36, 62–66) and liver stages (67, 68). More recently, assays for screening gametocytocidal drugs have been reported in response to the urgent need for drugs to block malaria transmission; however, the throughput of these assays was still relatively low (12–14). High-throughput drug screening requires a method to obtain large numbers of parasites, which is still the limiting step for assays using gametocytes or mosquito stages as screening targets. Methods to increase the yield of gametocyte production are necessary to improve the throughput level of drug assays for testing a large number of compounds against this important sexual stage. Our approach of screening parasites with a disrupted gene that was expressed at higher levels in gametocytes or mosquito stages than in asexual stages may represent a novel, productive approach to search for drugs that can preferentially inhibit nonerythrocytic stages of malaria parasites.
Supplementary Material
ACKNOWLEDGMENTS
We thank the NIAID intramural editor Brenda Rae Marshall for assistance and Paul Shinn for compound management.
This work was supported by the Divisions of Intramural Research at the National Institute of Allergy and Infectious Diseases and the National Human Genome Research Institute and by the Director's Challenge Award Program, all at the National Institutes of Health. The PATH/Malaria Vaccine Initiative supported the P. falciparum mosquito membrane feeding assays.
R.T.E., H.J., and D.R. participated in parasite culture, gene KO, drug assay and screen, data analysis, and writing of the manuscript. S.P. performed drug assays in mosquitoes, data analysis, and writing. S.D., Y.W., and L.L. performed tests of relapse in monkeys, data analysis, and writing. T.Q.T. and K.W. participated in gametocyte culture and the exflagellation assay; B.D., K.M., and C.L, performed in vitro mosquito feeding; J.Y., R.L.J., R.H., and C.P.A. performed high-throughput screening and data analysis; R.L.J. also participated in writing. X.-z.S. supervised the research, data analysis, and writing.
We declare we have no competing interests.
Footnotes
Published ahead of print 5 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00920-12.
REFERENCES
- 1. Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alonso PL, Collins FH, Duffy PE. 2008. Malaria: progress, perils, and prospects for eradication. J. Clin. Invest. 118:1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO 2010. World malaria report. WHO, Geneva, Switzerland: http://www.who.int/malaria/world_malaria_report_2010/en/index.html [Google Scholar]
- 3. White NJ. 2008. Qinghaosu (artemisinin): the price of success. Science 320:330–334 [DOI] [PubMed] [Google Scholar]
- 4. Kilama W, Ntoumi F. 2009. Malaria: a research agenda for the eradication era. Lancet 374:1480–1482 [DOI] [PubMed] [Google Scholar]
- 5. White NJ. 2008. The role of anti-malarial drugs in eliminating malaria. Malar. J. 7(Suppl. 1):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shekalaghe SA, Drakeley C, van den Bosch S, Ter Braak R, van den Bijllaardt W, Mwanziva C, Semvua S, Masokoto A, Mosha F, Teelen K, Hermsen R, Okell L, Gosling R, Sauerwein R, Bousema T. 2011. A cluster-randomized trial of mass drug administration with a gametocytocidal drug combination to interrupt malaria transmission in a low endemic area in Tanzania. Malar. J. 10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song J, Socheat D, Tan B, Dara P, Deng C, Sokunthea S, Seila S, Ou F, Jian H, Li G. 2010. Rapid and effective malaria control in Cambodia through mass administration of artemisinin-piperaquine. Malar. J. 9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baird JK, Surjadjaja C. 2011. Consideration of ethics in primaquine therapy against malaria transmission. Trends Parasitol. 27:11–16 [DOI] [PubMed] [Google Scholar]
- 9. Cohen RJ, Sachs JR, Wicker DJ, Conrad ME. 1968. Methemoglobinemia provoked by malarial chemoprophylaxis in Vietnam. N. Engl. J. Med. 279:1127–1131 [DOI] [PubMed] [Google Scholar]
- 10. Wells TN, Burrows JN, Baird JK. 2010. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 26:145–151 [DOI] [PubMed] [Google Scholar]
- 11. Olliaro P, Wells TN. 2009. The global portfolio of new antimalarial medicines under development. Clin. Pharmacol. Ther. 85:584–595 [DOI] [PubMed] [Google Scholar]
- 12. Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, Richman A, Sim BK, Lee MC, Hoffman SL, Fidock DA. 2011. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc. Natl. Acad. Sci. U. S. A. 108:E1214–E1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buchholz K, Burke TA, Williamson KC, Wiegand RC, Wirth DF, Marti M. 2011. A high-throughput screen targeting malaria transmission stages opens new avenues for drug development. J. Infect. Dis. 203:1445–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanaka TQ, Williamson KC. 2011. A malaria gametocytocidal assay using oxidoreduction indicator, alamarBlue. Mol. Biochem. Parasitol. 177:160–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dean M, Annilo T. 2005. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet. 6:123–142 [DOI] [PubMed] [Google Scholar]
- 16. Foote SJ, Kyle DE, Martin RK, Oduola AM, Forsyth K, Kemp DJ, Cowman AF. 1990. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature 345:255–258 [DOI] [PubMed] [Google Scholar]
- 17. Hayton K, Su X-Z. 2004. Genetic and biochemical aspects of drug resistance in malaria parasites. Curr. Drug Targets Infect. Disord. 4:1–10 [DOI] [PubMed] [Google Scholar]
- 18. Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906–909 [DOI] [PubMed] [Google Scholar]
- 19. Yuan J, Johnson RL, Huang R, Wichterman J, Jiang H, Hayton K, Fidock DA, Wellems TE, Inglese J, Austin CP, Su XZ. 2009. Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum. Nat. Chem. Biol. 5:765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenberg E, Litus I, Schwarzfuchs N, Sinay R, Schlesinger P, Golenser J, Baumeister S, Lingelbach K, Pollack Y. 2006. pfmdr2 confers heavy metal resistance to Plasmodium falciparum. J. Biol. Chem. 281:27039–27045 [DOI] [PubMed] [Google Scholar]
- 21. Mu J, Ferdig MT, Feng X, Joy DA, Duan J, Furuya T, Subramanian G, Aravind L, Cooper RA, Wootton JC, Xiong M, Su X-Z. 2003. Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol. Microbiol. 49:977–989 [DOI] [PubMed] [Google Scholar]
- 22. Raj DK, Mu J, Jiang H, Kabat J, Singh S, Sullivan M, Fay MP, McCutchan TF, Su X-Z. 2009. Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J. Biol. Chem. 284:7687–7696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin RE, Henry RI, Abbey JL, Clements JD, Kirk K. 2005. The ‘permeome’ of the malaria parasite: an overview of the membrane transport proteins of Plasmodium falciparum. Genome Biol. 6:R26 doi:10.1186/gb-2005-6-3-r26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doyle LA, Ross DD. 2003. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 22:7340–7358 [DOI] [PubMed] [Google Scholar]
- 25. Krishnamurthy P, Schuetz JD. 2006. Role of ABCG2/BCRP in biology and medicine. Annu. Rev. Pharmacol. Toxicol. 46:381–410 [DOI] [PubMed] [Google Scholar]
- 26. Huang R, Southall N, Wang Y, Yasgar A, Shinn P, Jadhav A, Nguyen DT, Austin CP. 2011. The NCGC Pharmaceutical Collection: a comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci. Transl. Med. 3:80ps16 doi:10.1126/scitranslmed.3001862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA. 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301:1503–1508 [DOI] [PubMed] [Google Scholar]
- 28. Young JA, Fivelman QL, Blair PL, de la Vega P, Le Roch KG, Zhou Y, Carucci DJ, Baker DA, Winzeler EA. 2005. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol. Biochem. Parasitol. 143:67–79 [DOI] [PubMed] [Google Scholar]
- 29. Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675 [DOI] [PubMed] [Google Scholar]
- 30. Furuya T, Mu J, Hayton K, Liu A, Duan J, Nkrumah L, Joy DA, Fidock DA, Fujioka H, Vaidya AB, Wellems TE, Su X-Z. 2005. Disruption of a Plasmodium falciparum gene linked to male sexual development causes early arrest in gametocytogenesis. Proc. Natl. Acad. Sci. U. S. A. 102:16813–16818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duraisingh MT, Triglia T, Cowman AF. 2002. Negative selection of Plasmodium falciparum reveals targeted gene deletion by double crossover recombination. Int. J. Parasitol. 32:81–89 [DOI] [PubMed] [Google Scholar]
- 32. Eastman RT, Dharia NV, Winzeler EA, Fidock DA. 2011. Piperaquine resistance is associated with a copy number variation on chromosome 5 in drug-pressured Plasmodium falciparum parasites. Antimicrob. Agents Chemother. 55:3908–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu Y, Sifri CD, Lei HH, Su XZ, Wellems TE. 1995. Transfection of Plasmodium falciparum within human red blood cells. Proc. Natl. Acad. Sci. U. S. A. 92:973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 48:1803–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. 2006. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc. Natl. Acad. Sci. U. S. A. 103:11473–11478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yuan J, Cheng KC, Johnson RL, Huang R, Pattaradilokrat S, Liu A, Guha R, Fidock DA, Inglese J, Wellems TE, Austin CP, Su XZ. 2011. Chemical genomic profiling for antimalarial therapies, response signatures, and molecular targets. Science 333:724–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Slater JW, Zechnich AD, Haxby DG. 1999. Second-generation antihistamines: a comparative review. Drugs 57:31–47 [DOI] [PubMed] [Google Scholar]
- 38. Grant SM, Goa KL, Fitton A, Sorkin EM. 1990. Ketotifen: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs 40:412–448 [DOI] [PubMed] [Google Scholar]
- 39. Gupta S, Popli A, Bathurst E, Hennig L, Droney T, Keller P. 1998. Efficacy of cyproheptadine for nightmares associated with post-traumatic stress disorder. Compr. Psychiatry 39:160–164 [DOI] [PubMed] [Google Scholar]
- 40. Milner E, Sousa J, Pybus B, Auschwitz J, Caridha D, Gardner S, Grauer K, Harris E, Hickman M, Kozar MP, Lee P, Leed S, Li Q, Melendez V, Moon J, Ngundam F, O'Neil M, Parriott S, Potter B, Sciotti R, Tangteung A, Dow GS. 5 February 2012. Ketotifen is an antimalarial prodrug of norketotifen with blood schizonticidal and liver-stage efficacy. Eur. J. Drug Metab. Pharmacokinet. [Epub ahead of print.] doi:10/1007/s13318-0080-2 [DOI] [PubMed] [Google Scholar]
- 41. Grahnen A, Lonnebo A, Beck O, Eckernas SA, Dahlstrom B, Lindstrom B. 1992. Pharmacokinetics of ketotifen after oral administration to healthy male subjects. Biopharm. Drug Dispos. 13:255–262 [DOI] [PubMed] [Google Scholar]
- 42. Basco LK, Ringwald P, Le Bras J. 1991. Chloroquine-potentiating action of antihistaminics in Plasmodium falciparum in vitro. Ann. Trop. Med. Parasitol. 85:223–228 [DOI] [PubMed] [Google Scholar]
- 43. Bitonti AJ, McCann PP. 1989. Desipramine and cyproheptadine for reversal of chloroquine resistance in Plasmodium falciparum. Lancet ii:1282–1283 [DOI] [PubMed] [Google Scholar]
- 44. Henry M, Alibert S, Baragatti M, Mosnier J, Baret E, Amalvict R, Legrand E, Fusai T, Barbe J, Rogier C, Pages JM, Pradines B. 2008. Dihydroethanoanthracene derivatives reverse in vitro quinoline resistance in Plasmodium falciparum malaria. Med. Chem. 4:426–437 [DOI] [PubMed] [Google Scholar]
- 45. Kyle DE, Milhous WK, Rossan RN. 1993. Reversal of Plasmodium falciparum resistance to chloroquine in Panamanian Aotus monkeys. Am. J. Trop. Med. Hyg. 48:126–133 [DOI] [PubMed] [Google Scholar]
- 46. Peters W, Ekong R, Robinson BL, Warhurst DC, Pan XQ. 1989. Antihistaminic drugs that reverse chloroquine resistance in Plasmodium falciparum. Lancet ii:334–335 [DOI] [PubMed] [Google Scholar]
- 47. Quan H, Tang LH. 2008. In vitro potentiation of chloroquine activity in Plasmodium falciparum by ketotifen and cyproheptadine. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 26:338–342 (In Chinese.) [PubMed] [Google Scholar]
- 48. Singh N, Puri SK. 2000. Interaction between chloroquine and diverse pharmacological agents in chloroquine resistant Plasmodium yoelii nigeriensis. Acta Trop. 77:185–193 [DOI] [PubMed] [Google Scholar]
- 49. Sowunmi A. 2003. A randomized comparison of chloroquine and chloroquine plus ketotifen in the treatment of acute, uncomplicated, Plasmodium falciparum malaria in children. Ann. Trop. Med. Parasitol. 97:103–117 [DOI] [PubMed] [Google Scholar]
- 50. Sowunmi A, Fateye BA. 2003. Plasmodium falciparum gametocytaemia in Nigerian children: before, during and after treatment with antimalarial drugs. Trop. Med. Int. Health 8:783–792 [DOI] [PubMed] [Google Scholar]
- 51. Targett G, Drakeley C, Jawara M, von Seidlein L, Coleman R, Deen J, Pinder M, Doherty T, Sutherland C, Walraven G, Milligan P. 2001. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 183:1254–1259 [DOI] [PubMed] [Google Scholar]
- 52. Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. 2012. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med. 9:e1001169 doi:10.1371/journal.pmed.1001169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dembele L, Gego A, Zeeman AM, Franetich JF, Silvie O, Rametti A, Le Grand R, Dereuddre-Bosquet N, Sauerwein R, van Gemert GJ, Vaillant JC, Thomas AW, Snounou G, Kocken CH, Mazier D. 2011. Towards an in vitro model of Plasmodium hypnozoites suitable for drug discovery. PLoS One 6:e18162 doi:10.1371/journal.pone.0018162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dean M. 2005. The genetics of ATP-binding cassette transporters. Methods Enzymol. 400:409–429 [DOI] [PubMed] [Google Scholar]
- 55. Sarkadi B, Homolya L, Szakacs G, Varadi A. 2006. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol. Rev. 86:1179–1236 [DOI] [PubMed] [Google Scholar]
- 56. Sidhu AB, Valderramos SG, Fidock DA. 2005. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 57:913–926 [DOI] [PubMed] [Google Scholar]
- 57. Sidhu AB, Verdier-Pinard D, Fidock DA. 2002. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298:210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu Y, Wellems TE. 1996. Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc. Natl. Acad. Sci. U. S. A. 93:1130–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Y, Berger SA. 2003. Ketotifen reverses MDR1-mediated multidrug resistance in human breast cancer cells in vitro and alleviates cardiotoxicity induced by doxorubicin in vivo. Cancer Chemother. Pharmacol. 51:407–414 [DOI] [PubMed] [Google Scholar]
- 60. Brenner C. 2004. Chemical genomics in yeast. Genome Biol. 5:240 doi:10.1186/gb-2004-5-9-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Szakacs G, Annereau JP, Lababidi S, Shankavaram U, Arciello A, Bussey KJ, Reinhold W, Guo Y, Kruh GD, Reimers M, Weinstein JN, Gottesman MM. 2004. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell 6:129–137 [DOI] [PubMed] [Google Scholar]
- 62. Chong CR, Chen X, Shi L, Liu JO, Sullivan DJ., Jr 2006. A clinical drug library screen identifies astemizole as an antimalarial agent. Nat. Chem. Biol. 2:415–416 [DOI] [PubMed] [Google Scholar]
- 63. Gamo FJ, Sanz LM, Vidal J, DE Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF. 2010. Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310 [DOI] [PubMed] [Google Scholar]
- 64. Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, Jimenez-Diaz MB, Martinez MS, Wilson EB, Tripathi AK, Gut J, Sharlow ER, Bathurst I, El Mazouni F, Fowble JW, Forquer I, McGinley PL, Castro S, Angulo-Barturen I, Ferrer S, Rosenthal PJ, Derisi JL, Sullivan DJ, Lazo JS, Roos DS, Riscoe MK, Phillips MA, Rathod PK, Van Voorhis WC, Avery VM, Guy RK. 2010. Chemical genetics of Plasmodium falciparum. Nature 465:311–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kato N, Sakata T, Breton G, Le Roch KG, Nagle A, Andersen C, Bursulaya B, Henson K, Johnson J, Kumar KA, Marr F, Mason D, McNamara C, Plouffe D, Ramachandran V, Spooner M, Tuntland T, Zhou Y, Peters EC, Chatterjee A, Schultz PG, Ward GE, Gray N, Harper J, Winzeler EA. 2008. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat. Chem. Biol. 4:347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weisman JL, Liou AP, Shelat AA, Cohen FE, Guy RK, DeRisi JL. 2006. Searching for new antimalarial therapeutics amongst known drugs. Chem. Biol. Drug Des. 67:409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chattopadhyay R, Velmurugan S, Chakiath C, Andrews Donkor L, Milhous W, Barnwell JW, Collins WE, Hoffman SL. 2010. Establishment of an in vitro assay for assessing the effects of drugs on the liver stages of Plasmodium vivax malaria. PLoS One 5:e14275 doi:10.1371/journal.pone.0014275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Meister S, Plouffe DM, Kuhen KL, Bonamy GM, Wu T, Barnes SW, Bopp SE, Borboa R, Bright AT, Che J, Cohen S, Dharia NV, Gagaring K, Gettayacamin M, Gordon P, Groessl T, Kato N, Lee MC, McNamara CW, Fidock DA, Nagle A, Nam TG, Richmond W, Roland J, Rottmann M, Zhou B, Froissard P, Glynne RJ, Mazier D, Sattabongkot J, Schultz PG, Tuntland T, Walker JR, Zhou Y, Chatterjee A, Diagana TT, Winzeler EA. 2011. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 334:1372–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.