Abstract
The combination of platelets and anidulafungin at 0.03 μg/ml significantly (P < 0.05) reduced the germination rate and hyphal elongation in Aspergillus fumigatus compared to those with either anidulafungin only or an untreated control. Platelets decreased the expression of the fks gene, which plays an important role in cell wall synthesis. Our results suggest that human platelets plus anidulafungin might contribute to defense against A. fumigatus.
TEXT
An intact immune system is essential for the defense against fungal pathogens (1). Human platelets are known to primarily play a key role in hemostasis; however, they are considered to be part of the innate immunity (2–4). They exert antimicrobial effects against bacteria, such as Staphylococcus aureus, and several other microorganisms in vitro (4, 5). Christin et al. showed that platelets damage hyphae of Aspergillus fumigatus (6). Recently, we observed that platelets have the capacity to attenuate the virulence of Aspergillus spp. and zygomycetes in vitro by reducing hyphal germination and elongation (7–9). In addition, the polysaccharide galactomannan, which is released by growing and vital hyphae of A. fumigatus, was significantly reduced under platelet treatment (8). Previously, we have reported that human platelets act beneficially with amphotericin B against A. fumigatus and other Aspergillus spp. (10, 11). Here we investigated whether platelets and anidulafungin in combination have an added effect on fungal germination, hyphal elongation, and hyphal damage of A. fumigatus. In addition, we analyzed the effects of human platelets in combination with anidulafungin on expression levels of the fks gene, which encodes the 1,3-β-d-glucan synthase. Echinocandins interfere with cell wall synthesis by inhibiting this enzyme, which forms glucan polymers, the major component of the fungal cell wall (12).
Two clinical isolates of A. fumigatus were used, and the strains were obtained from patients suffering from invasive aspergillosis. Platelet concentrates (storage time < 24 h) were provided by the local Department of Immunology and Blood Transfusion at Innsbruck Medical University. The minimum effective concentration (MEC) was determined according to the Antifungal Susceptibility Testing (AFST) committee, EUCAST, broth microdilution method (13), and it was found to be 0.03 μg/ml for both isolates tested. Subsequently, 0.03 μg/ml and a subinhibitory MEC of 0.0078 μg/ml of anidulafungin were applied for further tests. The determination of germination rate and hyphal elongation was performed as described elsewhere (7–9). Conidial suspensions were treated either with platelets or anidulafungin alone or with the combination of platelets and the drug. For investigation of hyphal elongation and the germination percentage, 100 μl of platelets (1 × 108/ml) and 100 μl of conidia (1 × 106/ml) suspended in RPMI 1640 (Sigma-Aldrich, Vienna, Austria) were mixed in an effector-to-target-cell (E:T) ratio of 100:1 and then anidulafungin was added and the mixture was incubated at 37°C. To calculate the germination rate, the percentage of conidia that did not germinate in comparison to the percentage that germinated was evaluated. Untreated and anidulafungin-treated fungi served as controls, and each assay was assessed in triplicate.
We also determined the antifungal activity of platelets, anidulafungin, and the combination of platelets and anidulafungin by a colorimetric assay with the dye 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2-H-tetrazolium-5-carboxynilide sodium salt (XTT; Sigma-Aldrich) plus 40 μg/ml coenzyme Q (Sigma-Aldrich). The Northern analysis and reverse transcription-quantitative PCR (qPCR) were performed according to a standard protocol (14), and the fold increase was evaluated by using the 2−ΔΔCT method (15). Repeated-measures analysis of variance (ANOVA) was used to evaluate differences between mean values, followed by Bonferroni's multiple-comparison test. A P value of <0.05 indicated statistical significance.
We found that the germination rate of A. fumigatus with platelets plus anidulafungin was 1.3% ± 0.5% at a concentration of 0.03 μg/ml and 2% ± 0.1% at 0.0078 μg/ml. These data revealed a significantly strong inhibitory effect on the germination rate (P < 0.05) compared to untreated (95.4% ± 4.3%) or exclusively platelet-treated (5.8% ± 3.0%) controls or anidulafungin treatment at 0.03 μg/ml (50.4% ± 17.2%) and at 0.0078 μg/ml (59% ± 8.9%).
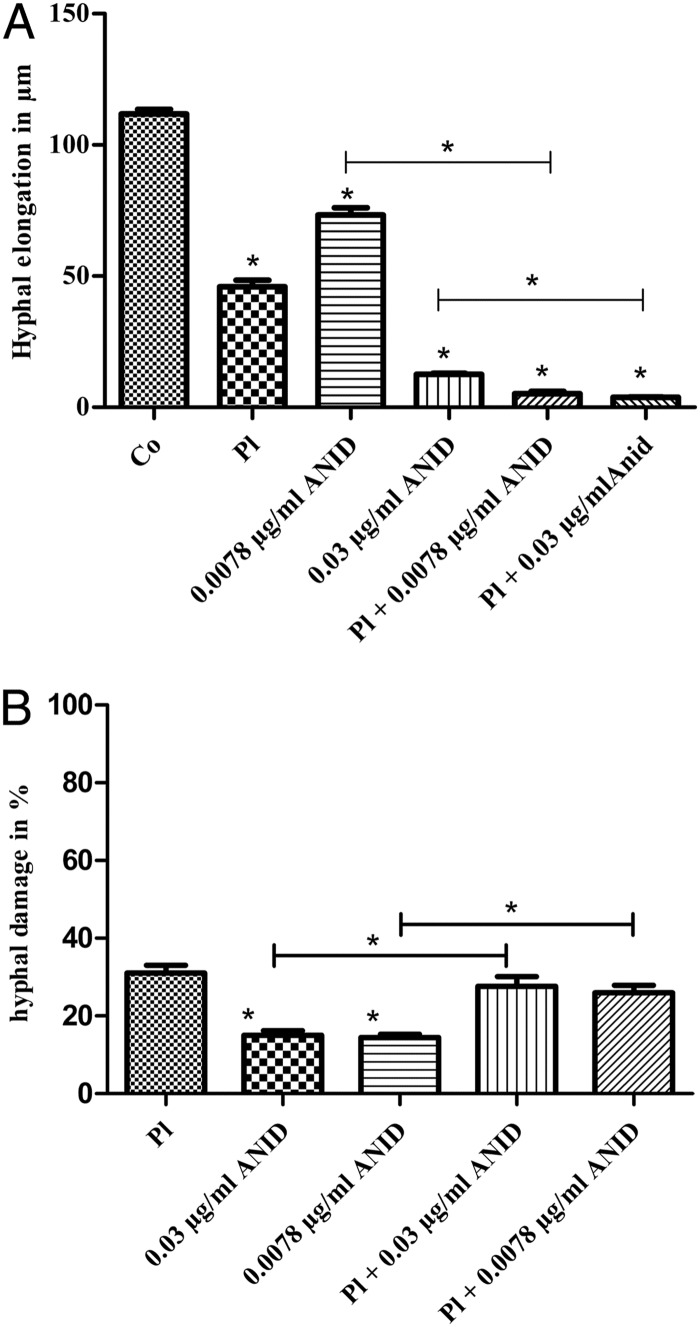
Fungal hyphae interact with platelets during angioinvasion, which often results in thrombosis and tissue infarction (16). As hyphal outgrowth is responsible primarily for the invasiveness of the fungus, it can be used as an indicator of antifungal activity of drugs or human cells (16). In this study, we found a significant reduction of hyphal elongation (P < 0.05) by platelets, anidulafungin, and combined administration of platelets and anidulafungin, as shown in Fig. 1A; the effect was strongest applying anidulafungin at 0.03 μg/ml. This is an important finding, since the changing of morphology from conidia to filaments is essential for the fungus to become invasive. Subsequently, the fungus is capable of invading the deep tissue of the target organs (16).
Fig 1.
(A) Effects of platelets, anidulafungin alone, and the combination of anidulafungin and platelets at an E:T ratio of 100:1 on hyphal elongation of A. fumigatus (n = 2). Aspergilli were incubated for 16 h in the absence of any platelets or drug (Co) or in the presence of platelets (Pl), 0.0078 μg/ml ANID, 0.03 μg/ml ANID, Pl and 0.0078 μg/ml ANID, or Pl and 0.03 μg/ml ANID. (B) Percentage of hyphal damage of aspergilli with platelets, anidulafungin, and the combination of anidulafungin and platelets at an E:T ratio of 100:1 by reduction of XTT. Data are representative of six independent experiments. Error bars show the standard error of the mean (SEM). An asterisk indicates a statistically significant difference with a P value of <0.05. Co, controls, growth of untreated fungi; Pl, platelets; ANID, anidulafungin.
Platelets damaged fungal hyphae significantly more than anidulafungin alone, as shown in Fig. 1B. Interestingly, only a modest increase in fungal injury resulted from the combination of platelets plus anidulafungin. These data support the idea that antifungal substances derived from activated platelets aid in prevention of fungal outgrowth rather than fungal impairment. So far, data on human phagocytes showed that anidulafungin supports polymorphonuclear (PMN) phagocyte-mediated hyphal damage in A. fumigatus (17). Also, in a recent study, micafungin was found to have an additive effect with PMN-induced damage in A. fumigatus hyphae (18). The exact mechanism of hyphal damage due to platelets is still unknown. However, a possible enhanced effect of immune effector cells combined with antifungal drugs against virulent fungi has been found in several studies (19–22).
Quantitative PCR and Northern blot showed that the combination of anidulafungin and human platelets induced downregulation of the target gene fks. Platelets were able to reduce the expression of the fks gene. Although platelets in combination with anidulafungin did not reveal a strong effect, platelets have the capacity to decrease the expression of fks (Fig. 2A). These data were confirmed with Northern analysis (Fig. 2B). The fks gene encodes 1,3-β-d-glucan synthase, which plays a leading role in fungal cell wall synthesis (12).
Fig 2.
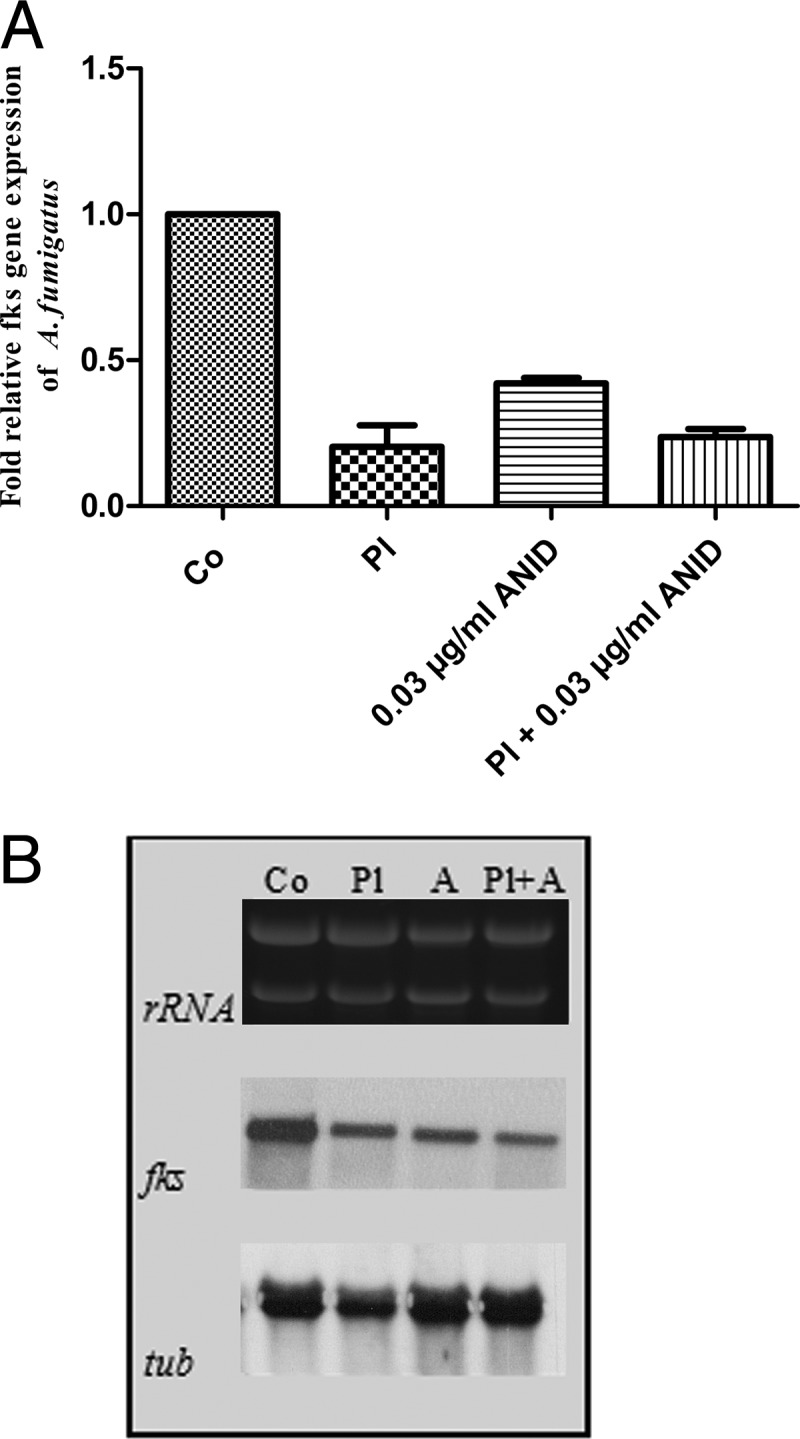
(A) Relative gene expression levels of the fks gene of untreated, platelet-treated, anidulafungin (0.03 μg/ml)-treated, and platelet-and-anidulafungin (E:T ratio, 100:1)-treated A. fumigatus. (B) Northern analysis of untreated and platelet-treated A. fumigatus fks and tub gene expression levels. Following platelet, anidulafungin (0.03 μg/ml), or combination treatment for 60 min, total RNA was isolated from A. fumigatus and hybridized with fks encoding 1,3-β-d-glucan synthase for gene expression analysis. As a loading control, blots were hybridized with the β-tubulin-encoding tub gene of A. fumigatus. (A) Error bars show the SEM. Co, controls, growth of untreated fungi; Pl, platelets; ANID, anidulafungin. (B) Co, control; Pl, platelets; A, anidulafungin; Pl + A, platelets plus anidulafungin.
In summary, our findings demonstrate that human platelets may potentiate the antifungal properties of anidulafungin against A. fumigatus. We showed that platelets augment antifungal activity of anidulafungin against A. fumigatus in vitro, as evidenced by the reduced fungal germination rate and hyphal elongation; both processes are important in fungal invasiveness and growth. The combination of anidulafungin and human platelets induced downregulation of the target gene fks in A. fumigatus. Further research is still required to clarify the exact roles that platelets have in the immune system against fungal infections and how exactly they can help antifungal therapies.
ACKNOWLEDGMENTS
This study was supported by a grant by Pfizer Austria.
S.P. has received grants from Gilead and Pfizer. C.L.-F. has worked as a consultant for Gilead Sciences, Astellas Pharma, and Pfizer, served at the speakers' bureau of Gilead Sciences, Pfizer, and MSD, and received grants from Pfizer and Gilead. All other authors report no conflict of interest.
The authors alone are responsible for the content and writing of the paper.
Footnotes
Published ahead of print 31 October 2012
REFERENCES
- 1. Denning DW. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781–805 [DOI] [PubMed] [Google Scholar]
- 2. Jurk K, Kehrel BE. 2005. Platelets: physiology and biochemistry. Semin. Thromb. Hemost. 31:381–392 [DOI] [PubMed] [Google Scholar]
- 3. Tang YQ, Yeaman MR, Selsted ME. 2002. Antimicrobial peptides from human platelets. Infect. Immun. 70:6524–6533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeaman MR. 1997. The role of platelets in antimicrobial host defense. Clin. Infect. Dis. 25:951–970 [DOI] [PubMed] [Google Scholar]
- 5. Fitzgerald JR, Foster TJ, Cox D. 2006. The interaction of bacterial pathogens with platelets. Nat. Rev. Microbiol. 4:445–457 [DOI] [PubMed] [Google Scholar]
- 6. Christin L, Wysong DR, Meshulam T, Hastey R, Simons ER, Diamond RD. 1998. Human platelets damage Aspergillus fumigatus hyphae and may supplement killing by neutrophils. Infect. Immun. 66:1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perkhofer S, Kainzner B, Kehrel BE, Dierich MP, Nussbaumer W, Lass-Flörl C. 2009. Potential antifungal effects of human platelets against zygomycetes in vitro. J. Infect. Dis. 200:1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perkhofer S, Kehrel BE, Dierich MP, Donnelly JP, Nussbaumer W, Hofmann J, Voneiff C, Lass-Flörl C. 2008. Human platelets attenuate Aspergillus species via granule-dependent mechanisms. J. Infect. Dis. 198:1243–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perkhofer S, Niederegger H, Blum G, Brugstaller W, Ledochowski M, Dierich MP, Lass-Flörl C. 2007. Interaction of 5-hydroxytryptamine (serotonin) against Aspergillus spp. in vitro. Int. J. Antimicrob. Agents 29:424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perkhofer S, Trappl K, Nussbaumer W, Dierich MP, Lass-Flörl C. 2010. Potential synergistic activity of antimycotic substances in combination with human platelets against Aspergillus fumigatus. J. Antimicrob. Chemother. 65:1309–1311 [DOI] [PubMed] [Google Scholar]
- 11. Perkhofer S, Trappl K, Striessnig B, Nussbaumer W, Lass-Flörl C. 2011. Platelets enhance activity of antimycotics substances against non-Aspergillus fumigatus Aspergillus species in vitro. Med. Mycol. 49:157–166 [DOI] [PubMed] [Google Scholar]
- 12. Denning DW. 2003. Echinocandin antifungal drugs. Lancet 362:1142–1151 [DOI] [PubMed] [Google Scholar]
- 13. Lass-Flörl C, Perkhofer S. 2008. In vitro susceptibility-testing in Aspergillus species. Mycoses 51:437–446 [DOI] [PubMed] [Google Scholar]
- 14. Arendrup MC, Perkhofer S, Howard SJ, Garcia-Effron G, Vishukumar A, Perlin D, Lass-Flörl C. 2008. Establishing in vitro-in vivo correlations for Aspergillus fumigatus: the challenge of azoles versus echinocandins. Antimicrob. Agents Chemother. 52:3504–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 16. Hogan LH, Klein BS, Levitz SM. 1996. Virulence factors of medically important fungi. Clin. Microbiol. Rev. 9:469–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brummer E, Chauhan SD, Stevens DA. 1999. Collaboration of human phagocytes with LY-303366 for antifungal activity against Aspergillus fumigatus. J. Antimicrob. Chemother. 43:491–496 [DOI] [PubMed] [Google Scholar]
- 18. Choi JH, Brummer E, Stevens DA. 2004. Combined action of micafungin, a new echinocandin, and human phagocytes for antifungal activity against Aspergillus fumigatus. Microbes Infect. 6:383–389 [DOI] [PubMed] [Google Scholar]
- 19. Dotis J, Simitsopoulou M, Dalakiouridou M, Konstantinou T, Taparkou A, Kanakoudi-Tsakalidou F, Walsh TJ, Roilides E. 2006. Effects of lipid formulations of amphotericin B on activity of human monocytes against Aspergillus fumigatus. Antimicrob. Agents Chemother. 50:868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gil-Lamaignere C, Roilides E, Mosquera J, Maloukou A, Walsh T. 2002. Antifungal triazoles and polymorphonuclear leukocytes synergize to cause increased hyphal damage to Scedosporium prolificans and Scedosporium apiospermum. Antimicrob. Agents Chemother. 46:2234–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lamaris GA, Lewis RE, Chamilos G, May GS, Safdar A, Walsh TJ, Raad II, Kontoyiannis DP. 2008. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J. Infect. Dis. 198:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tullio V, Mandras N, Scalas D, Allizond V, Banche G, Roana J, Greco D, Castagno F, Cuffini AM, Carlone NA. 2010. Synergy of caspofungin with human polymorphonuclear granulocytes for killing Candida albicans. Antimicrob. Agents Chemother. 54:3964–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]