Abstract
The objective of this study was to evaluate whether Candida albicans exhibits altered pathogenicity characteristics following sublethal antimicrobial photodynamic inactivation (APDI) and if such alterations are maintained in the daughter cells. C. albicans was exposed to sublethal APDI by using methylene blue (MB) as a photosensitizer (0.05 mM) combined with a GaAlAs diode laser (λ 660 nm, 75 mW/cm2, 9 to 27 J/cm2). In vitro, we evaluated APDI effects on C. albicans growth, germ tube formation, sensitivity to oxidative and osmotic stress, cell wall integrity, and fluconazole susceptibility. In vivo, we evaluated C. albicans pathogenicity with a mouse model of systemic infection. Animal survival was evaluated daily. Sublethal MB-mediated APDI reduced the growth rate and the ability of C. albicans to form germ tubes compared to untreated cells (P < 0.05). Survival of mice systemically infected with C. albicans pretreated with APDI was significantly increased compared to mice infected with untreated yeast (P < 0.05). APDI increased C. albicans sensitivity to sodium dodecyl sulfate, caffeine, and hydrogen peroxide. The MIC for fluconazole for C. albicans was also reduced following sublethal MB-mediated APDI. However, none of those pathogenic parameters was altered in daughter cells of C. albicans submitted to APDI. These data suggest that APDI may inhibit virulence factors and reduce in vivo pathogenicity of C. albicans. The absence of alterations in daughter cells indicates that APDI effects are transitory. The MIC reduction for fluconazole following APDI suggests that this antifungal could be combined with APDI to treat C. albicans infections.
INTRODUCTION
Management of infections caused by clinically relevant fungal pathogens is a challenge due to the incidence of resistance that can develop during therapy, especially in immunocompromised individuals (1). The increasing need for prolonged use of antifungal drugs, longer than usually recommended for antibiotics, is accompanied by a corresponding increased incidence of side effects. Furthermore, the limited number of available antifungal compounds and the need to determine the susceptibility profile of the organism also complicate the treatment of these infections (2).
The fungal species Candida albicans is part of the commensal flora of the human gastrointestinal and genitourinary tracts and can cause superficial infections of the mucosa and skin (3, 4). The infection depends on imbalances between increased C. albicans virulence attributes and impaired host defense systems. In immunocompromised individuals, however, C. albicans may invade deeper tissues, penetrate the blood vessels, and cause life-threatening systemic infections (3, 5).
Photodynamic therapy (PDT) is a light-based treatment platform that is under development for several applications in oncology, dermatology, and ophthalmology, and it has recently been investigated as an antimicrobial therapy. The combination of nontoxic dyes referred to as photosensitizers (PS) and harmless low-intensity visible light generates reactive oxygen species (ROS) that are toxic to microorganisms. The potent and broad-spectrum antimicrobial effect has highlighted this therapy as a promising alternative treatment for localized infections. The photodynamic effect depends on the type and concentration of PS employed, combined with the irradiation parameters that activate the dye. Many reports in the literature have confirmed efficient antimicrobial photodynamic inactivation (APDI) of various yeast and bacterial species following the proper light and PS dosimetries delivered to the cells.
The production of ROS in APDI has been implicated in two important aspects of microbial physiology: (i) changes in the expression of virulence determinants of yeasts (6, 7) and (ii) the impact of APDI on the overall survival of microorganisms. Moreover, some types of PS are able to penetrate the microbial cell and bind to cytoplasmic components and nuclear material. Methylene blue (MB), a widely studied PS (8), has an affinity to guanine bases of DNA (9). Consequently, generation of ROS activity near DNA may occur and it can induce mutations in a random form, since ROS can interact with nucleic acid bases.
In the present study, we evaluated whether C. albicans cells exposed under sublethal conditions of APDI exhibited altered pathogenicity characteristics. Different methods of analysis were employed to evaluate yeast cells following APDI and their further daughter cells. We report the effects of MB-mediated APDI on the ability of C. albicans to grow, to form germ tubes (GTs), and to cause a systemic infection, and also on its susceptibility to fluconazole and sensitivity to stress agents.
MATERIALS AND METHODS
Strain and inoculum preparation.
Candida albicans ATCC 90028 cells (10) were subcultured from vial stocks onto Sabouraud dextrose agar under aerobic conditions at 37°C. Yeast inocula were prepared from 24-h cultures, and the turbidity of cell suspensions was measured in a spectrophotometer at 540 nm in order to obtain suspensions with an optical density of 0.16 (1 × 106 to 2 × 106 CFU/ml) and 0.8 (1 × 107 CFU/ml).
PS and irradiation source.
A stock solution of 5 mM MB (Sigma-Aldrich) was prepared in distilled water. The PS solution was filtered by employing a sterile 0.22-μm membrane and stored in the dark before use. The PS was added to the yeast suspension to give a final working concentration of 50 μM. The concentration of MB was chosen from a preliminary study based on the criterion of effective photodynamic inactivation without dark toxicity (data not shown).
A GaAlAs diode laser (Photon Lase III; DMC, São Carlos, Brazil) with a wavelength of 660 nm and output power of 30 mW was used in this study. The laser probe was fixed on a holder that kept the beam area at 0.4 cm2, which coincided to a single well size from the 96-well microtiter plate.
APDI studies.
Parameters of irradiation that caused no reduction of viable cells were used to investigate the effects of sublethal APDI on growth curve, germ tube formation, in vivo pathogenicity, sensitivity to stress compounds, and fluconazole susceptibility. C. albicans cultures exposed to APDI and their further daughter cells were evaluated. Before all tests, daughter cells were generated by culturing yeast cells following APDI for 24 h. For all tests, we used a control group composed of cells incubated with MB but without irradiation.
C. albicans cells (optical density at 540 nm [OD540], 0.8) were incubated with 50 μM MB in phosphate-buffered saline (PBS) for 10 min at room temperature and in the dark. Aliquots were placed in wells of a 96-well microtiter plate and then irradiated with a fluence rate of 75 mW/cm2 over an area of 0.4 cm2, for time exposures of 2 to 6 min delivering fluences of 9 J/cm2 to 27 J/cm2, respectively (11). Yeast suspensions were serially diluted in PBS to give dilutions of 10−1 to 10−5 times the original concentration. Ten-microliter aliquots of each dilution were streaked onto Sabouraud agar plates in triplicate and incubated at 37°C overnight (12). The yeast colonies were counted and converted into CFU ml−1 for analysis. Two types of control conditions were used: without PS and irradiation, and with PS in the dark.
Growth curves.
For determination of growth curves, Sabouraud dextrose broth was inoculated with C. albicans cells at an OD600 of 0.01. The suspensions were placed in 96-well microtiter plates and incubated at 37°C. Growth was monitored in a spectrophotometer (Spectramax M4; Molecular Devices) at 600 nm with 30-min intervals (13). C. albicans cultures exposed under the three conditions of sublethal APDI were evaluated. In all groups, cells were incubated with 50 μM MB. Two independent experiments were performed in triplicate.
Germ tube formation.
We analyzed germ tube formation in order to verify whether sublethal APDI induced alterations in this virulence determinant that is important for C. albicans pathogenesis. Yeast cells were incubated with 10% fetal bovine serum at a concentration of approximately 106 cells ml−1 for 3 h at 37°C (14). After this period, cells were fixed in 1% formaldehyde, and then 5 μl of the yeast suspension was placed on a microscope slide and covered with a coverslip. The number of GTs was determined by examining 100 yeast cells under a light microscope, and percent GT formation was also determined. Seven samples of each group were analyzed.
In vivo pathogenicity assay.
The experimental procedures were approved by the Institutional Ethic Committee on Research Animal Care (IPEN-CNEN/SP). A mouse model of hematogenously disseminated candidiasis was used to investigate pathogenesis alterations caused by APDI (15, 16). Female BALB/c mice (9 to 11 weeks of age; body mass of about 21 g) were used in the study. All the animals were housed four per cage, maintained on a 12-h light and 12-h dark cycle, and had access to food and water ad libitum. Mice were infected via the lateral caudal vein with 0.1 ml of a C. albicans suspension containing 1 × 106 cells (17). Animal survival was evaluated daily (18).
Stress sensitivity test.
We decided to examine the sensitivity of C. albicans to different stress-inducing agents in order to determine the alterations induced by APDI that could alter in vivo pathogenicity. Sodium dodecyl sulfate (SDS), caffeine, hydrogen peroxide (H2O2), menadione, and NaCl sensitivity assays were carried out to evaluate cell wall integrity and responses to oxidative and osmotic stress following APDI. Aliquots of 5 μl of a 10-fold serial dilution of 103 to 101 cells were plated on yeast extract-peptone-dextrose (YPD) medium supplemented with the indicated stress compounds. Plates were incubated at 30°C and 37°C until colonies appeared.
Fluconazole susceptibility.
We tested whether sublethal APDI could affect fluconazole susceptibility by comparing the MICs of fluconazole for our C. albicans groups. This antifungal drug was chosen since it represents one of the most commonly used azoles for treatment of candidiasis (19). The MIC to the antifungal fluconazole was determined by the broth microdilution method according to the standards established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (20). Briefly, C. albicans samples cultured at 35°C for 24 h were suspended in sterile distilled water. A working inoculum with a final concentration of 1 × 105 to 2 × 105 CFU/ml was used. The assay medium used was RPMI 1640 medium (without sodium bicarbonate and with l-glutamine) supplemented with 2% glucose, buffered to pH 7.0 with 0.165 M 3-(N-morpholino)-propanesulfonic acid (MOPS). A stock solution of fluconazole (Sigma-Aldrich, São Paulo, Brazil) was prepared, and we considered the potency of the drug to be 98%. The range of concentrations tested was 0.125 to 64 μg/ml. MIC values were determined in a spectrophotometer (SpectraMax M4; Molecular Devices) at 530 nm, after 24 h of incubation. We determined the lowest drug concentration of each group (n = 7) that promoted an inhibition of growth of ≥50% compared to the drug-free control (n = 7). Two independent experiments were conducted. In all groups, yeast stock inocula were incubated with 50 μM MB before preparation of working suspensions.
Statistics.
The areas under the growth curves were obtained and normalized based on the mean area for the control group. Germ tube formation results are presented as the percent GT formation. Data were analyzed to verify the assumption of normality (Shapiro-Wilk test) and the equality of group variances (Levene test). Comparisons between groups were made by an analysis of variance followed by Tukey's post hoc test. The survival data were analyzed by the nonparametric log rank test. For all tests, the overall significance level was set at 5%.
RESULTS
Parameters for sublethal APDI.
In order to determine sublethal irradiation parameters for MB-mediated APDI, three different fluences were evaluated. A 50 μM concentration of MB did not show any toxicity for yeast cells after incubation for 10 min in the dark. Mean values of the CFU/ml (± standard deviations) of C. albicans cells treated only with MB (6.9 ± 0.09 logs) did not present a significant difference (P > 0.05) from the cells that were not exposed to MB or light (6.9 ± 0.3 logs). After irradiation with 9, 18, or 27 J/cm2 of 660-nm laser light in the presence of MB, no reduction of viable cells was observed (7 ± 0.2, 7 ± 0.2, 6.9 ± 0.2, respectively).
Sublethal APDI delays C. albicans growth.
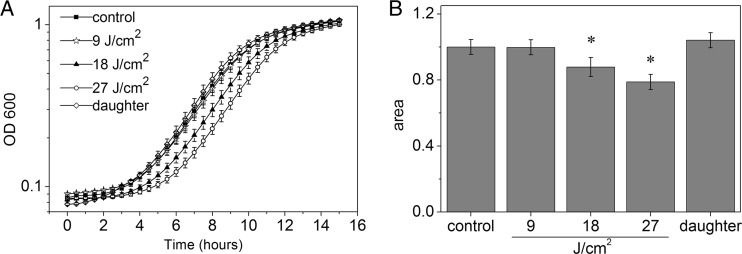
Similar growth curves (Fig. 1A) were obtained for control (C. albicans cells treated with MB) and MB-mediated APDI with 9 J/cm2 of irradiation. C. albicans exposed to 18 and 27 J/cm2 of APDI remained in the lag phase longer, and this alteration was time dependent. Daughter cells presented a shorter lag phase, whereas exponential growth was similar to the control. Furthermore, the areas under the growth curves were obtained to quantify the growth of C. albicans (Fig. 1B). Control cells, cells treated with MB-mediated APDI with 9 J/cm2 of irradiation, and daughter cells presented similar results. A significant reduction was observed in cells exposed to 18 and 27 J/cm2 of APDI, and the change was in a fluence-dependent manner.
Fig 1.
Effects of sublethal APDI on growth of C. albicans. (A) Growth curves; (B) normalized areas under the curves. Data represent mean values, and bars are standard deviations. Symbols represent statistically significant differences compared to the other groups (P < 0.05).
Inhibition of germ tube formation following sublethal APDI does not persist in daughter cells.
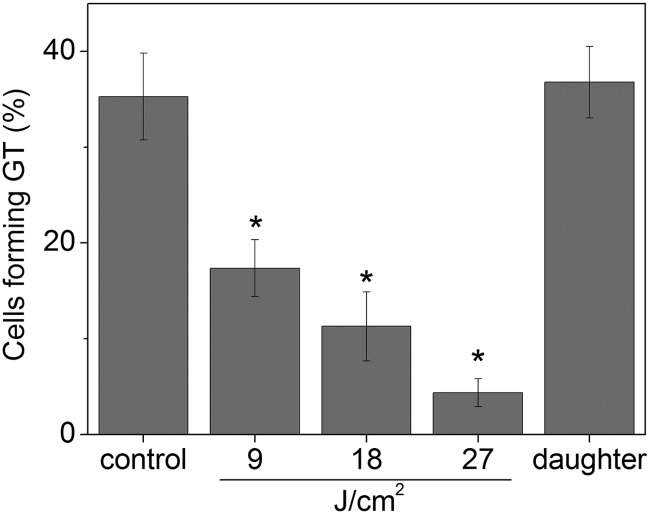
The ability of C. albicans to form germ tubes significantly decreased after exposition to sublethal APDI, and the reduction was higher with longer irradiation times (P < 0.05). Cells treated with APDI (9, 18, or 27 J/cm2) also formed fewer germ tubes than daughter cells of C. albicans cells submitted to APDI (P < 0.05). On the other hand, control and daughter cells showed similar GT formation (P > 0.05) (Fig. 2).
Fig 2.
Effect of antimicrobial photodynamic inactivation on GT formation. Each column represents the mean percentage of cells exhibiting germ tubes in the indicated group, and error bars show standard deviations. *, statistically significant difference compared to the other groups (P < 0.05).
Pathogenicity of C. albicans in a systemic infection model is affected only following APDI.
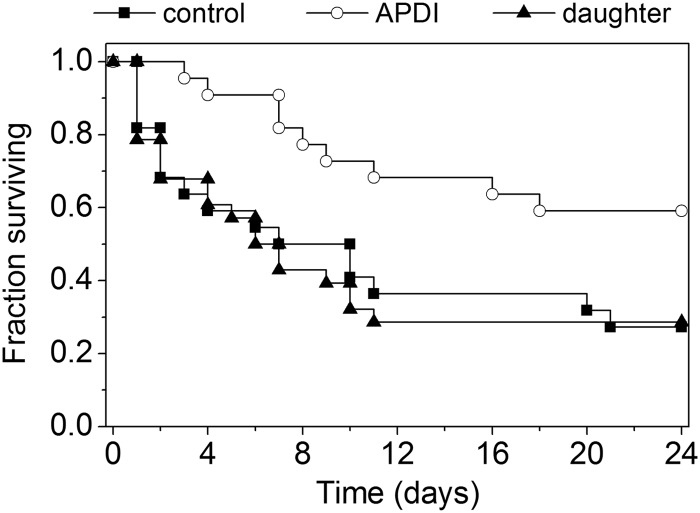
The infection with C. albicans pretreated with APDI was less aggressive than infection with the same dose of untreated yeast cells. The first animal from the APDI group died 3 days after inoculation, while 26% of the mice from the control group were deceased by this period (Fig. 3). Furthermore, the overall survival percentage was significantly increased in the APDI group compared to control mice (24-day survival, 59% versus 27%, APDI versus controls, respectively; P = 0.016). On the other hand, mice infected with daughter cells of C. albicans submitted to APDI showed a similar survival curve as animals infected with untreated yeast. No difference was observed between the two groups (24-day survival, 29% versus 27%, daughter cells of the APDI-treated group versus untreated cells, respectively; P = 0.88), whereas the survival percentage of animals from the APDI group was significantly higher than from the daughter group (24-day survival, 59% versus 29%, APDI versus daughter, respectively; P = 0.009).
Fig 3.
Kaplan-Meier survival curves for mice systemically infected with C. albicans. The graph shows results for control cells treated only with MB (n = 22), C. albicans cells exposed to sublethal MB-mediated APDI (27 J/cm2) (n = 22), and daughter cells submitted to sublethal APDI and cultured before injection (n = 28).
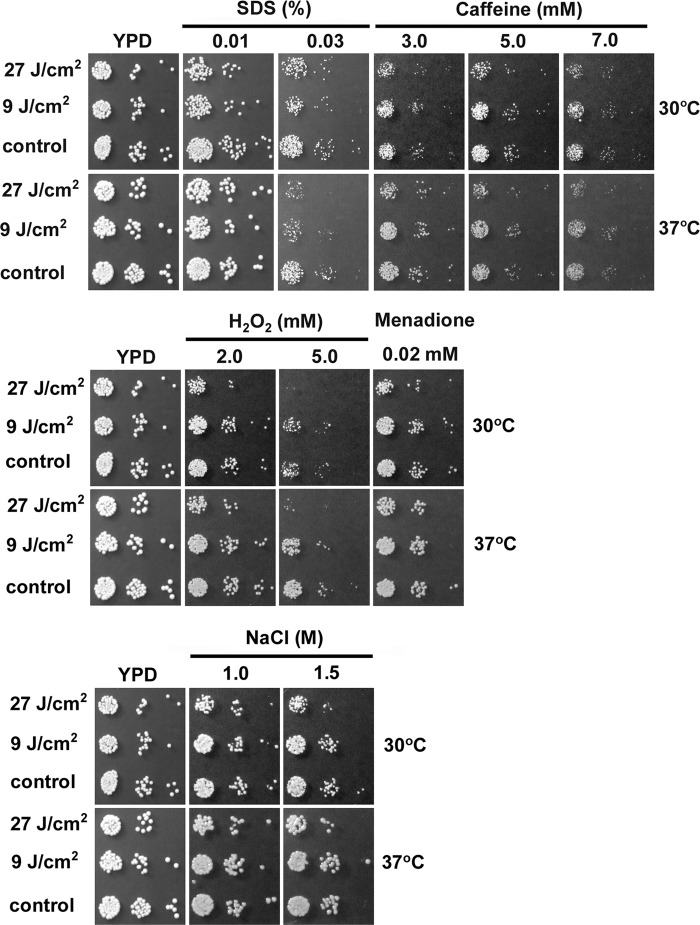
APDI increased C. albicans sensitivity to SDS and hydrogen peroxide.
Incubation with MB alone (control cells) did not influence growth under any of the stress conditions tested (Fig. 4); only when the PS was activated with light did the cells exhibit increased sensitivity to the stress conditions examined in our assays. Notably, photodynamic damage resulted in impaired resistance to the oxidative stress-inducing agent hydrogen peroxide, but not to the superoxide generator menadione. Following APDI, cells displayed increased sensitivity to the cell wall stressor SDS; however, only a marginal effect could be observed in response to caffeine (APDI with 27 J/cm2). On the other hand, APDI did not impair the growth of C. albicans in the presence of the osmotic stressor NaCl. Under stress conditions that altered the C. albicans phenotype, growth inhibition was more pronounced in cells irradiated with 27 J/cm2 than with 9 J/cm2.
Fig 4.
APDI affects the resistance of C. albicans to SDS and hydrogen peroxide. Untreated C. albicans cells were grown on YPD without any additional compound. Plates were incubated at 30°C and 37°C for 32 h.
Fluconazole susceptibility is altered by APDI.
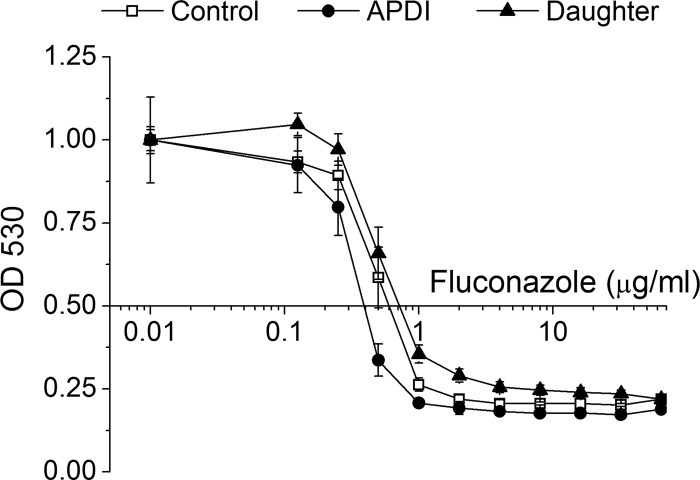
Although fluconazole activity against untreated yeast was slightly higher than against daughter cells of C. albicans submitted to APDI (Fig. 5), a similar MIC value was observed for both samples (MIC, 1 μg/ml). Confirming our hypothesis, fluconazole was more active against C. albicans following sublethal MB-mediated APDI. Within the range of 0.125 to 64 μg/ml fluconazole, inhibition of growth of these cells was higher, resulting in a reduction of the MIC to 0.5 μg/ml.
Fig 5.
Fluconazole susceptibility curves of C. albicans exposed to sublethal APDI. Cells from the APDI group were irradiated with 27 J/cm2. Where the x axis intersects with the y axis (at 0.5) indicates inhibition of growth of 50%.
DISCUSSION
The increasing incidence of localized and invasive fungal infections in addition to the rise of drug-resistant strains have led to the investigation of new antifungal approaches by several research groups (2, 21). Photodynamic therapy emerged as a promising modality due to the antimicrobial effectiveness against a broad range of microorganisms (8). Recently, studies have focused the investigation of APDI effects on key elements of the microbial phenotype. These aspects of APDI have been only sporadically explored; there are scattered reports that the production of ROS from APDI can change the virulence profiles of bacteria (22, 23) and fungi (6, 7). However, the consequences of APDI to further generations of the treated pathogens remain under investigation.
Besides the known fungicidal activity of APDI (7, 11, 24), we observed that sublethal photodynamic action had a temporarily fungistatic effect on C. albicans, and the extent of this activity was dependent on the amount of energy delivered to the cell-PS system, even under conditions bellow the photoinactivation threshold. The increase in lag phase observed immediately after APDI suggested that cell growth was arrested. Since exponential growth was similar to that in untreated cells, it is reasonable to assume that following APDI cells initiate a rescue response, which promotes repair and allows further cell cycle progression. These cell responses to photodynamic effects have been described in human cells subjected to PDT (25). Furthermore, decreases in cellular growth rates of C. albicans have also been reported after oxidative stress promoted by hydrogen peroxide (26) and by other oxidant agents (27).
Similar to oxidant agents, APDI can promote a temporary cell growth arrest in different phases of the cell cycle (25, 28, 29). This inhibition of proliferation can be caused by different mechanisms: the slowdown of cellular metabolism caused by a reduction of nutrient uptake, or the impaired bioenergetic function of mitochondria, via a direct signal generated in mitochondria related to growth inhibition. Cellular metabolism can be reduced after APDI due to decreased transport of carbohydrates, amino acids, or phosphate through membrane-bound carrier proteins (30). Photodynamic action can also disturb carbon metabolism and ATP production by knocking out intracellular enzymes (e.g., alcohol dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase, hexokinase, and cytochrome c oxidase) (31). In addition, photodynamic damage of mitochondria can increase the expression of p21, an inhibitor of cyclin-dependent kinases, leading to growth inhibition (29). The temporary cell growth arrest caused by oxidant exposure, with little or no cell death, is described as a defense mechanism against oxidant insult, as it enables the cell to induce antioxidant defense and repair systems to minimize the injury and to remove or replace whatever damaged cellular components are present before cycle progression (27). Nevertheless, further studies are necessary to elucidate which of these mechanisms are involved in MB-mediated APDI for C. albicans. The core of this effort will employ the generation and combinatorial analysis of physiological, biochemical, phenotypic, and gene expression data in yeast cells subjected to PDI, along the lines of similar studies that have emphasize other stressors and oxidative stress (32, 33).
C. albicans is a microorganism that has the ability to switch between distinct forms in response to external stimuli (34). This ability has been considered a virulence mechanism of this fungus, i.e., a trait of C. albicans that is needed to subvert host defenses and cause disease (35). Among morphogenetic forms of C. albicans, the germ tube is the initial elongating structure formed during the yeast-hypha transition. The development of germ tubes was reported to be inhibited as a response to stress, including oxidative stress, caused by exposure of C. albicans to immune system cells (36). An oxidative-tolerant mutant developed by exposure of C. albicans to an oxidant agent also caused reduction of germ tube and pseudohypha formation, less extracellular phospholipase production, and less pathogenicity in mice, as an adaptive response to oxidative stress (37). The inhibition of germ tube formation observed in our study suggests that sublethal MB-mediated APDI produces injuries that arrest cells in the yeast form. All tested sublethal conditions of APDI inhibited GT formation, indicating that this cell function is sensitive to MB-mediated APDI, in contrast with cell growth. Besides the fluence-dependent effects observed in our study, it has been reported that the decrease of GT formation can also be correlated to MB concentration after APDI (6).
Since cell growth and germ tube formation are characteristics related to the pathogenicity of C. albicans, we proposed to evaluate whether alterations observed in vitro could affect the ability of this fungus to cause a systemic infection. Notably, mice infected with C. albicans that had been preexposed to APDI developed a less aggressive infection, with increased mouse survival. This finding correlated with the inhibition of cell growth and germ tube formation caused by APDI. It has been reported that hypha development and hypha-associated factors can assist C. albicans in resisting the host immune defense factors, such as macrophages (38, 39) and neutrophils (36), and allow fungal cells to escape from the bloodstream (40) and to invade tissues (41). Furthermore, the germ tube is the dominant growth form of C. albicans in plasma (36). Therefore, it is plausible to assume that sublethal APDI affected the C. albicans capability to grow and to escape from the bloodstream and favored host defenses, rendering the fungus more susceptible to killing by immune cells. Another important aspect is that once C. albicans is arrested in the yeast form, the phagocytosis by immune system cells is facilitated (42). Besides the reduced ability to survive in a hostile environment such as the bloodstream, APDI can also inhibit C. albicans adhesion (7), which will also interfere with the establishment of infection.
The increased sensitivities to SDS and hydrogen peroxide indicate that APDI affects cell wall structure and lowers resistance to additional oxidative stress. The slight susceptibility differences found with caffeine and the lack of response with sodium chloride suggest that APDI promotes mild to moderate weakening of the cell wall. These detected alterations promoted by APDI could also be responsible for the reduced ability of C. albicans to infect and the lowered resistance to immune cells. The cell wall is a vital structure during interaction with the host, and it is involved in several functions, including protection, growth, and adherence (43). Furthermore, one of the strategies used by phagocytes to kill a pathogen is mediated by generation of ROS (44). Among of them, H2O2 contributes to antimicrobial activity by damaging lipids, DNA, and proteins of microbial cells (44). Besides that, the plasma membrane is another site of cellular damage that has been demonstrated in yeast following APDI (45). Since H2O2 does not diffuse freely across cell membranes (27), an increase of membrane permeability after MB-mediated APDI could also be responsible for the increased sensitivity to this agent.
In the present study, we also subjected C. albicans to an antifungal susceptibility test, in order to verify whether sublethal APDI could alter fluconazole activity against this yeast. Indeed we observed that fluconazole was more potent against C. albicans following MB-mediated APDI, as it promoted higher inhibition of growth and a reduction of the MIC. Another research group investigated the effects of APDI in C. albicans previously incubated with antifungal drugs (46). Contrary to our results, APDI was reported not to be augmented by fluconazole, whereas pretreatment of C. albicans with miconazole improved fungistasis and killing by APDI. Several methodological differences, such as strain, PS, and time of drug incubation, may be responsible for these distinct results. Besides, the treatment sequence could also have a big effect, as with any combination therapy. The PS MB can bind to several subcellular localizations (47), causing injury to different cellular structures. Changes in membrane permeability (45), cell wall damage, and lower resistance to additional oxidative stress by oxidizing agents observed following MB-mediated APDI could be responsible for the increased fluconazole activity observed in our study. Furthermore, APDI can induce oxidation of ergosterol and accumulation of oxidized ergosterol derivatives in the plasma membrane, which implies changes occur in the physical properties of the plasma membrane and could adversely affect membrane transport (48). Nevertheless, more studies are needed to clarify the role of the APDI-antifungal agent interaction.
Combination therapies are increasingly being studied in the area of infectious disease, and several compounds that improve the activity of conventional antimicrobial agents have been identified (49, 50). A successful synergistic effect was demonstrated with the combination of fluconazole together with compounds that perturbed membrane and/or cell wall permeability (49). Therefore, our results highlight the possibility of improving activity against C. albicans by combining APDI with conventional antifungal drugs. In addition to enhanced antimicrobial activity and the possibility of reduced drug doses, the decreased incidence of adverse effects and avoidance of selection of resistant strains could also be achieved. A more comprehensive approach is needed to confirm these hypotheses.
Because ROS are one of the major causes of DNA damage and mutations, fungal cells have evolved several repair mechanisms to counteract oxidative DNA damage (39). In addition, studies have failed to demonstrate that microorganisms exposed to APDI show characteristics of genotoxicity induced by APDI (51–53). Our evaluation of daughter cells of C. albicans subjected to APDI correlated with these studies. None of the alterations in the growth curve, germ tube formation, or the ability to cause a disseminated infection observed following APDI was preserved in daughter cells. Fluconazole activity against daughter cells was also similar to that in untreated cells, and the same MIC was observed for both groups.
In summary, our data showed that sublethal MB-mediated APDI inhibited pathogenicity-related characteristics, impaired resistance to the oxidative stress-inducing agent hydrogen peroxide, and damaged cell wall integrity. As a consequence, APDI reduced the ability of C. albicans to cause a systemic infection. On the other hand, the absence of alterations in daughter cells indicated that these effects are transitory. The reduction of the fluconazole MIC following APDI suggested that combination therapies could be a useful approach to treat C. albicans infections.
ACKNOWLEDGMENTS
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (grant 2010/13313-9) and Conselho Nacional de Pesquisa e Desenvolvimento. M. R. Hamblin was supported by U.S. NIH grant R01AI050875. G. Tegos was supported by the NIH (grant 5U54MH084690-02).
Footnotes
Published ahead of print 5 November 2012
REFERENCES
- 1. Cowen LE, Anderson JB, Kohn LM. 2002. Evolution of drug resistance in Candida albicans. Annu. Rev. Microbiol. 56:139–165 [DOI] [PubMed] [Google Scholar]
- 2. Gomez-Lopez A, Zaragoza O, Rodriguez-Tudela JL, Cuenca-Estrella M. 2008. Pharmacotherapy of yeast infections. Expert Opin. Pharmacother. 9:2801–2816 [DOI] [PubMed] [Google Scholar]
- 3. Gualco L, Debbia EA, Bandettini R, Pescetto L, Cavallero A, Ossi MC, Schito AM, Marchese A. 2007. Antifungal resistance in Candida spp. isolated in Italy between 2002 and 2005 from children and adults. Int. J. Antimicrob. Agents 29:179–184 [DOI] [PubMed] [Google Scholar]
- 4. Pfaller MA, Pappas PG, Wingard JR. 2006. Invasive fungal pathogens: current epidemiological trends. Clin. Infect. Dis. 43:S3–S14 [Google Scholar]
- 5. Pfaller MA, Diekema DJ, Jones RN, Messer SA, Hollis RJ. 2002. Trends in antifungal susceptibility of Candida spp. isolated from pediatric and adult patients with bloodstream infections: SENTRY Antimicrobial Surveillance Program, 1997 to 2000. J. Clin. Microbiol. 40:852–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munin E, Giroldo LM, Alves LP, Costa MS. 2007. Study of germ tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT). J. Photochem. Photobiol. B 88:16–20 [DOI] [PubMed] [Google Scholar]
- 7. Soares BM, da Silva DL, Sousa GR, Amorim JC, de Resende MA, Pinotti M, Cisalpino PS. 2009. In vitro photodynamic inactivation of Candida spp. growth and adhesion to buccal epithelial cells. J. Photochem. Photobiol. B 94:65–70 [DOI] [PubMed] [Google Scholar]
- 8. Dai T, Huang YY, Hamblin MR. 2009. Photodynamic therapy for localized infections: state of the art. Photodiagn. Photodyn. Ther. 6:170–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin XH, Wu P, Chen W, Zhang YF, Xia XH. 2007. Electrochemical DNA biosensor for the detection of short DNA species of chronic myelogenous leukemia by using methylene blue. Talanta 72:468–471 [DOI] [PubMed] [Google Scholar]
- 10. Asmundsdottir LR, Erlendsdottir H, Agnarsson BA, Gottfredsson M. 2009. The importance of strain variation in virulence of Candida dubliniensis and Candida albicans: results of a blinded histopathological study of invasive candidiasis. Clin. Microbiol. Infec. 15:576–585 [DOI] [PubMed] [Google Scholar]
- 11. Prates RA, da Silva EG, Yamada AM, Suzuki LC, Paula CR, Ribeiro MS. 2009. Light parameters influence cell viability in antifungal photodynamic therapy in a fluence and rate fluence-dependent manner. Laser Phys. 19:1038–1044 [Google Scholar]
- 12. Jett BD, Hatter KL, Huycke MM, Gilmore MS. 1997. Simplified agar plate method for quantifying viable bacteria. Biotechniques 23:648–650 [DOI] [PubMed] [Google Scholar]
- 13. Thewes S, Moran GP, Magee BB, Schaller M, Sullivan DJ, Hube B. 2008. Phenotypic screening, transcriptional profiling, and comparative genomic analysis of an invasive and non-invasive strain of Candida albicans. BMC Microbiol. 8:187 doi:10.1186/1471-2180-8-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mackenzie DW. 1962. Serum tube identification of Candida albicans. J. Clin. Pathol. 15:563–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Repentigny L. 2004. Animal models in the analysis of Candida host-pathogen interactions. Curr. Opin. Microbiol. 7:324–329 [DOI] [PubMed] [Google Scholar]
- 16. Spellberg B, Ibrahim AS, Edwards JE, Jr, Filler SG. 2005. Mice with disseminated candidiasis die of progressive sepsis. J. Infect. Dis. 192:336–343 [DOI] [PubMed] [Google Scholar]
- 17. Navarathna DH, Hornby JM, Krishnan N, Parkhurst A, Duhamel GE, Nickerson KW. 2007. Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans. Infect. Immun. 75:1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maidan MM, De Rop L, Relloso M, Diez-Orejas R, Thevelein JM, Van Dijck P. 2008. Combined inactivation of the Candida albicans GPR1 and TPS2 genes results in avirulence in a mouse model for systemic infection. Infect. Immun. 76:1686–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodriguez-Tudela JL, Arendrup MC, Barchiesi F, Bille J, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Denning DW, Donnelly JP, Dromer F, Fegeler W, Lass-Florl C, Moore C, Richardson M, Sandven P, Velegraki A, Verweij P. 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398–405 [DOI] [PubMed] [Google Scholar]
- 21. Dai T, Fuchs BB, Coleman JJ, Prates RA, Astrakas C, St Denis TG, Ribeiro MS, Mylonakis E, Hamblin MR, Tegos GP. 2012. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front. Microbiol. 3:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Komerik N, Wilson M, Poole S. 2000. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem. Photobiol. 72:676–680 [DOI] [PubMed] [Google Scholar]
- 23. Tubby S, Wilson M, Nair SP. 2009. Inactivation of staphylococcal virulence factors using a light-activated antimicrobial agent. BMC Microbiol. 9:211 doi:10.1186/1471-2180-9-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chabrier-Rosello Y, Foster TH, Perez-Nazario N, Mitra S, Haidaris CG. 2005. Sensitivity of Candida albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrob. Agents Chemother. 49:4288–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moor AC. 2000. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B 57:1–13 [DOI] [PubMed] [Google Scholar]
- 26. Alby K, Bennett RJ. 2009. Stress-induced phenotypic switching in Candida albicans. Mol. Biol. Cell 20:3178–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Temple MD, Perrone GG, Dawes IW. 2005. Complex cellular responses to reactive oxygen species. Trends Cell Biol. 15:319–326 [DOI] [PubMed] [Google Scholar]
- 28. Flattery-O'Brien JA, Dawes IW. 1998. Hydrogen peroxide causes RAD9-dependent cell cycle arrest in G2 in Saccharomyces cerevisiae whereas menadione causes G1 arrest independent of RAD9 function. J. Biol. Chem. 273:8564–8571 [DOI] [PubMed] [Google Scholar]
- 29. Sasnauskiene A, Kadziauskas J, Vezelyte N, Jonusiene V, Kirveliene V. 2009. Apoptosis, autophagy and cell cycle arrest following photodamage to mitochondrial interior. Apoptosis 14:276–286 [DOI] [PubMed] [Google Scholar]
- 30. Paardekooper M, De Bruijne AW, Van Steveninck J, Van den Broek PJ. 1993. Inhibition of transport systems in yeast by photodynamic treatment with toluidine blue. Biochim. Biophys. Acta 1151:143–148 [DOI] [PubMed] [Google Scholar]
- 31. Paardekooper M, De Bruijne AW, Van Steveninck J, Van den Broek PJ. 1995. Intracellular damage in yeast cells caused by photodynamic treatment with toluidine blue. Photochem. Photobiol. 61:84–89 [DOI] [PubMed] [Google Scholar]
- 32. Lemar KM, Aon MA, Cortassa S, O'Rourke B, Muller CT, Lloyd D. 2007. Diallyl disulphide depletes glutathione in Candida albicans: oxidative stress-mediated cell death studied by two-photon microscopy. Yeast 24:695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petti AA, Crutchfield CA, Rabinowitz JD, Botstein D. 2011. Survival of starving yeast is correlated with oxidative stress response and nonrespiratory mitochondrial function. Proc. Natl. Acad. Sci. U. S. A. 108:E1089–E1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whiteway M, Bachewich C. 2007. Morphogenesis in Candida albicans. Annu. Rev. Microbiol. 61:529–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calderone RA, Fonzi WA. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327–335 [DOI] [PubMed] [Google Scholar]
- 36. Fradin C, De Groot P, MacCallum D, Schaller M, Klis F, Odds FC, Hube B. 2005. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol. Microbiol. 56:397–415 [DOI] [PubMed] [Google Scholar]
- 37. Fekete A, Emri T, Gyetvai A, Gazdag Z, Pesti M, Varga Z, Balla J, Cserhati C, Emody L, Gergely L, Pocsi I. 2007. Development of oxidative stress tolerance resulted in reduced ability to undergo morphologic transitions and decreased pathogenicity in a t-butylhydroperoxide-tolerant mutant of Candida albicans. FEMS Yeast Res. 7:834–847 [DOI] [PubMed] [Google Scholar]
- 38. Ghosh S, Navarathna DH, Roberts DD, Cooper JT, Atkin AL, Petro TM, Nickerson KW. 2009. Arginine-induced germ tube formation in Candida albicans is essential for escape from murine macrophage line RAW 264.7. Infect. Immun. 77:1596–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lorenz MC, Bender JA, Fink GR. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 3:1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Filler SG, Sheppard DC. 2006. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2:e129 doi:10.1371/journal.ppat.0020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Felk A, Kretschmar M, Albrecht A, Schaller M, Beinhauer S, Nichterlein T, Sanglard D, Korting HC, Schafer W, Hube B. 2002. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect. Immun. 70:3689–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Collette JR, Lorenz MC. 2011. Mechanisms of immune evasion in fungal pathogens. Curr. Opin. Microbiol. 14:668–675 [DOI] [PubMed] [Google Scholar]
- 43. Ruiz-Herrera J, Elorza MV, Valentin E, Sentandreu R. 2006. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 6:14–29 [DOI] [PubMed] [Google Scholar]
- 44. Seider K, Heyken A, Luttich A, Miramon P, Hube B. 2010. Interaction of pathogenic yeasts with phagocytes: survival, persistence and escape. Curr. Opin. Microbiol. 13:392–400 [DOI] [PubMed] [Google Scholar]
- 45. Giroldo LM, Felipe MP, de Oliveira MA, Munin E, Alves LP, Costa MS. 2009. Photodynamic antimicrobial chemotherapy (PACT) with methylene blue increases membrane permeability in Candida albicans. Laser Med. Sci. 24:109–112 [DOI] [PubMed] [Google Scholar]
- 46. Snell SB, Foster TH, Haidaris CG. 2012. Miconazole induces fungistasis and increases killing of Candida albicans subjected to photodynamic therapy. Photochem. Photobiol. 88:596–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prates RA, Kato IT, Ribeiro MS, Tegos GP, Hamblin MR. 2011. Influence of multidrug efflux systems on methylene blue-mediated photodynamic inactivation of Candida albicans. J. Antimicrob. Chemother. 66:1525–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bocking T, Barrow KD, Netting AG, Chilcott TC, Coster HGL, Hofer M. 2000. Effects of singlet oxygen on membrane sterols in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 267:1607–1618 [DOI] [PubMed] [Google Scholar]
- 49. Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Ejim L, Rossi L, De Pascale G, Curak J, Brown E, Tyers M, Wright GD. 2011. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol. Syst. Biol. 7:499 doi:10.1038/msb.2011.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Uppuluri P, Nett J, Heitman J, Andes D. 2008. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob. Agents Chemother. 52:1127–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cassidy CM, Donnelly RF, Tunney MM. 2010. Effect of sub-lethal challenge with photodynamic antimicrobial chemotherapy (PACT) on the antibiotic susceptibility of clinical bacterial isolates. J. Photochem. Photobiol. B 99:62–66 [DOI] [PubMed] [Google Scholar]
- 52. Griffiths MA, Wren BW, Wilson M. 1997. Killing of methicillin-resistant Staphylococcus aureus in vitro using aluminium disulphonated phthalocyanine, a light-activated antimicrobial agent. J. Antimicrob. Chemother. 40:873–876 [DOI] [PubMed] [Google Scholar]
- 53. Lauro FM, Pretto P, Covolo L, Jori G, Bertoloni G. 2002. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem. Photobiol. Sci. 1:468–470 [DOI] [PubMed] [Google Scholar]