Abstract
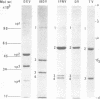
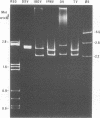
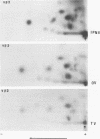
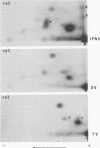
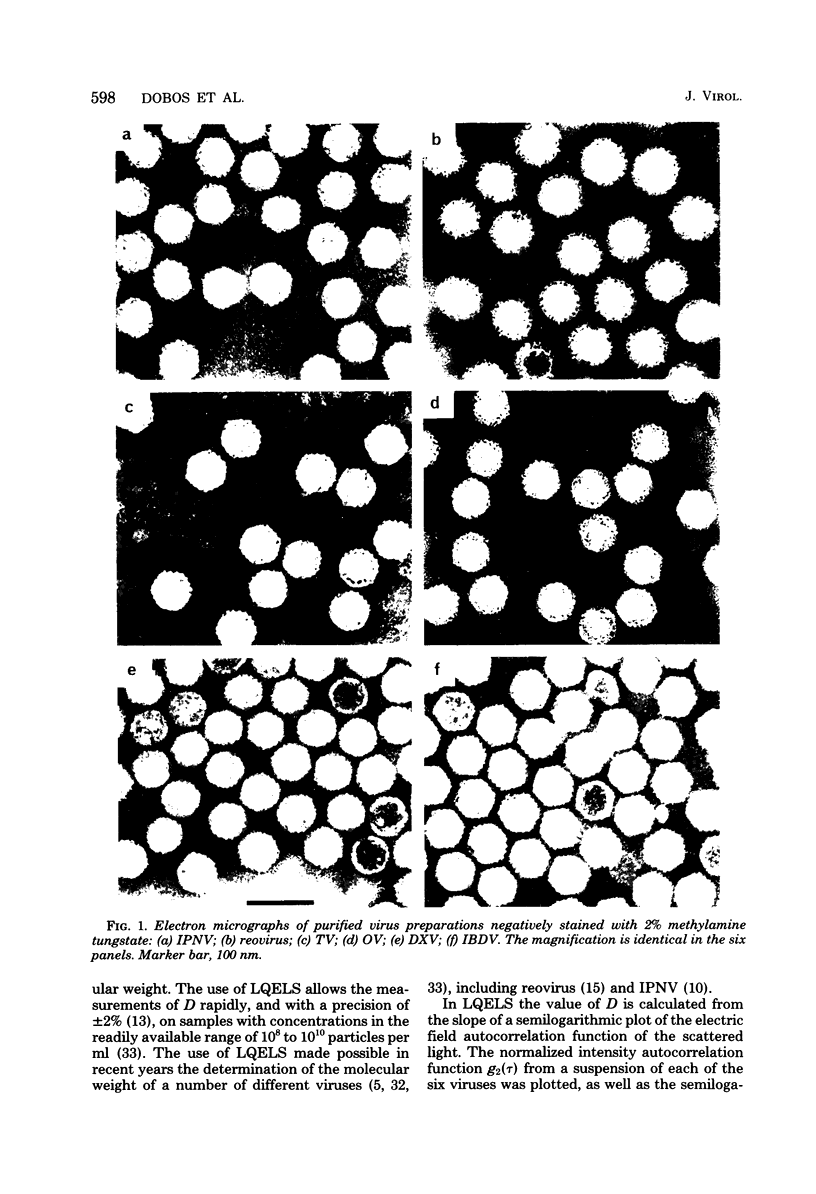
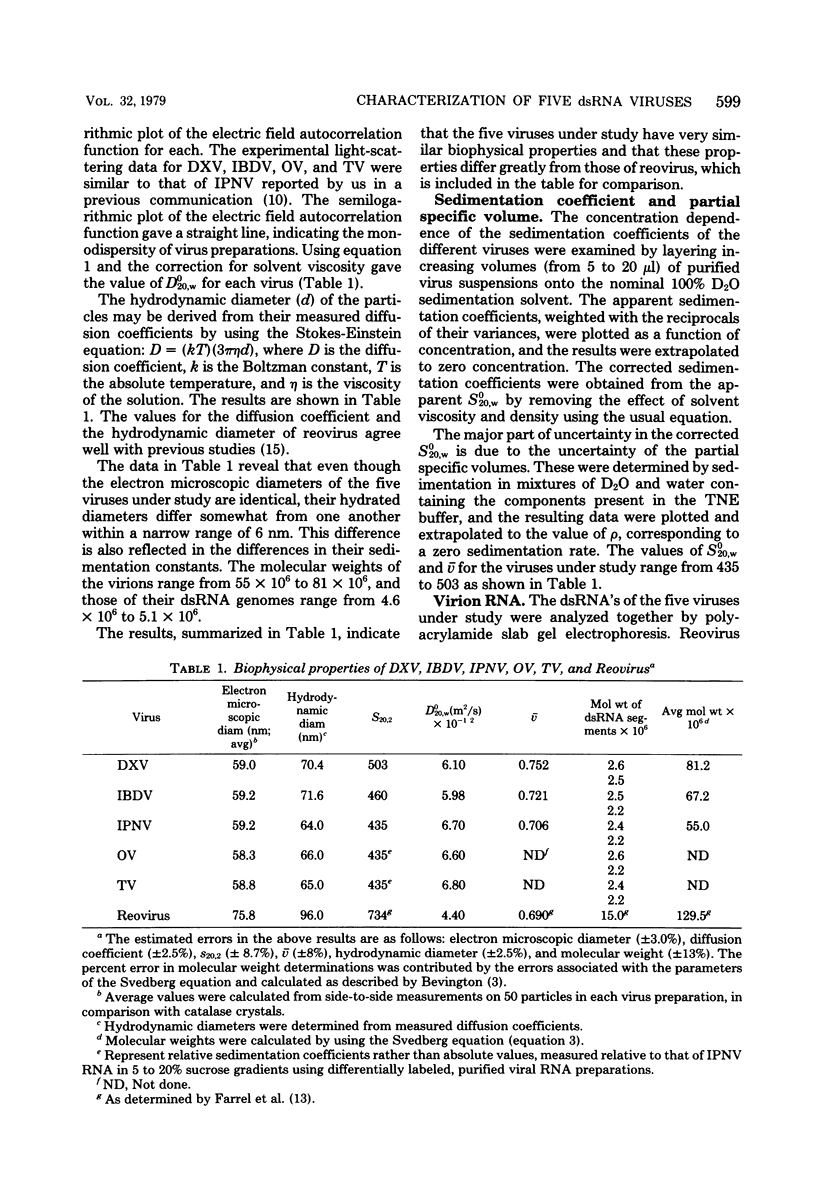
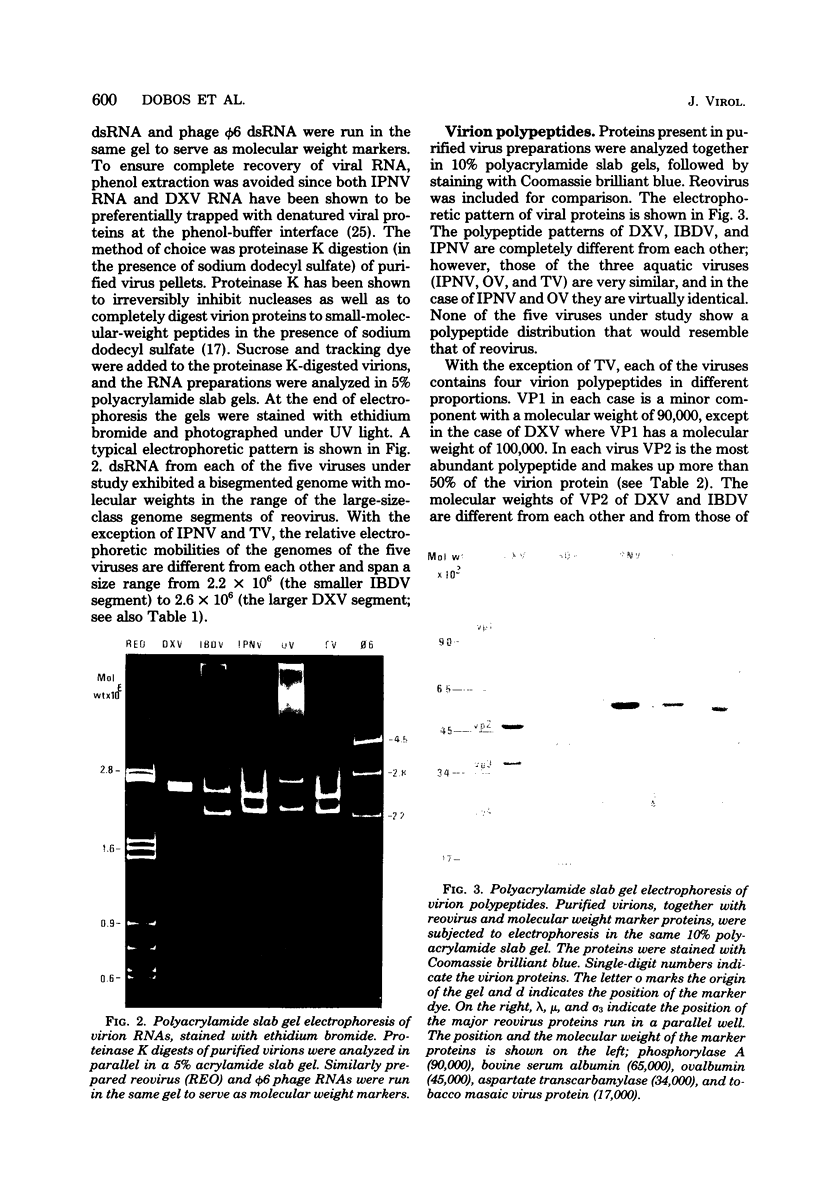
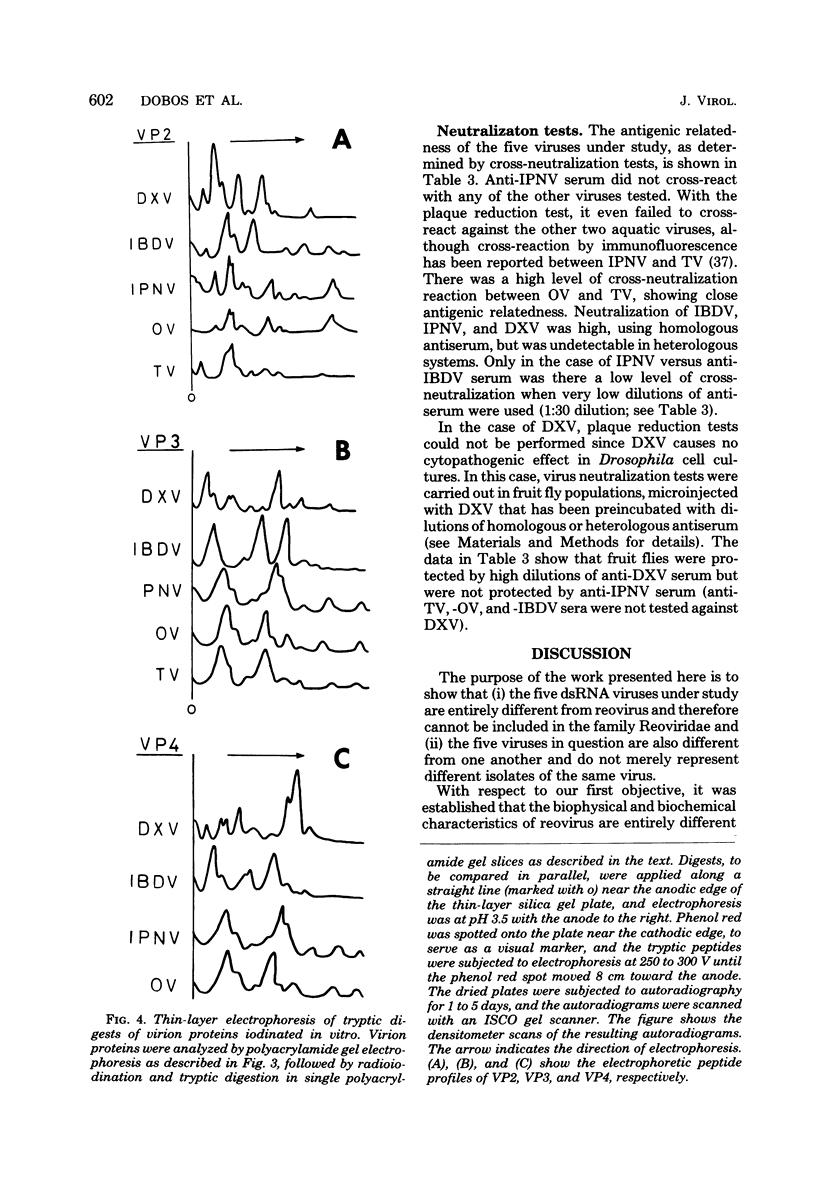
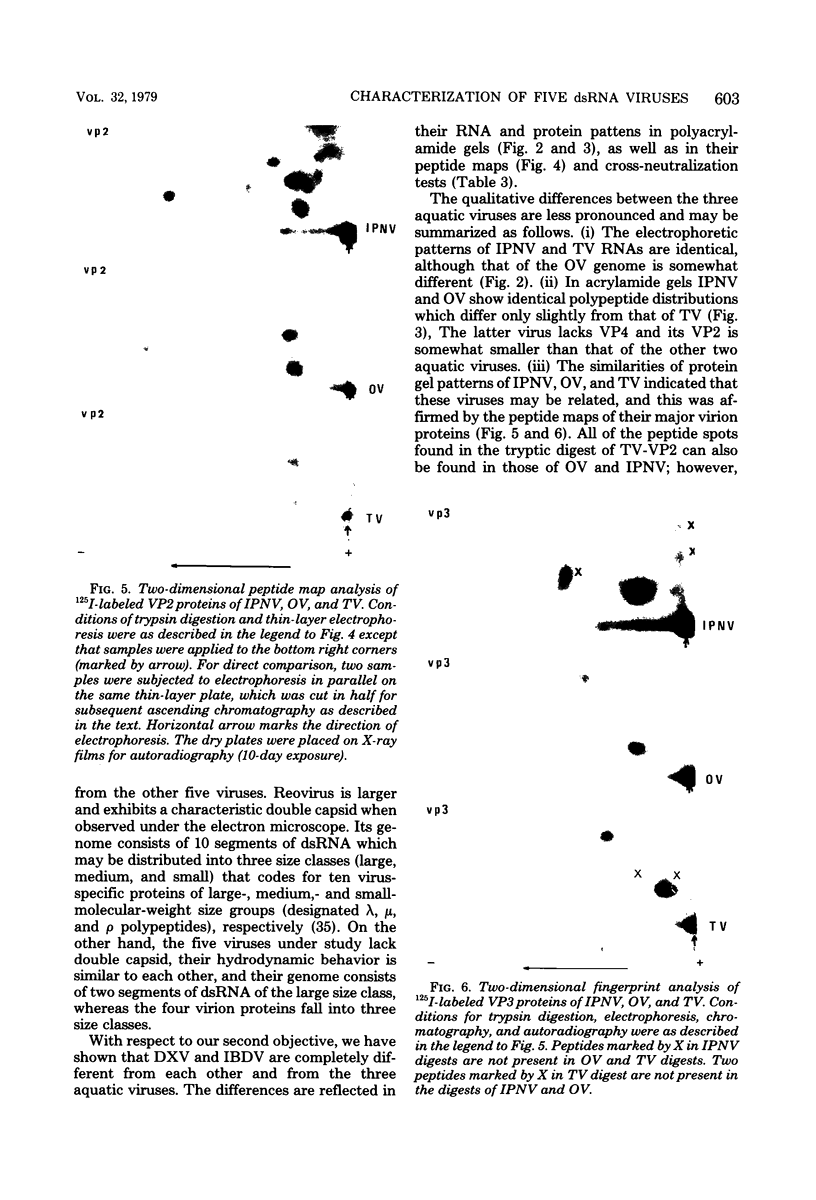
Infectious pancreatic necrosis virus of fish, infectious bursal disease virus of chickens, Tellina virus and oyster virus of bivalve molluscs, and drosophila X virus of Drosophila melanogaster are naked icosahedral viruses with an electron microscopic diameter of 58 to 60 nm. The genome of each of these viruses consists of two segments of double-stranded RNA (molecular weight range between 2.6 x 10(6) and 2.2 x 10(6), and the virion, capsid proteins fall into three size class categories (large, medium, and small; ranging from 100,000 to 27,000) as determined by polyacrylamide slab gel electrophoresis. The hydrodynamic properties of the five viruses are similar as determined by analytical ultracentrifugation and laser quasi-elastic, light-scattering spectroscopy. The calculated particle weights range between 55 x 10(6) and 81 x 10(6). Tryptic peptide comparisons of 125I-labeled virion proteins showed that five viruses are different from each other, although there was considerable overlap in the peptide maps of the three aquatic viruses, indicting a degree of relatedness. Cross-neutralization tests indicated that drosophila X, infectious pancreatic necrosis, and infectious bursal disease viruses were different from each other and from oyster and Tellina viruses. The same test showed oyster and Tellina viruses to be related. The biochemical and biophysical properties of the five viruses cannt be included in the family Reoviridae or in any of the present virus genera.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alayse A. M., Cohen J., Scherrer J. Etude de la synthèse des ARN viraus dans les cellules FHM et RTG-2 infectées par le virus de la nécrose pancréatique infectieuse (NPI) Ann Microbiol (Paris) 1975 Dec;126(4):471–483. [PubMed] [Google Scholar]
- Burness A. T., Clothier F. W. Particle weight and other biophysical properties of encephalomyocarditis virus. J Gen Virol. 1970 Mar;6(3):381–393. doi: 10.1099/0022-1317-6-3-381. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Pusey P. N., Koppel D. E., Schaefer D. W., Franklin R. M. Intensity fluctuation spectroscopy of laser light scattered by solutions of spherical viruses: R17, Q beta, BSV, PM2, and T7. II. Diffusion coefficients, molecular weights, solvation, and particle dimensions. Biochemistry. 1974 Feb 26;13(5):960–970. doi: 10.1021/bi00702a021. [DOI] [PubMed] [Google Scholar]
- Cohen J., Poinsard A., Scherrer R. Physico-chemical and morphological features of infectious pancreatic necrosis virus. J Gen Virol. 1973 Dec;21(3):485–498. doi: 10.1099/0022-1317-21-3-485. [DOI] [PubMed] [Google Scholar]
- Dobos P., Hallett R., Kells D. T., Sorensen O., Rowe D. Biophysical studies of infectious pancreatic necrosis virus. J Virol. 1977 Apr;22(1):150–159. doi: 10.1128/jvi.22.1.150-159.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P., Rowe D. Peptide map comparison of infectious pancreatic necrosis virus-specific polypeptides. J Virol. 1977 Dec;24(3):805–820. doi: 10.1128/jvi.24.3.805-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Size and structure of the genome of infectious pancreatic necrosis virus. Nucleic Acids Res. 1976 Aug;3(8):1903–1924. doi: 10.1093/nar/3.8.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Virus-specific protein synthesis in cells infected by infectious pancreatic necrosis virus. J Virol. 1977 Jan;21(1):242–258. doi: 10.1128/jvi.21.1.242-258.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Farrell J. A., Harvey J. D., Bellamy A. R. Biophysical studies of reovirus type 3. Virology. 1974 Nov;62(1):145–153. doi: 10.1016/0042-6822(74)90310-9. [DOI] [PubMed] [Google Scholar]
- Harvey J. D., Farrell J. A., Bellamy A. R. Biophysical studies of reovirus type 3. II. Properties of the hydrated particle. Virology. 1974 Nov;62(1):154–160. doi: 10.1016/0042-6822(74)90311-0. [DOI] [PubMed] [Google Scholar]
- Hilz H., Wiegers U., Adamietz P. Stimulation of proteinase K action by denaturing agents: application to the isolation of nucleic acids and the degradation of 'masked' proteins. Eur J Biochem. 1975 Aug 1;56(1):103–108. doi: 10.1111/j.1432-1033.1975.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Johnson P. T., Bodammer J. E. A disease of the blue crab, Callinectes sapidus, of possible viral etiology. J Invertebr Pathol. 1975 Jul;26(1):141–143. doi: 10.1016/0022-2011(75)90185-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Independent translation of the genes of bacteriophage f2 RNA. J Mol Biol. 1968 Mar 28;32(3):681–685. doi: 10.1016/0022-2836(68)90351-3. [DOI] [PubMed] [Google Scholar]
- Macdonald R. D., Roy K. L., Yamamoto T., Chang N. Oligonucleotide fingerprints of the RNAs from infectious pancreatic necrosis virus. Arch Virol. 1977;54(4):373–377. doi: 10.1007/BF01314782. [DOI] [PubMed] [Google Scholar]
- Macdonald R. D., Yamamoto T. The structure of infectious pancreatic necrosis virus RNA. J Gen Virol. 1977 Feb;34(2):235–247. doi: 10.1099/0022-1317-34-2-235. [DOI] [PubMed] [Google Scholar]
- Malsberger R. G., Cerini C. P. Multiplication of infectious pancreatic necrosis virus. Ann N Y Acad Sci. 1965 Aug 10;126(1):320–327. doi: 10.1111/j.1749-6632.1965.tb14283.x. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Müller H., Scholtissek C., Becht H. The genome of infectious bursal disease virus consists of two segments of double-stranded RNA. J Virol. 1979 Sep;31(3):584–589. doi: 10.1128/jvi.31.3.584-589.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick H., Cursiefen D., Becht H. Structural and growth characteristics of infectious bursal disease virus. J Virol. 1976 Apr;18(1):227–234. doi: 10.1128/jvi.18.1.227-234.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Electrophoretic separation of all components of the double-stranded RNA of wound tumor virus. Virology. 1973 Aug;54(2):557–562. doi: 10.1016/0042-6822(73)90168-2. [DOI] [PubMed] [Google Scholar]
- Salmeen I., Rimai L., Liebes L., Rich M. A., McCormick J. J. Hydrodynamic diameters of RNA tumor viruses. Studies by laser beat frequency light scattering spectroscopy of avian myeloblastosis and Rauscher murine leukemia viruses. Biochemistry. 1975 Jan 14;14(1):134–141. doi: 10.1021/bi00672a023. [DOI] [PubMed] [Google Scholar]
- Salmeen I., Rimai L., Luftig R. B., Libes L., Retzel E., Rich M., McCormick J. J. Hydrodynamic diameters of murine mammary, Rous sarcoma, and feline leukemia RNA tumor viruses: studies by laser beat frequency light-scattering spectroscopy and electron microscopy. J Virol. 1976 Feb;17(2):584–596. doi: 10.1128/jvi.17.2.584-596.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Underwood B. O., Smale C. J., Brown F., Hill B. J. Relationship of a virus from Tellina tenuis to infectious pancreatic necrosis virus. J Gen Virol. 1977 Jul;36(1):93–109. doi: 10.1099/0022-1317-36-1-93. [DOI] [PubMed] [Google Scholar]