Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) has become a major source of infection in hospitals and in the community. Increasing antibiotic resistance in S. aureus strains has created a need for alternative therapies to treat disease. A component of the licorice root Glycyrrhiza spp., 18β-glycyrrhetinic acid (GRA), has been shown to have antiviral, antitumor, and antibacterial activity. This investigation explores the in vitro and in vivo effects of GRA on MRSA pulsed-field gel electrophoresis (PFGE) type USA300. GRA exhibited bactericidal activity at concentrations exceeding 0.223 μM. Upon exposure of S. aureus to sublytic concentrations of GRA, we observed a reduction in expression of key virulence genes, including saeR and hla. In murine models of skin and soft tissue infection, topical GRA treatment significantly reduced skin lesion size and decreased the expression of saeR and hla genes. Our investigation demonstrates that at high concentrations GRA is bactericidal to MRSA and at sublethal doses it reduces virulence gene expression in S. aureus both in vitro and in vivo.
INTRODUCTION
Staphylococcus aureus is a significant cause of morbidity and mortality worldwide. This ubiquitous Gram-positive bacterium can cause diseases ranging from mild to life threatening (1, 2). Methicillin-resistant S. aureus (MRSA), once restricted to the hospital setting, is now also common in the community (1). MRSA is a leading cause of infections presenting to emergency departments across the United States, accounting for ∼59% of skin and soft tissue infections (3). In addition, mortality caused by MRSA in the United States from 2001 to 2005 exceeded deaths caused by AIDS and tuberculosis combined (4). Infections caused by MRSA limit treatment options, since these strains are resistant to the entire class of β-lactam antibiotics. In addition, these strains can demonstrate additional resistance to other antibiotics such as clindamycin and erythromycin (1). Vancomycin remains the antibiotic used for definitive therapy (1). However, in 2002 the United States recorded its first case of vancomycin-resistant S. aureus (5). Thus, it is readily apparent that the continued use of currently available antibiotics against this bacterium runs the risk of completely exhausting their efficacies (1).
As an alternative to antibiotic use, there has been increased interest in finding nontraditional treatment methods, including natural compounds, that are effective against bacterial infections (1, 6). One natural substance that has shown some antimicrobial promise is a triterpenoid saponin isolated from Glycyrrhiza spp., of the licorice family (7). The isolated glycyrrhizic acid (GA) is considered metabolically inactive in vivo and is hydrolyzed to 18-β-glycyrrhetinic acid (GRA) by commensal gut bacteria (8–10), at which point it can be absorbed into the bloodstream (9, 10). GA, GRA, and various licorice extracts have previously been shown to have antitumor, antiviral, antibacterial, and anti-inflammatory activities. GA inhibits growth of herpes simplex type 1 in human cells, prevents the hepatitis A virus from penetrating cells, and inhibits HIV replication (11, 12). Glycyrrhizin was shown to have anti-inflammatory properties as demonstrated by decreased production of reactive oxygen species (ROS) in human neutrophils (13). GRA can induce cell death in some tumor cells, such as primary effusion lymphoma cells transformed by Kaposi sarcoma-associated herpesvirus, possibly through the release of mitochondrial apoptotic factors (14–16). Additionally, licorice flavonoids were shown to increase the susceptibility of MRSA strains to oxacillin, a β-lactam antibiotic (17). A crude chloroform fraction of licorice root decreased the expression of the methicillin resistance genes mecA, mecI, and mecRI (18). However, no clear mode of action has been defined for any of these effects. Collectively, these studies illustrate that various types of licorice extracts have antipathogen activity and may modulate the host and pathogen response.
In this study, we increase our understanding of the antimicrobial potential of GRA and demonstrate that GRA has potent bactericidal activity against MRSA in vitro. Additionally, we show that GRA reduces lesion size in vivo using a mouse model of S. aureus skin infection and demonstrate that GRA reduces expression of key staphylococcal virulence factors both in vitro and in vivo, including hla and saeRS. These data show potential for GRA to treat MRSA skin infections and demonstrate that in addition to having antimicrobial activity, GRA reduces expression of key virulence factors that may attenuate pathology caused by MRSA.
MATERIALS AND METHODS
Bacterial strains and culture.
S. aureus cultures were grown in tryptic soy broth (TSB) containing 0.5% glucose. Cultures were inoculated from overnight growth at a dilution of 1/200 and grown at 37°C with shaking at 250 rpm. For all experiments, cultures were grown to mid-exponential phase (optical density at 600 nm [OD600] = 1.5). Two strains were used for the studies: pulsed-field gel electrophoresis types USA300 (LAC) and USA400 (MW2). For in vitro studies, cultures were washed and resuspended in TSB or RPMI 1640 medium (Cellgro) containing 10 mM HEPES to 108/ml. For in vivo studies, cultures were washed and resuspended in sterile Dulbecco's phosphate-buffered saline (DPBS) (Cellgro).
Glycyrrhizic acid and 18-β-glycyrrhetinic acid.
Stocks of GA (Fluka Analytical) and GRA (Aldrich) were suspended in 100% dimethyl sulfoxide (DMSO) (Sigma). The stocks were frozen in aliquots of 25 μl at 25 mg/ml. Stocks were diluted to the appropriate concentrations in the media indicated by the experiment. For in vivo studies, GA and GRA were prepared in DMSO at 60 mg/ml and 120 mg/ml.
Bactericidal assays.
Bacteria (2 ×105) were resuspended in TSB with varied concentrations of GA and GRA, diluted in TSB, in a 96-well tissue culture plate. The plate was incubated for 1 h at 37°C, and S. aureus was plated onto tryptic soy agar (TSA) (EMD). At indicated time points, samples were serially diluted (1:10) in water, and CFU were enumerated the next day. The MIC of GRA was determined using the agar plate dilution method (19).
Bacterial growth kinetics.
Overnight cultures were diluted 1:200 in 20 ml TSB with varied concentrations of GA and GRA, in a 100-ml flask. Cultures were incubated at 37°C with shaking (250 rpm) for 6 h. The OD600 of cultures was recorded at 1-h intervals using a Nanodrop 2000; bacteria were plated onto tryptic soy agar (TSA), and CFU were enumerated the next day.
In vitro gene expression.
Bacteria (∼2 × 107) were resuspended in TSB containing 52.5 μg/ml of GA or GRA or in TSB alone. Samples were incubated at 37°C for 1 h, and RNA was harvested and subjected to TaqMan real-time reverse transcriptase PCR (RT-PCR), as described previously (20–22). The relative quantification of genes was determined by changes in expression of transcripts relative to expression in untreated bacteria. Samples were normalized to the housekeeping gene gyrB. Data are expressed as change in transcript level after treatment with GA or GRA. The sequences of the primers and probes used for analysis are listed in Table 1.
Table 1.
List of probes and primers
| Gene | Probe or primer sequence |
|---|---|
| gyrB probe | 5′-AATCGGTGGCGACTTTGATCTAGCGAAAG-3′ |
| gyrB fwd | 5′-CAAATGATCACAGCTTTGGTACAG-3′ |
| gyrB rvs | 5′-CGGCATCAGTCATAATGACGAT-3′ |
| saeR probe | 5′-ATTTACGCCTTAACTTTAGGTGCAGAT-3′ |
| saeR fwd | 5′-CTGCCAAAACACAAGAACATGATAC-3′ |
| saeR rvs | 5′-ATTTACGCCTTAACTTTAGGTGCAGAT-3′ |
| hla probe | 5′-ATGAATCCTGTCGCTAATGCCGCAGA-3′ |
| hla fwd | 5′-CAACAACACTATTGCTAGGTTCCATATT-3′ |
| hla rvs | 5′-CCTGTTTTTACTGTAGTATTGCTTCCA-3′ |
| RNAIII probe | 5′-TGCACAAGATATCATTTCAACAATCAGTGACTTAGTAAAA-3′ |
| RNAIII fwd | 5′-GTGATGGAAAATAGTTGATGAGTTGTTT-3′ |
| RNAIII rvs | 5′-GAATTTGTTCACTGTGTCGATAATCC-3′ |
| mecA probe | 5′-ATCTATAGCGCATTAGAAAA-3′ |
| mecA fwd | 5′-ACTGATTAACCCAGTACAGATCCTTTC-3′ |
| mecA rvs | 5′-TCCAAACTTTGTTTTTCGTGTCTTT-3′ |
| sbi probe | 5′-CAGGTAGCTTTATGGTTGCTACAAAAAT-3′ |
| sbi fwd | 5′-ATACATCAAAACATTACGCGAACAC-3′ |
| sbi rvs | 5′-CTGGGTTCTTGCTGTCTTTAAGTG-3′ |
Mouse model of skin infection.
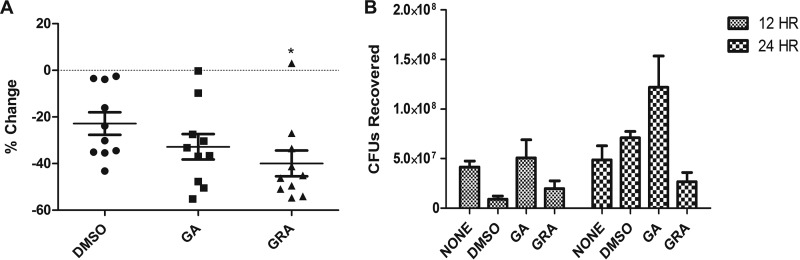
All animal studies conformed to the National Institutes of Health guidelines and were approved by the Montana State University Institutional Animal Care and Use Committee. Female Crl;SKH1-hrBR hairless mice were purchased from Charles River Laboratories. Mice were inoculated subcutaneously with 2 × 107 CFU of S. aureus, LAC strain, in sterile PBS. Mice were treated daily beginning on day 3 with topical application of 600 μg GA or GRA in DMSO or DMSO alone. Abscess area was calculated daily, and the percent change shown in Fig. 2 was calculated over a four-day period with the formula % change = (size on day 7 − size on day 3)/size on day 3 × 100.
Fig 2.
GRA attenuates MRSA severity during skin infection. Mice (n = 10) were inoculated subcutaneously with 2 × 107 CFU of LAC. (A) Percent reduction in abscess size following daily treatment with DMSO, GRA (600 μg/ml), or GA (600 μg/ml) from day 3 to day 7. Percent change in size was calculated over a 4-day period (day 3 through day 7) using the formula % change = (size on day 7 − size on day 3)/size on day 3 × 100 (*, P < 0.05 as determined by ANOVA and Tukey's posttest when comparing DMSO- and GRA-treated mice). (B) Survival of LAC in skin at 12 and 24 h postinfection. Mice (n = 3) were infected with 2 × 107 CFU of LAC and treated with topical application of DMSO, GRA (600 μg/ml), or GA (600 μg/ml) 1 h postinfection, and tissues were harvested at 12 or 24 h postinfection. There was no significant difference in numbers of CFU recovered in GRA-treated mice compared to numbers of CFU from DMSO-treated or untreated mice.
For gene expression and cytokine assays, mice were treated immediately after inoculation and then daily. Mice were then sacrificed at designated time points, and abscesses were excised using a 9-mm punch and weighed. Excised material was homogenized in sterile PBS and used to determine CFU, to analyze changes in gene expression, and to measure cytokine expression. For gene expression analysis, homogenate was centrifuged for 5 min at 7,000 rpm, the supernatant was removed, and the pellet was resuspended in RNA Protect (Qiagen) until purified and analyzed as previously described (20–22).
Cytokine assays.
A cytometric bead array (CBA) (BD Biosciences) was used to determine the concentration of interleukin 10 (IL-10), IL-17A, TNF-α, gamma interferon (IFN-γ), IL-6, IL-4, and IL-2, and Bio-Plex cytokine assays (Bio-Rad) were used to determine the levels of IL-1α, IL-1β, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), KC, MIP-1α, and MIP-1β from supernatants of excised infected material. Abscesses were homogenized in sterile PBS, and the supernatant from the resulting homogenate was subjected to a CBA or Bio-Plex assay to determine the presence of cytokines in the abscess. All assays were performed according to the manufacturer's instructions.
Statistical analyses.
All data sets were analyzed using GraphPad Prism, version 5 for PC (GraphPad Software, San Diego, CA), using one-way analysis of variance (ANOVA) with posttest or two-tailed t test as indicated.
RESULTS
GRA attenuates survival of MRSA in vitro.
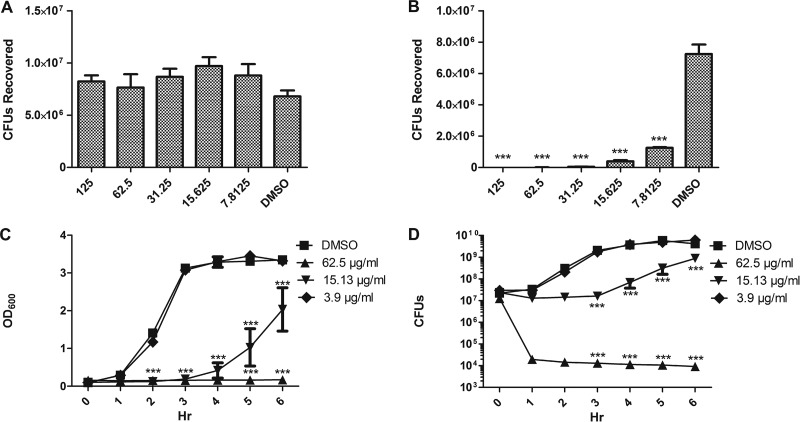
To further investigate the antimicrobial activities of purified GA and GRA, we incubated S. aureus pulsed-field gel electrophoresis (PFGE) type USA300 strain LAC (a MRSA strain), for 1 h in TSB with varied concentrations of GA or GRA. (DMSO was used for control wells.) Following incubation with GA, growth exceeded initial inoculum at all concentrations (Fig. 1A). However, following incubation with GRA, there was a significant decrease in the numbers of CFU recovered from all concentrations tested, compared to the numbers of CFU recovered following incubation with DMSO (Fig. 1B). The effect of bacterial growth was dose dependent, ranging from an average of 5.7 ×102 CFU recovered after exposure to 125 μg/ml to an average of 1.3 ×106 CFU recovered after exposure to 7.8125 μg/ml. Bacterial recovery in the control group averaged 7 × 106 CFU. Similar results were found upon investigating the effects of GA and GRA on bacterial growth using an additional strain of MRSA, PFGE type USA400 (strain MW2; data not shown). These data suggest that GRA has antimicrobial activity against MRSA in vitro.
Fig 1.
GRA inhibits MRSA growth in a dose-dependent manner. MRSA strain LAC was grown to mid-exponential phase and resuspended in TSB (2 ×105 CFU) and incubated with varied concentrations of GA or GRA. CFU recovered following incubation for 1 h with varied concentrations of GA (A) or GRA (B). (C and D) OD600 (C) and CFU (D) values recovered at each hour following incubation of 2 × 107 CFU LAC with varied concentrations of GRA over a 6-h time course. ***, P < 0.001, as determined by one-way ANOVA with Tukey's posttest (A and B) or two-way ANOVA with Bonferroni comparison (C and D) compared to bacteria grown in DMSO (data are from three separate experiments).
To determine if GRA demonstrated antimicrobial activity over a 6-h time course, bacteria were incubated in TSB with DMSO or TSB containing varied concentrations of GA and GRA for 6 h at 37°C, with shaking. The optical density at 600 nm (OD600) was measured every hour, and the bacteria were plated to determine numbers of CFU. The growth over a 6-h time period is shown in Fig. 1C and D, along with correlated optical densities. As observed in the previous experiment, GA had no effect on bacterial survival (data not shown). GRA demonstrated concentration-dependent inhibition of growth (Fig. 1C and D). At the highest concentration of 62.5 μg/ml, the numbers of CFU recovered following the 6-h incubation were significantly decreased compared to those of the control at 3, 4, 5, and 6 h. This trend was also evident when bacteria were incubated with 15.625 μg/ml GRA. At 3.9 μg/ml, the antimicrobial effect of GRA was no longer evident and the numbers of bacteria recovered (at every time point) were similar to those of the control group. Similar results were found with strain MW2 (data not shown). Finally, we used the agar plate dilution method to determine the MIC of GRA and GA. The MIC for GRA was found to be 60 μg/ml, based on three independent experiments, and GA did not inhibit growth at any of the concentrations tested (data not shown). This correlated with data obtained from the growth curves, demonstrating no visible growth of bacteria at 62.5 μg/ml GRA. These results are congruent with previously published results on the antibacterial effects of crude licorice extracts (18, 23–25).
GRA reduces severity of MRSA skin infection.
Since GRA had antimicrobial activity in vitro, we used a mouse model of staphylococcal skin and soft tissue infection to determine the effect of topical treatment of GRA on the severity of skin infections. Mice were infected subcutaneously with S. aureus (USA300). The injection site was treated daily with a topical application of 600 μg GA or GRA suspended in 5 μl DMSO or with DMSO alone. To calculate the effect of GRA on staphylococcal skin disease, we determined the percent change in abscess size daily. Treatment with GRA reduced the size of the abscess over time versus DMSO treatment. From day 3 to day 7, GRA treatment significantly reduced the size of the abscesses by 39.97% ± 5.53% compared to DMSO treatment (Fig. 2A).
Since GRA reduced bacterial survival in vitro, we next investigated whether GRA reduced bacterial survival in vivo. One hour after infection, mice were treated with 600 μg GA or GRA suspended in DMSO or DMSO alone or were given no treatment. At 12 and 24 h after infection, mice were euthanized, the site of infection was excised, and CFU were enumerated. There was no significant difference between the numbers of bacterial CFU recovered from any of the other treatment groups at 12 h and 24 h (Fig. 2B).
GRA modulates the local host response to MRSA infection.
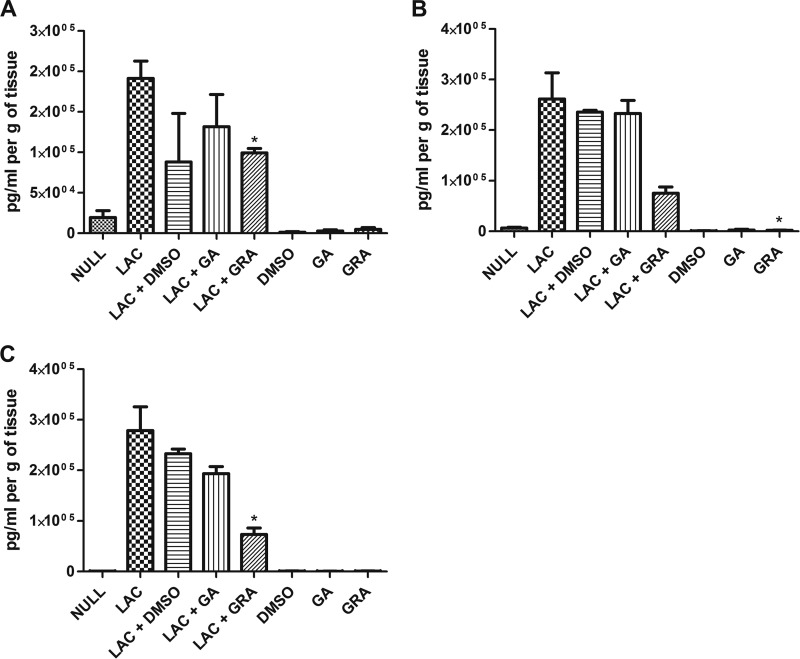
Previous studies have shown that GA has little effect on neutrophil function and the proinflammatory cytokines IL-1β and IL-6 but contributes to anti-inflammatory responses by inhibition of signaling pathways such as the PI3K/Akt pathway and by inhibiting the induction of IL-12 and IFN-γ (13, 26, 27). GRA has been shown to inhibit the classical complement pathway, reduce IL-6 expression, and inhibit inflammation also via the PI3K/Akt pathway (9, 28). However, no studies have been published that explore the effects of either of these compounds or of crude licorice root extracts on immune responses in the skin. Since treatment with GRA reduced the severity of infection but did not significantly reduce bacterial burden, we hypothesized that GRA could modulate the host response. Following skin infection and treatment with GRA, GA, or DMSO alone, we measured the expression of 13 cytokines via cytometric bead array, including IL-10, IL-17A, TNF-α, IFN-γ, IL-6, IL-4, IL-2, IL-1α, IL-1β, G-CSF, GM-CSF, KC, and MIP-1β. Of the 13 cytokines investigated, only two, KC and G-CSF, demonstrated significant differences between GRA-treated mice and DMSO-treated mice. At 12 h postinoculation, the level of KC in the tissue homogenate of the infected but untreated mice was significantly increased compared to the expression of KC in the infected and GRA-treated mice (191,447 ± 21,386 pg/ml per g of tissue and 99,301 ± 5,414 pg/ml per g of tissue, respectively) (Fig. 3A). At 24 h posttreatment, the level of KC in the tissue homogenate of the uninfected and untreated mice was significantly increased compared to the level of KC in the uninfected but GRA-treated mice (6,572 ± 1,101 pg/ml per g of tissue and 2,141 ± 466.3 pg/ml per g of tissue, respectively) (Fig. 3B). At 24 h postinoculation, the expression of G-CSF in the infected but untreated mice was significantly increased compared to the level of G-CSF in the infected and GRA-treated mice (278,877 ± 46,797 pg/ml per g tissue and 73,083 ± 13,091 pg/ml per g of tissue, respectively) (Fig. 3C). The decrease in KC and G-CSF in infected and GRA-treated mice suggests an anti-inflammatory effect of GRA during MRSA skin and soft tissue infection, supporting previously published data that show the anti-inflammatory effects of licorice root compounds, including reducing the expression of inflammatory cytokines such as IL-6 and IL-1, inhibition of complement, and reducing reactive oxygen species levels in neutrophils (9, 13, 26, 28).
Fig 3.
GRA induces altered cytokine responses in skin. (A and B) Concentration of KC at site of inoculation/treatment 12 h postinfection with 2 × 107 LAC (*, P < 0.05 as determined by two-tailed t test when comparing infected and untreated mice [LAC] to infected and GRA-treated mice [LAC+GRA]) (A) and 24 h postinfection (*, P < 0.05 as determined by two-tailed t test when comparing uninfected and untreated mice [NULL] to uninfected and GRA-treated mice [GRA]) (B). (C) Concentration of G-CSF at site of inoculation/treatment 24 h postinoculation (*, P < 0.05 as determined by two-tailed t test when comparing infected and untreated mice [LAC] to infected and GRA-treated mice [LAC+GRA]). Results are from two to three mice per treatment group.
GRA alters gene expression of MRSA in vitro.
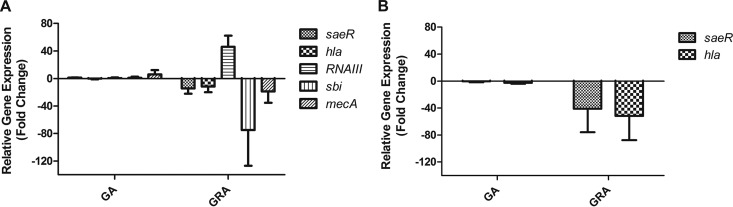
Previous studies have shown the effects of various licorice extracts on MRSA gene expression in vitro, including changes in genes such as mecA and hla (18, 29). To determine if GA or GRA had any influence on virulence factor expression, we incubated USA300 with a sublethal dose of GRA or GA (52.5 μg/ml) and measured transcript abundance of key S. aureus virulence factors saeR, hla, RNAIII, sbi, and mecA 1 h postexposure. GA had a negligible effect on transcript abundance of all genes investigated in both strains (Fig. 4A and B). Transcript abundance of saeR, the regulatory gene component of the global virulence regulatory system SaeR/S, which is essential in the development of staphylococcal skin lesions in mice (21), was downregulated 14-fold following incubation with GRA versus incubation with control in USA300 (Fig. 4A). hla, encoding alpha-toxin, a virulence factor responsible for dermonecrosis in mouse skin infections (30), was downregulated 11-fold compared to that in the control in USA300 (Fig. 4A). sbi, a gene encoding an immunomodulatory protein important in antibody and complement evasion (31), was downregulated 74-fold (Fig. 4A). In addition, mecA, which encodes altered penicillin-binding protein 2a to confer resistance to β-lactam antibiotics, was downregulated 18-fold compared to that in controls (Fig. 4A). The only gene tested that was upregulated upon incubation with GRA was RNAIII transcript, the effector gene of the quorum-sensing Agr two-component regulatory system (over 46-fold increase) (Fig. 4A). To confirm GRA-downregulated transcription of key virulence factors, we measured the fold change in saeR and hla transcripts in an additional MRSA strain, PFGE type USA400 (MW2). saeR was downregulated 40-fold and hla was downregulated 50-fold after incubation with GRA compared to incubation with DMSO (Fig. 4B). These results further support our observations that GRA reduces S. aureus virulence. The reduction in expression of S. aureus virulence genes, including the SaeR/S virulence regulatory system, suggests that GRA may differentially regulate an even larger number of S. aureus genes and supports the hypothesis that reduced virulence gene expression may correlate with attenuated pathology observed in the skin infection model.
Fig 4.
GRA alters S. aureus virulence gene expression in vitro. (A) Fold change of five S. aureus virulence genes after 1-h incubation of 2 × 107 LAC with GA or GRA. (B) Fold change of saeR and hla after 1-h incubation of 2 × 107 MW2 cells with GA or GRA. Data are normalized to gyrB transcript abundance, and fold change is relative to S. aureus incubated in medium alone (time matched). Data are from five (saeR) or three (all other genes) experiments.
GRA alters gene expression of key MRSA virulence factors in vivo during murine skin infection.
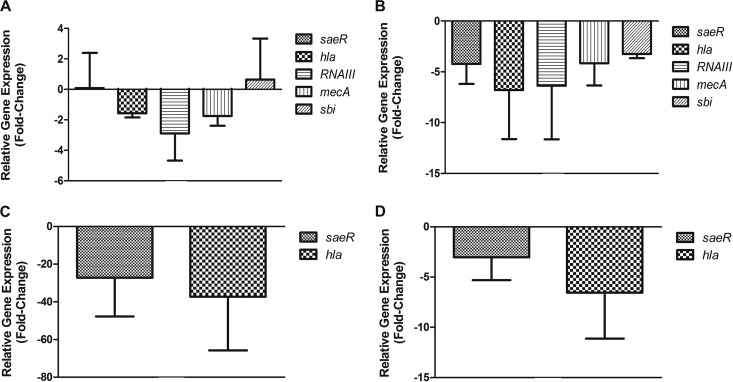
Based on the size of the abscesses, the severity of MRSA skin infection was decreased upon treatment with GRA (Fig. 2A), and this reduction was not due to a significant decrease in bacterial burden (Fig. 2B). GRA reduced expression of key neutrophil recruiting cytokines which may have contributed to diminished disease severity. To further investigate GRA-mediated reduction in the severity of infection, mice were infected subcutaneously with MRSA and were then treated topically with GRA (600 μg) or DMSO. Due to the lack of an effect of GA in vitro, we did not analyze the effect of GA on expression of virulence genes in vivo. At designated time points (12, 24, 72, and 96 h postinfection), mice were euthanized, and bacterial mRNA was extracted from infected tissue and subjected to TaqMan RT-PCR with primers and probes specific for the same genes investigated in vitro. Twelve hours postinfection, GRA slightly downregulated expression of hla, RNAIII, and mecA (Fig. 5A). At 24 h postinfection, all five virulence genes tested were downregulated in the GRA-treated mice (−4.225 ± 1.957 for saeR, −6.3575 ± 5.283 for hla, −3.243 ± 0.412 for sbi, −4.169 ± 2.170 for mecA, and −6.798 ± 4.816 for hla) (Fig. 5B). Seventy-two hours postinfection, saeR and hla were downregulated in the GRA-treated mice (−27.204 ± 20.596 and −37.297 ± 28.558, respectively) (Fig. 5C). At 96 h postinfection, saeR and hla were still slightly downregulated in the GRA-treated mice (−3.016 ± 2.280 and −6.538 ± 4.590, respectively) (Fig. 5D). These data suggest that reduction in virulence gene expression in MRSA upon treatment with GRA contributes to reduced severity of MRSA skin infection in the mouse model.
Fig 5.
GRA alters S. aureus virulence gene expression in vivo. Mice were inoculated subcutaneously with LAC (2 × 107) and treated with GRA (600 μg) 1 h postinoculation and then once per day. At given time points, the site of inoculation was excised and bacterial RNA was isolated. Gene expression of five S. aureus virulence genes was investigated at 12 (A) and 24 h (B) postinoculation. Gene expression of saeR and hla was also investigated at 72 h (C) and 96 h (D) postinoculation. Gene expression is normalized to gyrB transcript abundance and relative to S. aureus isolated from untreated mice (data are from three mice per treatment group).
DISCUSSION
In the current study, we show that GRA inhibits MRSA growth in vitro in a dose-dependent manner, as determined by colony counts and optical density in liquid culture (Fig. 1A to D). The data indicate a dose-dependent bactericidal effect over both short and extended time courses. In contrast, GA showed no bactericidal activity and actually increased the growth of MRSA relative to that in controls (Fig. 1A). These data in part support previously published work showing the bactericidal effects of various crude fractions of licorice root extracts—such as hexane-fractionated licorice root or acetone extraction (18, 23–25). However, these data are in contrast to a recently published study showing no antibacterial effect of GRA against various strains of S. aureus (29). While the basis for these differences currently is not clear, they could be due to variation in the preparation and delivery of the compound.
The increased prevalence of skin and soft tissue infections caused by MRSA, combined with increased resistance to other classes of antibiotics and enhanced virulence, demonstrates a need for the development of new therapies (4, 32, 33). Although we did not see a significant reduction in bacterial survival in the wound, there was a significant decrease in the size of the wound over time when treated with GRA (Fig. 2A and B). However, we are currently optimizing the treatment protocol to ensure a maximum absorption of the compounds into the skin in order to test if higher doses of GRA are bactericidal. Notably, GA treatment resulted in a slight decrease in abscess size, although this was not statistically significant. This may result from GA being metabolized in the liver, followed by hydrolysis to GRA in the gut, and then reabsorbed to reach the site of infection (10).
The skin contains a vast array of cells that initiate an immediate innate immune response once the epidermal layer is breached. Antimicrobial peptides contained in the skin, along with keratinocytes, Langerhans cells, and many other immune cell types, are set to respond immediately upon infection (34, 35). Neutrophils are one of the most important cells combating skin infection caused by S. aureus (34). Neutrophils are recruited to the site of infection through cytokine and chemokine signaling. However, there is a delicate balance of neutrophil recruitment and killing, and the inflammatory response of neutrophils in response to S. aureus can be detrimental (36). We investigated the effect of GRA treatment on the levels of various pro- and anti-inflammatory molecules in the skin of infected and uninfected mice. Upon treating mice with GRA, we observed a decrease in two neutrophil recruitment factors, KC and G-CSF (Fig. 3A, B, and C). Studies have shown that a reduction, but not elimination, of neutrophils reduces bacterial burden and contributes to host survival (36). The reduction in neutrophil chemotactic factors may correlate with reduced neutrophil numbers at the site of infection, which may account for less tissue damage and smaller lesion sizes. These data suggest that GRA does have an immunomodulatory effect but may not fully account for the reduction in abscess size. Future studies will determine if reduced KC and G-CSF correlate with reduced neutrophil numbers at the site of infection.
To further explore the effect of licorice extracts on S. aureus, we evaluated virulence gene expression in S. aureus after incubation with the two compounds. We assayed five important S. aureus virulence genes: saeR, hla, RNAIII, mecA, and sbi. saeR, the regulatory gene of the SaeR/S two-component system, is important for the development of dermonecrosis in a murine skin model of infection and regulates the production of numerous virulence factors, including hemolysins, leukotoxins, and adhesins (21, 37, 38). hla, which encodes alpha-hemolysin, is regulated by the SaeR/S and Agr two-component gene regulatory systems and has been shown to be an important factor in the development of community-associated MRSA skin infections (21, 30, 39, 40). The Agr two-component system is known for its role in quorum sensing but also plays a role in regulating virulence factors through the functions of the RNAIII transcript (41, 42) and in regulation of methicillin resistance through the gene mecA (43). sbi, an immunoglobulin-binding protein of S. aureus, inhibits complement and has been shown to be regulated by the SaeR/S two-component system (21, 31, 44). In our in vitro model, we observed a decrease in the expression of saeR, hla, mecA, and sbi after incubating S. aureus with GRA (Fig. 4). The decrease in saeR expression correlates well with the observed downregulation of hla and sbi, since these two genes are under strong transcriptional control of SaeR/S (21). Others have shown that exposure of MRSA to fractionated licorice root extracts can increase oxacillin susceptibility, which may stem from the reduction in mecA transcription, an observation supported by our data demonstrating reduction in mecA expression following exposure to GRA (24). In contrast to the other genes reduced by exposure to GRA in vitro, RNAIII transcripts were upregulated following GRA exposure. The upregulation of RNAIII contradicts recent findings that show downregulation of RNAIII transcript after MRSA incubation with GRA, but the differences between these studies could be due to the variation in technique and formulation of GRA (29). The same trend in gene expression was seen in vivo after treatment with GRA; however, RNAIII transcripts were downregulated at levels similar to those of the other four genes assayed 24 h after initial treatment, supporting observations made by others and suggesting that GRA modulates RNAIII under specific conditions, since we did not observe downregulation of RNAIII in vitro (Fig. 5A to D) (29). The downregulation of virulence genes tested correlated to the decrease in abscess size after GRA treatment and may be the reason behind a reduction in abscess size even though bacterial burden is not affected.
Collectively, this study provides a foundation for future work to determine if repeated exposure to GRA results in resistance to the antimicrobial effects of GRA on S. aureus. Moreover, this study justifies future work to determine the precise mechanism of action of GRA on both the host and pathogen that results in reduced disease severity.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health grants NIH-RR020185, GM-103500, and AT-004986, equipment from the Murdoch Charitable Trust, and the Montana State University Agriculture Experiment Station. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
We thank Mark Jutila and Mark Quinn for critical review of the manuscript, Robert Watkins for technical assistance with CBA analyses, and Kyler B. Pallister, Shannon Griffith, and Cassy Cooper for assistance with mouse studies. All acknowledged individuals are in the Department of Immunology and Infectious Diseases, Montana State University.
Footnotes
Published ahead of print 31 October 2012
REFERENCES
- 1. Deleo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 [DOI] [PubMed] [Google Scholar]
- 3. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674 [DOI] [PubMed] [Google Scholar]
- 4. Johnson JK, Khoie T, Shurland S, Kreisel K, Stine OC, Roghmann MC. 2007. Skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus USA300 clone. Emerg. Infect. Dis. 13:1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, Kolonay JF, Shetty J, Killgore GE, Tenover FC. 2003. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302:1569–1571 [DOI] [PubMed] [Google Scholar]
- 6. Stapleton PD, Taylor PW. 2002. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci. Prog. 85:57–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asl MN, Hosseinzadeh H. 2008. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 22:709–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hattori T, Ikematsu S, Koito A, Matsushita S, Maeda Y, Hada M, Fujimaki M, Takatsuki K. 1989. Preliminary evidence for inhibitory effect of glycyrrhizin on HIV replication in patients with AIDS. Antiviral Res. 11:255–261 [DOI] [PubMed] [Google Scholar]
- 9. Kao TC, Shyu MH, Yen GC. 2010. Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation. J. Agric. Food Chem. 58:8623–8629 [DOI] [PubMed] [Google Scholar]
- 10. Ploeger B, Mensinga T, Sips A, Seinen W, Meulenbelt J, DeJongh J. 2001. The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling. Drug Metab. Rev. 33:125–147 [DOI] [PubMed] [Google Scholar]
- 11. Ito M, Sato A, Hirabayashi K, Tanabe F, Shigeta S, Baba M, De CE, Nakashima H, Yamamoto N. 1988. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV). Antiviral Res. 10:289–298 [DOI] [PubMed] [Google Scholar]
- 12. van Rossum TG, Vulto AG, de Man RA, Brouwer JT, Schalm SW. 1998. Review article: glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment. Pharmacol. Ther. 12:199–205 [DOI] [PubMed] [Google Scholar]
- 13. Akamatsu H, Komura J, Asada Y, Niwa Y. 1991. Mechanism of anti-inflammatory action of glycyrrhizin: effect on neutrophil functions including reactive oxygen species generation. Planta Med. 57:119–121 [DOI] [PubMed] [Google Scholar]
- 14. Cohen JI. 2005. Licking latency with licorice. J. Clin. Invest. 115:591–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fiore C, Salvi M, Palermo M, Sinigaglia G, Armanini D, Toninello A. 2004. On the mechanism of mitochondrial permeability transition induction by glycyrrhetinic acid. Biochim. Biophys. Acta 1658:195–201 [DOI] [PubMed] [Google Scholar]
- 16. Horigome H, Homma M, Hirano T, Oka K. 2001. Glycyrrhetinic acid induced apoptosis in murine splenocytes. Biol. Pharm. Bull. 24:54–58 [DOI] [PubMed] [Google Scholar]
- 17. Hatano T, Kusuda M, Inada K, Ogawa TO, Shiota S, Tsuchiya T, Yoshida T. 2005. Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry 66:2047–2055 [DOI] [PubMed] [Google Scholar]
- 18. Lee JW, Ji YJ, Yu MH, Bo MH, Seo HJ, Lee SP, Lee IS. 2009. Antimicrobial effect and resistant regulation of Glycyrrhiza uralensis on methicillin-resistant Staphylococcus aureus. Nat. Prod. Res. 23:101–111 [DOI] [PubMed] [Google Scholar]
- 19. Andrews JM. 2001. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48(Suppl 1):5–16 [DOI] [PubMed] [Google Scholar]
- 20. Nygaard TK, Deleo FR, Voyich JM. 2008. Community-associated methicillin-resistant Staphylococcus aureus skin infections: advances toward identifying the key virulence factors. Curr. Opin. Infect. Dis. 21:147–152 [DOI] [PubMed] [Google Scholar]
- 21. Nygaard TK, Pallister KB, Ruzevich P, Griffith S, Vuong C, Voyich JM. 2010. SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J. Infect. Dis. 201:241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, Deleo FR. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175:3907–3919 [DOI] [PubMed] [Google Scholar]
- 23. Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T. 2002. Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia 73:536–539 [DOI] [PubMed] [Google Scholar]
- 24. Hatano T, Shintani Y, Aga Y, Shiota S, Tsuchiya T, Yoshida T. 2000. Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoisoflavanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. (Tokyo) 48:1286–1292 [DOI] [PubMed] [Google Scholar]
- 25. Nitalikar MM, Munde KC, Dhore BV, Shikalgar SN. Studies of antibacterial activities of Glycyrrhiza glabra root extract. Int. J. PharmTech Res. 2:899–901 [Google Scholar]
- 26. Shamsa F, Ohtsuki K, Rezazadeh S. 2010. The anti-inflammatory and anti-viral effects of an ethnic medicine: glycyrrhizin. J. Med. Plants 9(Suppl 6):1–28 [Google Scholar]
- 27. Thiyagarajan P, Chandrasekaran CV, Deepak HB, Agarwal A. 2011. Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents. Inflammopharmacology 19:235–241 [DOI] [PubMed] [Google Scholar]
- 28. Kroes BH, Beukelman CJ, van den Berg AJ, Wolbink GJ, van Dijk H, Labadie RP. 1997. Inhibition of human complement by beta-glycyrrhetinic acid. Immunology 90:115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li HE, Qiu JZ, Yang ZQ, Dong J, Wang JF, Luo MJ, Pan J, Dai XH, Zhang Y, Song BL, Deng XM. 2012. Glycyrrhetinic acid protects mice from Staphylococcus aureus pneumonia. Fitoterapia 83:241–248 [DOI] [PubMed] [Google Scholar]
- 30. Kennedy AD, Bubeck WJ, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, Deleo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J. Infect. Dis. 202:1050–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith EJ, Visai L, Kerrigan SW, Speziale P, Foster TJ. 2011. The Sbi protein is a multifunctional immune evasion factor of Staphylococcus aureus. Infect. Immun. 79:3801–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dryden MS. 2010. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 65(Suppl 3):iii35–iii44 [DOI] [PubMed] [Google Scholar]
- 33. Moellering RC., Jr 2010. The problem of complicated skin and skin structure infections: the need for new agents. J. Antimicrob. Chemother. 65(Suppl 4):iv3–iv8 [DOI] [PubMed] [Google Scholar]
- 34. Miller LS, Cho JS. 2011. Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 11:505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Otto M. 2010. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev. Dermatol. 5:183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713–3722 [DOI] [PubMed] [Google Scholar]
- 37. Giraudo AT, Mansilla C, Chan A, Raspanti C, Nagel R. 2003. Studies on the expression of regulatory locus sae in Staphylococcus aureus. Curr. Microbiol. 46:246–250 [DOI] [PubMed] [Google Scholar]
- 38. Montgomery CP, Boyle-Vavra S, Daum RS. 2010. Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS One 5:e15177 doi:10.1371/journal.pone.0015177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giraudo AT, Cheung AL, Nagel R. 1997. The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Arch. Microbiol. 168:53–58 [DOI] [PubMed] [Google Scholar]
- 40. Xiong YQ, Willard J, Yeaman MR, Cheung AL, Bayer AS. 2006. Regulation of Staphylococcus aureus alpha-toxin gene (hla) expression by agr, sarA, and sae in vitro and in experimental infective endocarditis. J. Infect. Dis. 194:1267–1275 [DOI] [PubMed] [Google Scholar]
- 41. Morfeldt E, Taylor D, von Gabain A, Arvidson S. 1995. Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J. 14:4569–4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yarwood JM, Schlievert PM. 2003. Quorum sensing in Staphylococcus infections. J. Clin. Invest. 112:1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. 2011. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 79:1927–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang L, Jacobsson K, Vasi J, Lindberg M, Frykberg L. 1998. A second IgG-binding protein in Staphylococcus aureus. Microbiology 144(Pt 4):985–991 [DOI] [PubMed] [Google Scholar]