Abstract
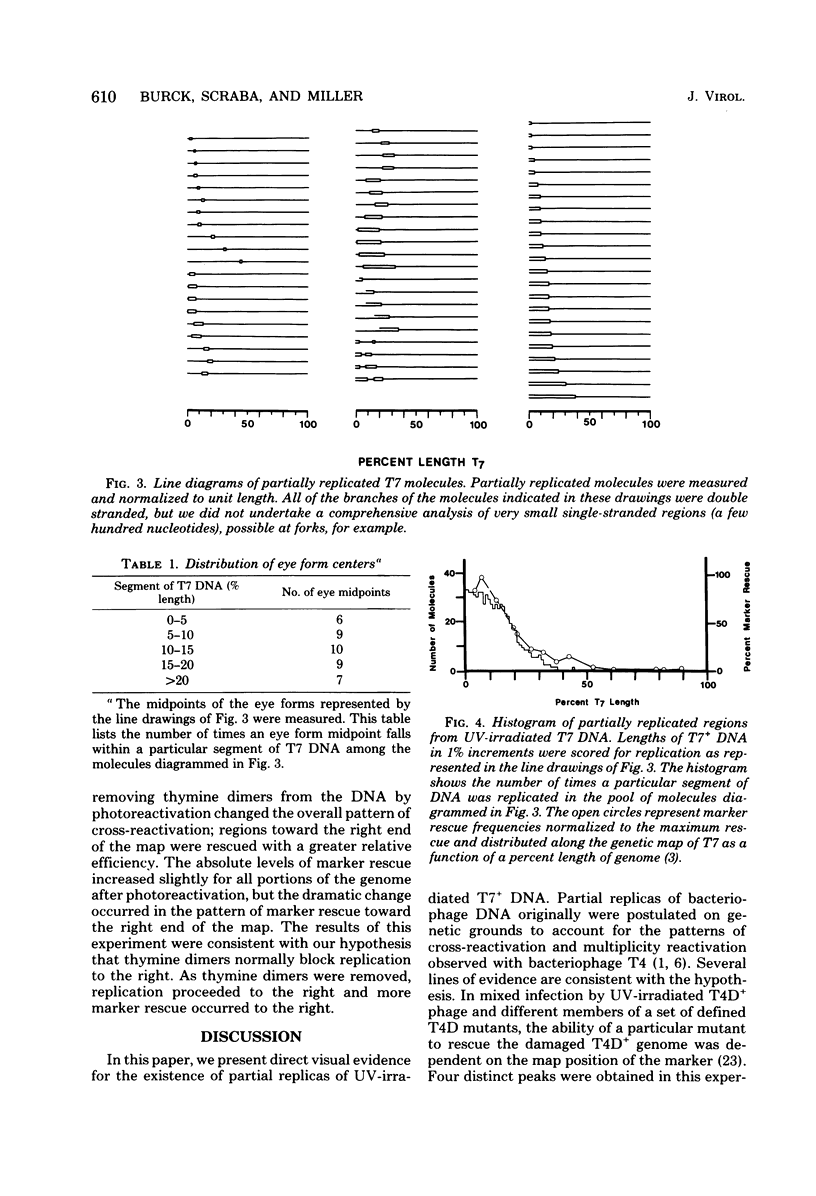
Partially replicated bacteriophage T7 DNA was isolated from Escherichia coli infected with UV-irradiated T7 bacteriophage and was analyzed by electron microscopy. The analysis determined the distribution of eye forms and forks in the partially replicated molecules. Eye forms and forks in unit length molecules were aligned with respect to the left end of the T7 genome, and segments were scored for replication in each molecule. The resulting histogram showed that only the left 25 to 30% of the molecules was replicated. Several different origins of DNA replication were used to initiate replication in the UV-irradiated experiments in which 32P-labeled progeny DNA from UV-irradiated phage was annealed with ordered restriction fragments of T7 DNA (K. B. Burck and R. C. Miller, Jr., Proc. Natl. Acad. Sci. U.S.A. 75:6144--6148, 1978). Both analyses support partial-replica hypotheses (N. A. Barricelli and A. H. Doermann, Virology 13:460--476, 1961; Doermann et al., J. Cell. comp. Physiol. 45[Suppl.]:51--74, 1955) as an explanation for the distribution of marker rescue frequencies during cross-reactivation; i.e., replication proceeds in a bidirectional manner from an origin to a site of UV damage, and those regions of the genome which replicate most efficiently are rescued most efficiently by a coinfecting phage. In addition, photoreactivation studies support the hypothesis that thymine dimers are the major UV damage blocking cross-reactivation in the right end of the T7 genome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRICELLI N. A., DOERMANN A. H. An analytical approach to the problems of phage recombination and reproduction. III. Cross reactivation. Virology. 1961 Apr;13:460–476. doi: 10.1016/0042-6822(61)90277-x. [DOI] [PubMed] [Google Scholar]
- Benbasat J. A., Burck K. B., Miller R. C., Jr Superinfection exclusion and lack of conservative transfer of bacteriophage T7 DNA. Virology. 1978 Jun 1;87(1):164–171. doi: 10.1016/0042-6822(78)90168-x. [DOI] [PubMed] [Google Scholar]
- Burck K. B., Miller R. C., Jr Marker rescue and partial replication of bacteriophage T7 DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6144–6148. doi: 10.1073/pnas.75.12.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOERMANN A. H., CHASE M., STAHL F. W. Genetic recombination and replication in bacteriophage. J Cell Physiol Suppl. 1955 May;45(Suppl 2):51–74. doi: 10.1002/jcp.1030450505. [DOI] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler D., Wolfson J., Magazin M. Initiation and reinitiation of DNA synthesis during replication of bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Apr;69(4):998–1002. doi: 10.1073/pnas.69.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez B., Lang D. Denaturation map of bacteriophage T7 DNA and direction of DNA transcription. J Mol Biol. 1972 Sep 28;70(2):239–251. doi: 10.1016/0022-2836(72)90536-0. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Dressler D. Site-specific initiation of a DNA fragment. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1652–1656. doi: 10.1073/pnas.75.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C. C., Buckley P. J., Carlson K. M., Kozinski A. W. Multiple and specific initiation of T4 DNA replication. J Virol. 1973 Jul;12(1):130–148. doi: 10.1128/jvi.12.1.130-148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamune Y. Effect of ultraviolet irradiation of bacteriophage f1 DNA on its conversion to replicative form by extracts of Escherichia coli. Mol Gen Genet. 1976 Dec 22;149(3):335–345. doi: 10.1007/BF00268536. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr, Lee M. The role of bacteriophage T7 exonuclease (gene 6) in genetic recombination and production of concatemers. J Mol Biol. 1976 Feb 25;101(2):223–234. doi: 10.1016/0022-2836(76)90374-0. [DOI] [PubMed] [Google Scholar]
- Rayssiguier C., Vigier P. R. Genetic evidence for the existence of partial replicas of T4 genomes inactivated by irradiation under ultraviolet light.?*ULTRAVIOLET RAYS. Virology. 1977 May 15;78(2):442–452. doi: 10.1016/0042-6822(77)90121-0. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B. PHYSICAL CHANGES AND MUTAGENESIS. J Cell Physiol. 1964 Oct;64:SUPPL 1–1:68. [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. The photochemistry, photobiology, and repair of polynucleotides. Prog Nucleic Acid Res Mol Biol. 1968;8:257–295. doi: 10.1016/s0079-6603(08)60548-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic analysis of non-essential bacteriophage T7 genes. J Mol Biol. 1973 Sep 15;79(2):227–236. doi: 10.1016/0022-2836(73)90002-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D., Magazin M. Bacteriophage T7 DNA replication: a linear replicating intermediate (gradient centrifugation-electron microscopy-E. coli-DNA partial denaturation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):499–504. doi: 10.1073/pnas.69.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack F. C. Cross-reactivation differences in bacteriophage T4D. Virology. 1965 Aug;26(4):758–760. [PubMed] [Google Scholar]




