Abstract
For treating Chagas disease (CD), a current worldwide health problem, only benznidazole and nifurtimox have been approved to be used. In both cases, unwanted drug-related adverse events (ADRs) are frequent when these drugs are used in adults in the chronic stage. The main objective of this study was to establish benznidazole ADRs and their relationship to serum concentrations in patients with chronic Trypanosoma cruzi infection in order to perform more accurate dosages to minimize ADRs. A total of 54 patients were recruited over 12 months. Of these 54 patients, 53 (98%) experienced at least one ADR during follow-up, and the overall average ADR incidence was 2.4 episodes/patient/month. Benznidazole treatment was discontinued in 11 patients, 7 among them due to severe adverse effects. The mean duration of treatment before withdrawal was 11 days. Benznidazole serum concentrations were recorded on days 15, 30, 45, and 60 of follow-up and evaluated according to clinical and epidemiological variables and ADR severity. No relationship was found between the benznidazole serum concentration and the ADRs. The mean (standard deviation) trough serum benznidazole concentrations (all below 20 mcg/ml) on days 15, 30, 45, and 60 were 6.4 (1.9), 6.1 (1.8), 6.2 (2.2), and 5.7 (1.7) μg/ml, respectively. Benznidazole serum concentrations do not appear to be related to the appearance of serious ADRs. Further, well-controlled studies are necessary to establish the optimal regimen for benznidazole in adults with chronic CD.
INTRODUCTION
Chagas disease (CD) is an endemic zoonotic disease caused by the hemoflagellate protozoan parasite, Trypanosoma cruzi. It affects 8 to 10 million people in Latin America (1) and is a worldwide public health problem due to migration flows (2).
The disease has two phases: an initial acute phase, which is usually asymptomatic, and a lifelong chronic phase, which in 60 to 70% of patients is clinically silent, but 20 to 30% of them will develop in years or decades heart problems (20 to 30%), digestive problems, or a combination of both (10 to 15%). Neurological symptoms are also seen in a small proportion of patients (5%) (3).
Current treatments for CD include nifurtimox and benznidazole, but only the latter is available in Spain. The efficacy of benznidazole in patients with T. cruzi infection is extremely variable and depends on the disease stage, the drug dose, and the age and geographic origin of the patient. Treatment in the acute phase of infection is highly effective with both benznidazole and nifurtimox, with cure rates of close to 100% in infants (4) and 60% in children and adults with recent infections (5, 6). The efficacy of drug therapy decreases with the duration of infection and is still a controversial issue (7, 8). The efficacy rates published in different studies of chronic T. cruzi infection vary due to methodological differences and high reinfection rates in areas of endemicity (9). The gold standard for evaluating efficacy is serologic testing, but even in successful treatments it may take from years to decades for seroreversion to occur (10). Thus, there is a pressing need for early markers of treatment response that can be used in clinical settings.
Data on the pharmacokinetics of benznidazole are limited. In vitro culture studies have shown that the trypanosomicidal concentration of benznidazole ranges from 3 to 6 μg/ml (12), and concentrations of 20 μg/ml seem to be related to a higher risk of toxicity, which mainly manifests as skin disorders (12). Benznidazole has good oral bioavailability, an apparent volume of distribution of 0.56 liter/kg, and an elimination half-life of 12 to 15 h (11, 12). The recommended dosage of benznidazole for the treatment of T. cruzi infection is 5 mg/kg/day, divided into two doses.
The reported frequency of benznidazole-related adverse drug reactions (ADRs) is ca. 50% for moderate reactions and 10% for serious ones (8, 13), although plasma concentrations of >20 μg/ml are not expected with the standard twice-daily dose regimen. The possibility of relating benznidazole serum concentrations to the appearance of ADRs is an appealing challenge that could help to further define the optimal dosage to attain the highest effectiveness with the lowest risk of toxicity.
The hypothesis of the present study was that ADRs in patients with T. cruzi infection treated with benznidazole could be related to the benznidazole serum concentrations. If this is true, clinicians would be equipped with a tool that would help to reduce the incidence of ADRs and improve the management of CD by tailoring dose regimens to individual needs.
The main objective of the present study was to establish a relationship between benznidazole serum concentrations and the appearance of ADRs attributable to this drug in patients with chronic T. cruzi infection. A secondary objective was to add to the pool of knowledge of the adverse-effect spectrum of benznidazole.
MATERIALS AND METHODS
Design and setting.
A 12-month observational prospective study was conducted of individuals from Latin American countries where CD is endemic and who had visited the Centre for International Health at Hospital Clínic, a university hospital, in Barcelona, Spain.
Recruitment and participants.
Fifty-four patients with T. cruzi infection who fulfilled the criteria to start benznidazole (14) were invited to participate in the study. They were all living in Barcelona and were between 18 and 50 years old. We excluded patients who were unable to comply with the follow-up requirements (fortnightly visits and mandatory unscheduled visits in the event of an unexpected treatment-emergent adverse reaction).
Procedure.
Clinical and epidemiological data were collected during patient interviews following the signing of an informed consent form. The information collected included age, sex, area of origin, history of residence in rural environments and mud houses, previous contact with the triatomine bug vector, history of blood donation or transfusion, and history—including information on toxic habits and other vascular risk factors.
Serologic tests for T. cruzi and HIV infection, hematology, biochemistry (including renal and liver function: creatinine, urea, and transaminases) were performed in all participants. T. cruzi-infected patients were studied using a protocol that included 12-lead electrocardiography, chest radiography, and echocardiography. Additional tests were performed according to individual symptoms.
Specific treatment with benznidazole (5 mg/kg/day for 60 days) was prescribed to all patients regardless of clinical stage. The follow-up protocol included fortnightly clinical and analytical tests for the duration of treatment. Treatment was considered to be complete when at least 80% of the total dose of benznidazole prescribed had been reached.
The fortnightly follow-up visits included routine hematology and biochemistry evaluations and the completion of a questionnaire on common ADRs and treatment adherence. In addition, predose serum samples to test benznidazole concentrations were collected at each visit. All of these tests were repeated during unscheduled visits (appearance of a new ADR between routine visits). In such cases, postdose serum samples were also taken, with evaluation of drug administration and blood sampling times. The simplified medication adherence questionnaire adapted to patients with T. cruzi infection was used to assess treatment adherence.
Two serum enzyme-linked immunosorbent assays (ELISAs) were used for the laboratory diagnosis of T. cruzi infection: a commercial ELISA with recombinant antigens (BioELISA Chagas; Biokit S.A., Lliçà d'Amunt, Barcelona, Spain) and a conventional ELISA (Orthoclinical Diagnostics, Inc.). Diagnosis was confirmed in patients with positive results in the two tests (15).
Benznidazole was quantified by high-performance liquid chromatography (16), with powder supplied by F. Hoffmann-La Roche, Ltd. (Sao Paulo, Brazil). All of the human plasma samples were spiked with 45 μl of benzocaine (Fagron Iberica S.A.U, Terrassa, Spain) as an internal standard to a final concentration of 300 mg/liter. The mixture was precipitated with 150 μl of 0.3 M (1/1 [vol/vol]) trichloroacetic acid and centrifuged at 8,000 × g for 10 min. Next, 100 μl of the supernatant was injected into the chromatographic system. The stationary phase was a Kromasil 100–5 C18 silica-based column (250 by 4.0 mm), and the mobile phase consisted of 60% ultrafiltered water and 40% acetonitrile. The flow rate was set at 0.9 ml/min, and the injection volume was 100 μl. The absorbance was monitored at 324 nm (16).
Statistical analysis.
Quantitative variables were determined as means and standard deviations or as medians and interquartile ranges (IQRs), and categorical variables were described by frequencies and percentages. A Student t test was used to compare normally distributed continuous variables and the Wilcoxon rank sum test to compare non-normally distributed variables. A Fisher exact test or the chi-square test was used to compare qualitative variables. All of the tests were two-tailed. Statistical significance was set at P < 0.05 and at P < 0.0125 for multiple comparisons for repeated measures over follow-up. The analyses were performed using Stata12 (Stata Corp., College Station, TX) (17).
RESULTS
Starting in November 2009, we recruited 17 male and 37 female patients, with mean ages of 37 years (IQR = 9) and 35 years (IQR = 9), respectively (P = 0.4191). The mean age at the start of treatment was 36 (IQR = 9) years. Some patients were under treatment with drugs other than benznidazole for concomitant conditions, including anemia, eosinophilia, dyslipidemia, arthrosis, migraine, mycosis, and chronic hepatitis B (one patient each), high blood pressure (two patients), thyroid disease and chronic gastritis (three patients each), and articular pain (four patients). The most common drug used during treatment with benznidazole was omeprazole (eight patients). Nonsteroidal anti-inflammatory drugs were being taken by six patients, levothyroxine by three patients, enalapril and benzodiazepines by two each, and acetaminophen, domperidone, entecavir, gabapentin, and ketoconazole by one patient each.
The incidence, time of appearance, and duration of the ADRs recorded during follow-up are shown in Table 1, and the frequency of each type of ADR is shown in Table 2. Of the 54 patients examined, 53 (98%) had at least one ADR during follow-up, and the average incidence of ADRs was 2.4 episodes/patient/month.
Table 1.
Characterization of ADRs to benznidazole in patients with T. cruzi infection: incidence, time of appearance, and durationa
| Symptom | No. (%) of patients | No. of patients |
No. of patients with recurrent ADRs | Median no. of days (IQR) of treatment before the appearance of ADR, no. of patients |
No. of patients with persistent ADRs | Median duration of ADR in days (IQR) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 ADR | 2–3 ADRs | >3 ADRs | First ADR | Second ADR | Third ADR | Last ADR | |||||
| General symptoms | 48/54 (89) | 16 | 17 | 15 | 4 | 15 (15), 48 | 30 (15), 32 | 45 (11), 22 | 60 (0), 15 | 25 | 30 (15) |
| Skin symptoms | 40/54 (74) | 22 | 18 | 0 | 5 | 15 (15), 40 | 30 (43), 18 | 52 (30), 6 | 8 | 15 (0) | |
| Fever | 8/54 (15) | 8 | 0 | 0 | 14 (15), 8 | ||||||
| Pruritus | 38/54 (70) | 18 | 14 | 6 | 6 | 15 (15), 38 | 30.5 (26.5), 20 | 54 (15), 6 | 9 | 15 (0) | |
| Gastrointestinal symptoms | 41/54 (76) | 19 | 15 | 7 | 7 | 15 (15), 41 | 30 (15), 22 | 45 (15), 18 | 60 (0), 7 | 14 | 30 (15) |
| Neurological/musculoskeletal symptoms | 40/54 (74) | 17 | 15 | 8 | 8 | 15 (15), 40 | 30 (15), 23 | 47 (15), 15 | 60 (0), 8 | 13 | 30 (30) |
IQR, interquartile range.
Table 2.
Number of ADRs during follow-up
| Type of ADR | No. of episodes during follow-up |
|---|---|
| General (48/54 patients [89%]) | |
| Headache | 25 |
| Anorexia | 8 |
| Asthenia | 17 |
| Headache + anorexia | 4 |
| Headache + asthenia | 28 |
| Asthenia + anorexia | 10 |
| Headache + anorexia + asthenia | 34 |
| Skin (40/54 patients [74%]) | |
| Urticaria | 9 |
| Macular rash | 32 |
| Palmar-plantar rash | 5 |
| Erythematosus rash | 5 |
| Facial rash | 6 |
| Gastrointestinal (41/54 patients [76%]) | |
| Epigastralgia | 34 |
| Nausea | 17 |
| Vomiting (with nausea) | 32 |
| Paresthesia | 23 |
| Myalgia/arthralgia | 40 |
| Paresthesia + myalgia/arthralgia | 26 |
The laboratory results during follow-up are shown in Table 3. Blood tests showed several cases of mild anemia and leucopenia and one case of severe lymphopenia at the beginning of treatment. There were no cases of hyperemia or leukocytosis. Eosinophilia was mild or moderate in all patients but one, who developed severe eosinophilia at the end of treatment. Biochemistry analyses showed several cases of mild liver function alterations, and there was also one case in which the transaminase levels were three times above normal values at day 15 of follow-up. No renal function or ionogram alterations were found. The clinical ADRs noted were not significantly related to hematologic or biochemical alterations.
Table 3.
Hematologic and biochemical alterations during follow-up
| Parametera | No. (%) of alterations during follow-up |
No. of patients with severe clinical ADRs and hematologic alterations | |||
|---|---|---|---|---|---|
| Day 15 | Day 30 | Day 45 | Day 60 | ||
| Complete blood count | |||||
| RBC* | 6 (13) | 12 (29) | 15 (38) | 14 (33) | 0 |
| WBC* | |||||
| Leukopenia | 3 (7) | 3 (7) | 1 (3) | 2 (5) | 0 |
| Neutropenia | 7 (15) | 11 (26) | 14 (35) | 10 (24) | 0 |
| Lymphopeniab | 2 (4)c | 3 (7) | 3 (8) | 1 (2) | 1 |
| Eosinophiliac | |||||
| Mild | 7 (15) | 7 (17) | 5 (13) | 11 (26) | 1 |
| Moderate | 5(11) | 1 (2) | 3 (8) | 2 (4)c | |
| Biochemistry | |||||
| Transaminases* | 8 (17)d | 9 (21) | 10 (24) | 9 (21) | 0 |
| Bilirubin* | 1 (2) | 2 (5) | 1 (2) | 0 | |
| LDH* | 4 (9) | 3 (7) | 3 (7) | 5 (12) | 1 |
| Total proteins* | 2 (4) | 3 (7) | 1 (2) | 1 | |
RBC, red blood cells; WBC, white blood cells; LDH, lactate dehydrogenase. *, Mild alterations: anemia (hemoglobin, 10 to 12 mg/dl), leukopenia (3,000 to 35,000 WBC/ml), neutropenia (1,000 to 1,200 neutrophils/ml, transaminases (<3 × ULN), bilirubin (0.2 to 1.2 mg/dl), LDH (250 to 450 U/liter), and total proteins (63 to 80 g/liter). Eosinophilia was mild (500 to 999 eosinophils/ml) or moderate (1,000 to 3,000 eosinophils/ml).
All cases were moderate (500 to 1,000 lymphocytes/ml), except for one severe case (<500 lymphocytes/ml).
There was one case of severe eosinophilia (>3,000 eosinophils/ml).
In one case, more than ×3 ULN.
Benznidazole treatment was discontinued in 11 patients, all women (P = 0.0104); 7 of these patients had severe adverse effects. The mean duration of treatment before withdrawal was 11 days (IQR = 35): four patients stopped treatment between days 9 and 11, one stopped treatment on day 21, and two stopped treatment on day 45. The benznidazole serum levels were within the recommended therapeutic range in all but one of the seven patients. In the seventh patient, the level was 7.3 μg/ml on the day treatment was stopped (day 10). No significant differences in serum concentrations of benznidazole were observed for patients taking other drugs (P = 0.1415) or for those who had concomitant conditions (P = 0.4705). However, the independent analysis of each ADR showed that treatment interruption was more common in patients with drug-related fever (P = 0.0064) and skin disorders (P = 0.0261) than in those without these reactions.
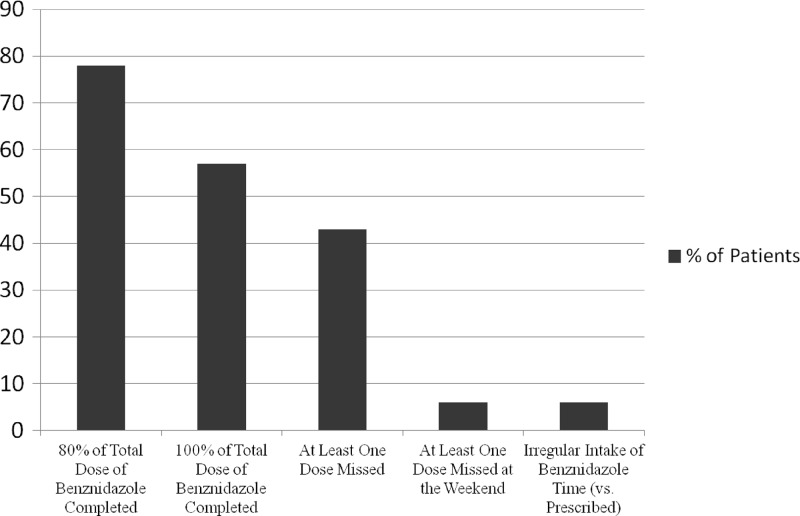
Data concerning treatment adherence are presented in Fig. 1. Full adherence (100% of total dose) was achieved in 31 (57%) patients; rates of >80% were reported by 11 patients, and rates of <80% were reported by 12 patients, 11 of whom had to stop treatment due to ADRs. Of the 23 (43%) patients who had forgotten to take at least one dose of benznidazole during the 60 days, only 3 (6%) had missed a dose during the weekend.
Fig 1.
Adherence to treatment.
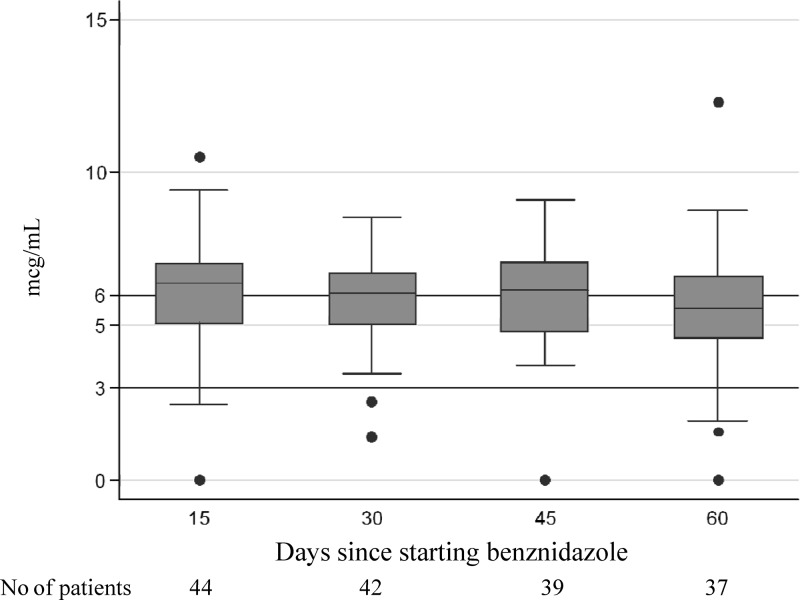
Benznidazole serum concentrations were recorded on days 15, 30, 45, and 60 of follow-up. No relationship was found between benznidazole serum concentration and ADRs evaluated according to sex, age, treatment completion, the presence of concomitant conditions, the use of concomitant medication (Table 4), and ADR severity (Table 5).The mean trough serum benznidazole concentrations on days 15, 30, 45, and 60 were 6.4 (1.9), 6.1 (1.8), 6.2 (2.2), and 5.7 (1.7) μg/ml, respectively (Fig. 2). No significant differences were observed on analyzing the relationship between these predose values and the appearance of each type of ADR (P > 0.05).
Table 4.
Correlation between benznidazole serum concentrations and treatment interruption, sex, other conditions, and concomitant drug use
| Time point (days) | Median benznidazole serum concn in μg/ml (IQR) [no. of patients] and associated P valuea |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment completed |
Sex |
Other diseases |
Concomitant drug use |
|||||||||
| Yes | No | P | Male | Female | P | No | Yes | P | No | Yes | P | |
| 15 | 6.5 (1.8) [38] | 5.2 (3.4) [6] | 0.0811 | 6.4 (1.7) [14] | 6.0 (2.0) [31] | 0.8445 | 6.57 (2.0) [30] | 5.7 (1.7) [15] | 0.3005 | 6.5 (2.1) [31] | 5.6 (1.6) [14] | 0.2918 |
| 30 | 6.18 (1.86) [39] | 4.4 (4.8) [3] | 0.0919 | 6.3 (1.7) [15] | 6.1 (2.2) [28] | 0.3589 | 6.2 (2.3) [28] | 5.9 (1.4) [15] | 0.8186 | 6.3 (1.4) [29] | 5.3 (1.2) [14] | 0.1327 |
| 45 | 6.3 (1.99) [37] | 4.8 (2.2) [2] | 0.1613 | 6.0 (2.2) [15] | 6.3 (2.2) [24] | 0.6236 | 6.1 (1.8) [25] | 6.3 (2.6) [14] | 0.7035 | 6.1 (1.8) [25] | 6.4 (3–0) [14] | 0.8148 |
| 60 | 5.7 (1.7) [37] | 5.9 (2.9) [15] | 5.3 (1.7) [22] | 0.8649 | 5.9 (2.3) [25] | 5.2(1.7) [12] | 0.2363 | 5.9 (2.0) [24] | 5.0 (1.9) [13] | 0.3318 | ||
Statistical significance was set at P < 0.0125.
Table 5.
Relationship between benznidazole serum concentrations and appearance of ADRs
| Time point (days) | Median benznidazole serum concn in μg/ml (IQR) [no. of patients] and associated P valuea |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severe ADR |
Skin symptoms |
Fever |
Pruritus |
Gastrointestinal symptoms |
Neurological/musculoskeletal symptoms |
|||||||||||||
| No | Yes | P | No | Yes | P | No | Yes | P | No | Yes | P | No | Yes | P | No | Yes | P | |
| 15 | 6.4 (1.9) [41] | 5.8 (3.1) [4] | 0.6322 | 6.79 (2.31) [12] | 6.38 (1.88) [33] | 0.5046 | 6.4 (1.8) [40] | 5.3 (0.7) [5] | 0.1814 | 5.7 (2.3) [14] | 6.4 (1.7) [31] | 0.7499 | 6.7 (3.3) [10] | 6.4 (1.8) [35] | 0.4949 | 6.8 (1.5) [9] | 6.0 (1.9) [36] | 0.2171 |
| 30 | 6.1 (1.8) [41] | 5.3 (1.8) [2] | 0.4534 | 5.52 (1.45) [12] | 6.20 (1.91) [31] | 0.3572 | 6.1 (1.8) [39] | 4.8 (3.5) [4] | 0.2096 | 5.6 (1.8) [13] | 6.1 (1.9) [30] | 0.3023 | 6.2 (2.4) [11] | 6.1 (1.8) [32] | 0.3030 | 6.4(1.0) [10] | 5.9 (1.8) [33] | 0.4376 |
| 45 | 6.3 (1.9) [37] | 4.8 (2.2) [2] | 0.1613 | 6.16 (2.66) [12] | 6.32 (2.51) [27] | 0.5129 | 6.1 (2.2) [36] | 7.5 (1.5) [3] | 0.0651 | 7.1 (1.8) [11] | 5.9 (2.1) [28] | 0.0045b | 6.3 (2.3) [9] | 6.2 (1.9) [30] | 0.8676 | 6.5(1.5) [9] | 6.1 (2.4) [30] | 0.3506 |
| 60 | 5.7 (1.7) [37] | 5.78 (2.59) [12] | 5.48 (1.59) [25] | 0.9741 | 5.6 (1.7) [34] | 6.8 (5.0) [3] | 0.5043 | 6.1 (2.1) [10] | 5.2 (2.3) [27] | 0.7067 | 6.5 (1.6) [9] | 5.2 (1.8) [28] | 0.4054 | 6.5 (1.1) [9] | 5.0 (2.1) [28] | 0.0177 | ||
Statistical significance was set at P < 0.0125 (Wilcoxon rank sum test).
Uncertain clinical significance due to the small number of cases compared.
Fig 2.
Median (IQR) serum benznidazole concentrations (trough concentrations) during follow-up (days 15, 30, 45, and 60).
In several cases, serum concentrations were determined after the administration of the drug (i.e., during visits requested by the patients normally due to the appearance of a new ADR). All of the concentrations were <20 μg/ml, and no significant differences were found between patients with or without each type of ADR.
DISCUSSION
Benznidazole is currently the treatment of choice for T. cruzi infection, despite the limited data available on its pharmacokinetics properties and effectiveness in chronic infection.
The data from the present study show that a dose regimen of benznidazole at 5 mg/kg/day results in mean serum concentrations that are at the top of the trypanocidal range (3 to 6 μg/ml), indicating that this dose is appropriate to obtain therapeutic drug concentrations. Studies performed by Altcheh et al. (19) have shown higher hepatic clearance of benznidazole and cure rates in children than in adults, suggesting that the therapeutic response achieved with 5 mg/kg/day could be achieved with lower doses in adults and in children in >7 years of age. However, in our series, the usual doses of benznidazole correspond with sufficient trypanocidal levels of the drug and well below the toxic concentrations. Based on our present findings, it is clear that the recommended benznidazole dose of 5 mg/kg/day allows optimal trypanocidal levels in adult chronic patients. However, the conventional duration of treatment (60 days) is not universally accepted (8, 10) and a rigorous evaluation of both the efficacy and tolerability of shorter treatments (e.g., 30 days) should be carried out in randomized clinical studies.
Benznidazole (like other nitroimidazoles) appears to interact with alcohol (20), but no interactions with alcohol were recorded among our patients, since alcohol intake was forbidden in all them during the treatment with benznidazole. In our study, the serum concentrations did not appear to be modified by the use of other drugs or the presence of concomitant conditions.
It has been reported that benznidazole concentrations of >20 μg/ml result in toxicity, but these levels were established in studies involving healthy volunteers (11, 12, 18). Toxic concentrations might be different in patients with T. cruzi infection, but no studies have yet been designed to explore this possibility. All of the patients in our series received the recommended dosage of 5 mg/kg/day, and the maximum concentration noted (including peak concentrations measured just a few hours after administration) was 12.5 μg/ml, which is considerably less than the toxic concentration of 20 μg/ml
Paresthesia and arthralgia have been linked to high levels of benznidazole at the end of treatment due to accumulation of the drug (21). Our data show no significant differences in benznidazole serum concentrations between patients with these ADRs and those without. Nonetheless, to improve the management of patients treated with benznidazole, in-depth, well-designed analyses are necessary to establish the mechanisms underlying adverse reactions and to investigate their relationship to hypersensitivity or idiosyncrasy.
Treatment adherence was excellent during follow-up. This is relevant for two reasons: good adherence provides reliable data on drug serum concentrations and close follow-up leads to improved knowledge and thus improved management of ADRs. We believe that the fact that our patients were provided with detailed information on the treatment schedule and duration and with a description of potential adverse reactions might have had a positive effect on adherence. Although 53 of the 54 patients experienced ADRs, these were severe in only 7 cases, and only 11 patients had to discontinue treatment. These data support previous findings that even with a high incidence of ADRs, these reactions can be successfully controlled with close follow-up and symptomatic treatment (8, 13).
Laboratory results during follow-up showed several cases of mild and moderate hematologic alterations and mild biochemical abnormalities. There was, however, one case of severe lymphopenia (associated with cutaneous, gastrointestinal, and general symptoms, arthralgia, pruritus, and fever) and another case of severe eosinophilia (general symptoms, nausea and epigastric pain, macular rash, and arthralgia). In both cases, the symptoms were well controlled, and the laboratory values returned to normal when benznidazole was withdrawn. There was no correlation between the clinical reactions and the hematologic or biochemical alterations.
A significant limitation of the present study is that we did not measure the serum concentrations of benznidazole metabolites, which might play an important role in toxicity.
To summarize, benznidazole serum concentrations below 20 mcg/ml do not appear to be related to the appearance of serious ADRs.
ACKNOWLEDGMENTS
We thank Professor Julio Urbina for his critical review of this work. We also thank Fundación Mundo Sano España and the Generalitat de Catalunya (Program 2009SGR38) for supporting our research in Chagas disease. This research was also funded by our own institution, the Barcelona Centre for International Health Research, Barcelona, Spain.
M.-J.P., L.G., E.P., D.S., and J.G. designed the study. M.-J.P., E.P., and J.G. oversaw the clinical management of the patients. L.G. and D.S. determined the benznidazole serum drug concentrations. E.R. performed the analysis and interpreted the data. All authors contributed to the literature search and writing the text.
Footnotes
Published ahead of print 31 October 2012
REFERENCES
- 1. Pan American Health Organization 2006. Estimación cuantitativa de la enfermedad de Chagas en las Americas. Organización Panamericana de la Salud, Montevideo, Uruguay [Google Scholar]
- 2. Gascon J, Bern C, Pinazo MJ. 2010. Chagas disease in Spain, the United States, and other non-endemic countries. Acta Trop. 115:22–27 [DOI] [PubMed] [Google Scholar]
- 3. Prata A. 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 1:92–100 [DOI] [PubMed] [Google Scholar]
- 4. Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin MJ, Freilij H. 2003. Aetiological treatment of congenital Chagas' disease diagnosed and monitored by the polymerase chain reaction. J. Antimicrob. Chemother. 52:441–449 [DOI] [PubMed] [Google Scholar]
- 5. de Andrade AL, Zicker de Oliveira FRM, Almeida Silva S, Luquetti A, Travassos LR, Almeida IC, de Andrade SS, de Andrade JG, Martelli CM. 1996. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 348:1407–1413 [DOI] [PubMed] [Google Scholar]
- 6. Sosa Estani S, Segura EL, Ruiz AM, Velazquez E, Porcel BM, Yampotis C. 1998. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. Am. J. Trop. Med. Hyg. 59:526–529 [DOI] [PubMed] [Google Scholar]
- 7. Marin-Neto JA, Rassi A, Jr, Morillo CA, Avezum A, Connolly SJ, Sosa-Estani S, Rosas F, Yusuf S. 2008. Rationale and design of a randomized placebo-controlled trial assessing the effects of etiologic treatment in Chagas' cardiomyopathy: the BENznidazole Evaluation For Interrupting Trypanosomiasis (BENEFIT). Am. Heart J. 156:37–43 [DOI] [PubMed] [Google Scholar]
- 8. Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertochi G, Armenti A. 2009. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev. Anti-Infect. Ther. 7:157–163 [DOI] [PubMed] [Google Scholar]
- 9. Rodriques Coura J, de Castro SL. 2002. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 97:3–24 [DOI] [PubMed] [Google Scholar]
- 10. Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, Postan M, Armenti A. 2006. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann. Inter. Med. 144:724–734 [DOI] [PubMed] [Google Scholar]
- 11. Raaflaub J, Ziegler WH. 1979. Single-dose pharmacokinetics of the trypanosomicide benznidazole in man. Drug Res. 29:1611–1614 [PubMed] [Google Scholar]
- 12. Raaflaub J. 1980. Multiple-dose kinetics of the trypanosomicide benznidazole in man. Drug Res. 30:2192–2194 [PubMed] [Google Scholar]
- 13. Pinazo MJ, Munoz J, Posada E, López-Chejade P, Gallego M, Ayala E, del Cacho E, Soy D, Gascon J. 2010. Tolerance of benznidazole in treatment of Chagas' disease in adults. Antimicrob. Agents Chemother. 54:4896–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bern C, Montgomery SP, Herwaldt BL, Rassi A, Jr, Marin-Neto JA, Dantas RO, Maguire JH, Acquatella H, Morillo C, Kirchhoff LV, Gilman RH, Reyes PA, Salvatella R, Moore AC. 2007. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA 298:2171–2181 [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization 2002. Control of Chagas' disease. World Health Organ. Tech. Rep. Ser. 905:1–109 [PubMed] [Google Scholar]
- 16. Guerrero L, Pinazo MJ, Posada E, Gascon J, Ribas J, Soy D. 2011. A high-performance liquid chromatographic method for benznidazole quantitation in plasma of patients with Chagas disease. Clin. Chem. Lab. Med. 49:77–82 [DOI] [PubMed] [Google Scholar]
- 17. StataCorp 2011. Stata, release 12: statistical software. StataCorp, College Station, TX [Google Scholar]
- 18. Morilla MJ, Benavidez PE, Lopez MO, Romero EL. 2003. Liposomal benznidazole: a high-performance liquid chromatographic determination for biodistribution studies. J. Chromatogr. Sci. 41:405–409 [DOI] [PubMed] [Google Scholar]
- 19. Altcheh J, Moscatelli G, Moroni S, Mastrantonio G, Marson E, Garcia-Bournissen F. 2012. Estudios farmacológicos de Benznidazol y Nifurtimox en niños y mujeres lactantes con enfermedad de Chagas, p 40–44 VIII Taller Sobre la Enfermedad de Chagas Importada, Barcelona, Spain [Google Scholar]
- 20. Anderson KE. 1981. Pharmacokinetics of nitroimidazoles: spectrum of adverse reactions. Scand. J. Infect. Dis. 26:60–67 [PubMed] [Google Scholar]
- 21. Cancado JR. 2002. Long term evaluation of etiological treatment of Chagas disease with benznidazole. Rev. Inst. Med. Trop. Sao Paulo 44:29–37 [PubMed] [Google Scholar]