Abstract
Fidaxomicin (FDX) is approved to treat Clostridium difficile-associated diarrhea and is superior to vancomycin in providing a sustained clinical response (cure without recurrence in the subsequent 25 days). The mechanism(s) behind the low recurrence rate of FDX-treated patients could be multifactorial. Here, we tested effects of FDX, its metabolite OP-1118, and vancomycin on spore germination and determined that none affected the initiation of spore germination but all inhibited outgrowth of vegetative cells from germinated spores.
TEXT
Clostridium difficile, a Gram-positive, spore-forming, obligate anaerobe, causes intestinal infections, usually in people who have recently completed antibiotic therapies for unrelated conditions (1). Antibiotics cause alterations in the normally protective colonic microbiota, creating a niche for C. difficile to colonize (2, 3). To cause disease, C. difficile spores, which are unaffected by inciting antibiotics, must germinate to vegetative, or actively growing, bacteria in the anaerobic environment of the colon in order to produce the toxins that are responsible for the primary disease symptoms (4–6). Therefore, C. difficile spores, which are highly resistant to chemical disinfectants and antibiotics, are the source of infection. Although the exact mechanism and receptors involved in C. difficile spore germination are not clearly defined, both taurocholic acid and glycine have been identified as factors that synergistically stimulate germination of spores into virulent vegetative cells that secrete potent toxins (7, 8).
Vancomycin and metronidazole are commonly prescribed to treat C. difficile infections (CDI) (1). However, patients treated with vancomycin or metronidazole frequently relapse with C. difficile disease (1). Recently, fidaxomicin (FDX) was approved in the United States, Europe, and Canada as an alternative for the treatment of CDI. During phase 3 clinical trials, FDX was shown to be superior to vancomycin in sustaining clinical response without recurrence for up to 25 days following treatment (9, 10).
Multiple factors may lie behind the reduced rate of relapsing CDI in FDX-treated patients. Both FDX and its main metabolite OP-1118 have been shown to strongly inhibit C. difficile spore formation (11). FDX also has a reduced impact on the normally protective colonic microbiota (12, 13). In this study, we evaluated whether FDX might block germination of C. difficile spores.
To test the effect of FDX on C. difficile spore germination, we purified spores from C. difficile strains CD196 (14) and UK1 (15), as described previously (16). To provide a quantitative measure of the effects of FDX, OP-1118, and vancomycin on C. difficile spore germination, we analyzed the kinetics of the initiation of spore germination. By measuring the maximum rate of spore germination under different conditions, we are able to determine an apparent Km, defined as the concentration that provides a half-maximal germination rate, for taurocholic acid (TA), a C. difficile spore germinant. This strategy has been used to test the effects of different compounds on C. difficile spore germination (16–19), C. sordellii spore germination (18, 20), and Bacillus anthracis spore germination (21). If the drugs were to affect the initiation of spore germination, a change in apparent Km should be detected.
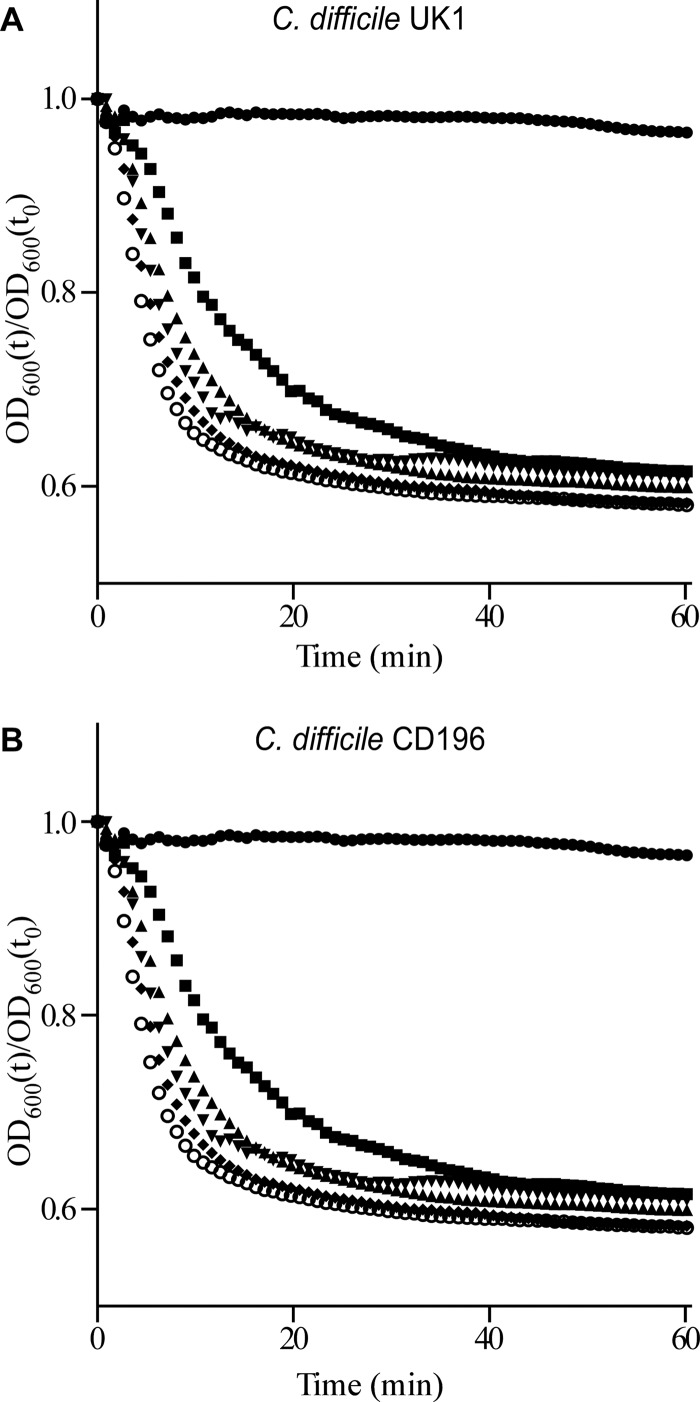
C. difficile UK1 and C. difficile CD196 spores were suspended in BHIS (brain heart infusion–5 g/liter yeast extract–0.1% l-cysteine) medium alone or in BHIS medium supplemented with 2 mM TA or 5 mM TA or 10 mM TA or 20 mM TA or 50 mM TA, and germination was assayed at room temperature by following changes in A600 using a PerkinElmer Lambda 25 spectrophotometer. As expected, C. difficile UK1 spores (Fig. 1A) and C. difficile CD196 spores (Fig. 1B) did not germinate in BHIS medium alone; TA is required for in vitro C. difficile spore germination (7, 8). Moreover, we observed a concentration-dependent, TA-dependent increase in the rate of C. difficile spore germination. To analyze the effects of FDX, OP-1118, and vancomycin on C. difficile spore germination, we added 0.25 μg/ml FDX (2× MIC) or 2.5 μg/ml OP-1118 (2.5× MIC) or 2.5 μg/ml vancomycin (2.5× MIC) and measured the kinetics of C. difficile spore germination. Germination in the above conditions was followed in triplicate samples; a representative sample is shown in Fig. 1. The apparent Km values for TA in the presence or absence of the tested drugs, as determined from the data in Fig. 1 and two additional replicates, are shown in Table 1. The addition of FDX, OP-1118, or vancomycin had no effect on the apparent Km for TA, which was similar to the previously reported value (16). Moreover, none of these compounds induced germination, suggesting that these antibiotics do not either positively or negatively affect the initiation of spore germination. Similar findings on spore germination were observed with C. difficile strain UK14 (15) with concentrations that are suprainhibitory for vegetative growth: 200× the MIC of FDX and 25× the MIC of OP-1118 (data not shown).
Fig 1.
Analyzing the initiation of spore germination in C. difficile UK1 and C. difficile CD196. Purified C. difficile UK1 spores (A) and C. difficile CD196 spores (B) were suspended in BHIS medium alone (•) or in BHIS medium supplemented with 2 mM TA (■) or 5 mM TA (▲) or 10 mM TA (▼) or 20 mM TA (⧫) or 50 mM TA (○). The initiation of germination was followed at A600, and values were normalized to t0. The experiment was performed in triplicate, and quantified values of the apparent Km for TA are listed in Table 1.
Table 1.
Apparent Km values of C. difficile spores for taurocholic acid
| Condition |
Km value (mM ± SD) for straina: |
|
|---|---|---|
| C. difficile UK1 | C. difficile CD196 | |
| No drug | 1.97 ± 0.13 | 2.16 ± 0.31 |
| 0.25 μg/ml FDX | 1.93 ± 0.30 | 2.56 ± 1.10 |
| 2.5 μg/ml OP-1118 | 1.89 ± 0.51 | 3.49 ± 0.97 |
| 2.5 μg/ml vancomycin | 2.9 ± 0.49 | 2.89 ± 0.36 |
Values represent the averages of the results of three independent experiments and are listed in millimolar ± 1 standard deviation from the mean. Differences are not statistically significant (Student's t test).
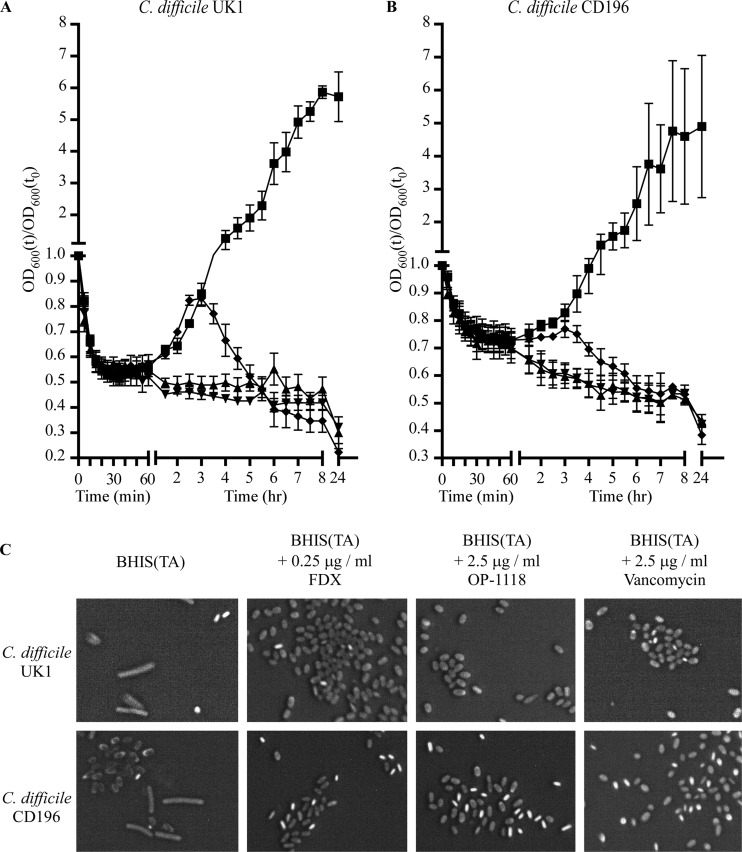
Since these drugs do not affect the initiation of spore germination, we tested whether later stages of germination would be affected. We assayed outgrowth by following changes in A600 in an anaerobic environment (10% hydrogen, 5% CO2, 85% nitrogen) over a 24-h period. C. difficile UK1 spores (Fig. 2A) or C. difficile CD196 spores (Fig. 2B) were suspended in BHIS medium alone or BHIS medium supplemented with 2 mM TA [BHIS(TA)] or BHIS(TA) supplemented with 0.25 μg/ml FDX or 2.5 μg/ml OP-1118 or 2.5 μg/ml vancomycin. Spores suspended in BHIS medium alone did not germinate during the duration of the experiment (data not shown). Spores suspended in BHIS(TA) initially showed reduced absorbance, indicating spore germination, but showed an increase in the A600 at approximately 2 h, and this increase continued for the duration of the experiment. We did not detect outgrowth (i.e., the rise in A600 subsequent to the initial drop) when C. difficile UK1 spores or C. difficile CD196 spores were suspended in BHIS(TA) with FDX or OP-1118. However, when outgrowth was measured in the presence of vancomycin, we observed an increase followed by a decrease in A600, suggesting that outgrowth had begun but was then inhibited by vancomycin. We confirmed by phase-contrast microscopy that the increase in absorbance observed for C. difficile UK1 (Fig. 2A) and C. difficile CD196 (Fig. 2B) was correlated with the disappearance of phase-bright spores and appearance of phase-dark spores and vegetative cells in the suspension (Fig. 2C). Spores incubated with any of the antibiotics transitioned from the phase-bright to the phase-dark state, but no vegetative cells appeared when antibiotics were present (Fig. 2C). These results suggest that FDX, OP-1118, and vancomycin inhibit outgrowth of vegetative cells from C. difficile spores, though FDX and OP-1118 may inhibit outgrowth at an earlier stage than does vancomycin, because spores germinated in the presence of FDX or OP-1118 did not show any increase in optical density (OD) during germination. This result is in agreement with the different mechanisms of action of the two drugs and supports previous observations that compounds which inhibit cell wall synthesis inhibit later stages of outgrowth (22). In contrast, FDX, which targets RNA polymerase and therefore inhibits production of RNA and proteins, completely inhibited outgrowth throughout the duration of the experiments (23).
Fig 2.
Measuring outgrowth of C. difficile spores during antibiotic exposure. Purified C. difficile UK1 spores (A) and C. difficile CD196 spores (B) were suspended in BHIS medium supplemented with 2 mM TA alone (■) or with 0.25 μg/ml FDX (▲) or 2.5 μg/ml OP-1118 (▼) or 2.5 μg/ml vancomycin (⧫). A600 was followed at 5-min intervals for 60 min and then every 30 min for another 7 h and finally determined at 24 h. Germination and outgrowth were followed at 37°C in an anaerobic environment and normalized to t0. Data points represent averages of the results of 3 independent experiments, and error bars represent 1 standard deviation from the mean and, at times, are smaller than the data point itself. (C) Samples were taken at the 150-min time point (above) and analyzed by phase-contrast microscopy. Dormant spores appear bright; germinated spores appear dark. Magnification: ×1,000. Contrast was adjusted using Canvas X and applied equally to all images.
In addition to inhibition of spore formation, inhibiting the conversion of C. difficile spores to actively growing vegetative cells could be a powerful way to prevent relapsing CDI. We found that FDX, OP-1118, and vancomycin do not affect the initiation of C. difficile spore germination but do inhibit outgrowth of a vegetative cell from the germinating spore. This action would also prevent the synthesis of toxins and any downstream effects. Thus, FDX, OP-1118, and vancomycin inhibit some of the earliest stages in C. difficile pathogenesis but the effects of FDX alone on C. difficile spore germination cannot explain the reduced frequency of relapsing CDI. Use of a narrow-spectrum antibiotic that inhibits spore formation and also prevents the outgrowth of germinated spores could be especially powerful, since it would allow most of the microbiota time to recover and reestablish colonization resistance.
ACKNOWLEDGMENTS
We thank A. L. Sonenshein for critical comments during preparation of the manuscript.
This project was funded through an Optimer Pharmaceuticals, Inc., funded contract.
Footnotes
Published ahead of print 12 November 2012
REFERENCES
- 1. Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7:526–536 [DOI] [PubMed] [Google Scholar]
- 2. Raibaud P, Ducluzeau R, Dubos F, Hudault S, Bewa H, Muller MC. 1980. Implantation of bacteria from the digestive tract of man and various animals into gnotobiotic mice. Am. J. Clin. Nutr. 33:2440–2447 [DOI] [PubMed] [Google Scholar]
- 3. Wilson KH, Perini F. 1988. Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect. Immun. 56:2610–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713 [DOI] [PubMed] [Google Scholar]
- 5. Lawley TD, Croucher NJ, Yu L, Clare S, Sebaihia M, Goulding D, Pickard DJ, Parkhill J, Choudhary J, Dougan G. 2009. Proteomic and genomic characterization of highly infectious Clostridium difficile 630 spores. J. Bacteriol. 191:5377–5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176–1179 doi:10.1038/nature07822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorg JA, Sonenshein AL. 2008. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 190:2505–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilson KH, Kennedy MJ, Fekety FR. 1982. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J. Clin. Microbiol. 15:443–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK. 2011. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 364:422–431 [DOI] [PubMed] [Google Scholar]
- 10. Venugopal AA, Johnson S. 2012. Fidaxomicin: a novel macrocyclic antibiotic approved for treatment of Clostridium difficile infection. Clin. Infect. Dis. 54:568–574 [DOI] [PubMed] [Google Scholar]
- 11. Babakhani F, Bouillaut L, Gomez A, Sears P, Nguyen L, Sonenshein AL. 2012. Fidaxomicin inhibits spore production in Clostridium difficile. Clin. Infect. Dis. 55(Suppl 2):S162–S169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finegold SM, Molitoris D, Vaisanen ML, Song Y, Liu C, Bolanos M. 2004. In vitro activities of OPT-80 and comparator drugs against intestinal bacteria. Antimicrob. Agents Chemother. 48:4898–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tannock GW, Munro K, Taylor C, Lawley B, Young W, Byrne B, Emery J, Louie T. 2010. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology 156:3354–3359 [DOI] [PubMed] [Google Scholar]
- 14. Popoff MR, Rubin EJ, Gill DM, Boquet P. 1988. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect. Immun. 56:2299–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Killgore G, Thompson A, Johnson S, Brazier J, Kuijper E, Pepin J, Frost EH, Savelkoul P, Nicholson B, van den Berg RJ, Kato H, Sambol SP, Zukowski W, Woods C, Limbago B, Gerding DN, McDonald LC. 2008. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J. Clin. Microbiol. 46:431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorg JA, Sonenshein AL. 2010. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J. Bacteriol. 192:4983–4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howerton A, Ramirez N, Abel-Santos E. 2011. Mapping interactions between germinants and C. difficile spores. J. Bacteriol. 193:274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liggins M, Ramirez N, Magnuson N, Abel-Santos E. 2011. Progesterone analogs influence germination of Clostridium sordellii and Clostridium difficile spores in vitro. J. Bacteriol. 193:2776–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramirez N, Liggins M, Abel-Santos E. 2010. Kinetic evidence for the presence of putative germination receptors in Clostridium difficile spores. J. Bacteriol. 192:4215–4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramirez N, Abel-Santos E. 2010. Requirements for germination of Clostridium sordellii spores in vitro. J. Bacteriol. 192:418–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akoachere M, Squires RC, Nour AM, Angelov L, Brojatsch J, Abel-Santos E. 2007. Indentification of an in vivo inhibitor of Bacillus anthracis spore germination. J. Biol. Chem. 282:12112–12118 [DOI] [PubMed] [Google Scholar]
- 22. Gut IM, Blanke SR, van der Donk WA. 2011. Mechanism of inhibition of Bacillus anthracis spore outgrowth by the lantibiotic nisin. ACS Chem. Biol. 6:744–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Artsimovitch I, Seddon J, Sears P. 2012. Fidaxomicin is an inhibitor of the initiation of bacterial RNA synthesis. Clin. Infect. Dis. 55(Suppl 2):S127–S131 [DOI] [PMC free article] [PubMed] [Google Scholar]