Abstract
Two different chelator-based antimicrobial catheter lock solutions, methylene blue-citrate-parabens (MB-CIT) and minocycline-EDTA-25% ethanol (M-EDTA-25% ETOH), were compared in 2-h biofilm eradication experiments. Eradication of both mature and immature Gram-positive, Gram-negative, and fungal biofilms was assessed. M-EDTA-25% ETOH was able to fully eradicate all biofilms within 2 h. MB-CIT was only effective against immature biofilms but was unable to fully eradicate most of the mature biofilms tested.
TEXT
The lumen of central venous catheters (CVC) is an important route for central line-associated bloodstream infections (CLABSI) and is usually locked with heparin or flushed with saline. However, heparin has recently been shown to stimulate Staphylococcus aureus biofilm adherence to the catheter surface (1, 2). Chelators (such as citrate or EDTA), on the other hand, have been shown to have anticoagulant/antithrombotic activity equivalent to that of heparin with the advantage of disrupting biofilm and enhancing antimicrobial agents that penetrate biofilm, such as minocycline (3–5). Chelator-based catheter lock solutions consisting of either methylene blue with citrate (MB-CIT) (Zuragen; Ash Access Technology, Lafayette, IN) or minocycline with EDTA (M-EDTA) have been shown to have activity against organisms embedded in biofilm and were also found to be effective in preventing a CLABSI after a dwell time of at least 24 h (6–10). However, a dwell time of 24 h is not practical as it cannot be accomplished in sick, hospitalized patients requiring different intravenous agents and blood products through various lumens. Previously, we have shown that adding 25% ethanol (ETOH) to M-EDTA would enhance its activity and rapidly eradicate methicillin-resistant Staphylococcus aureus (MRSA) and Candida parapsilosis within 2 h in immature biofilm (11). In the current study, we compare the activities of M-EDTA-25% ETOH and MB-CIT against various resistant bacterial and fungal (Candida species) pathogens in immature and mature biofilm after 2 h of exposure.
Biofilm was grown on sterile silicone discs (1-cm diameter) following a modified Kuhn's method (12). Silicone discs were placed into a 24-well tissue culture plate and incubated overnight at 37°C with 1 ml of human plasma. The plasma was removed and replaced with 1 ml of a 5.5 × 105 CFU/ml inoculum of various organisms. For immature biofilm, the plates were incubated for 24 h at 37°C. For mature biofilm, the plates were incubated for 48 h, with the medium being replaced after 24 h. The inoculum was then removed, and the discs were washed by shaking for 30 min in 0.9% sterile saline. After washing, the discs were placed in 1 ml of lock solution and incubated at 37°C for 2 h. The discs were then removed and placed in 5 ml of 0.9% sterile saline and sonicated to disrupt any remaining biofilm. The resulting solution was then quantitatively cultured by making serial dilutions in 0.9% sterile saline and plating on agar plates (tryptic soy agar [TSA] plus 5% sheep blood for all bacterial organisms and Sabouraud dextrose agar for yeasts). The challenge organisms were methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecium (VRE), multidrug-resistant (MDR) Pseudomonas aeruginosa (PS), MDR Acinetobacter baumanni (An), Candida albicans (CA), and a clinical strain of Candida glabrata. The lock solutions tested were the quadruple combination MB-CIT (0.05% methylene blue, 7.0% sodium citrate, 0.15% methylparaben, and 0.015% propylparaben) and the triple combination M-EDTA-25% ETOH (0.1% minocycline, 3% calcium EDTA, and 25% ethanol). No lock solution (broth) was applied to the controls.
Quantitative recoveries (CFU/disk) from biofilms following exposure to the lock solutions for 2 h are presented in Fig. 1A to C for the Gram-positive, Gram-negative, and fungal organisms, respectively. Reductions in recoveries for MB-CIT versus controls were significant (P < 0.05) for A. baumannii at 24 and 48 h, P. aeruginosa at 24 and 48 h, VRE E. faecium at 24 and 48 h, MRSA at 24 h, C. glabrata at 24 h, and C. albicans at 24 and 48 h. There were no significant reductions (P > 0.05) of C. albicans at 48 h and MRSA at 48 h. M-EDTA-25% ETOH gave significant reductions versus the results for controls (P < 0.01) against both 24- and 48-h biofilms of all organisms. The reductions in viable organisms with M-EDTA-25% ETOH treatment versus the reductions with MB-CIT treatment were significant (P < 0.01) for C. albicans at 24 and 48 h, C. glabrata at 48 h, and MRSA at 24 and 48 h. Trends were present but underpowered to show significance for VRE E. faecium at 48 h (P = 0.07) and A. baumannii at 48 h (P = 0.07). It is notable that the number of organisms recovered in some of the assays was below the lower limit of detection in the serial dilution and quantitative cultures.
Fig 1.
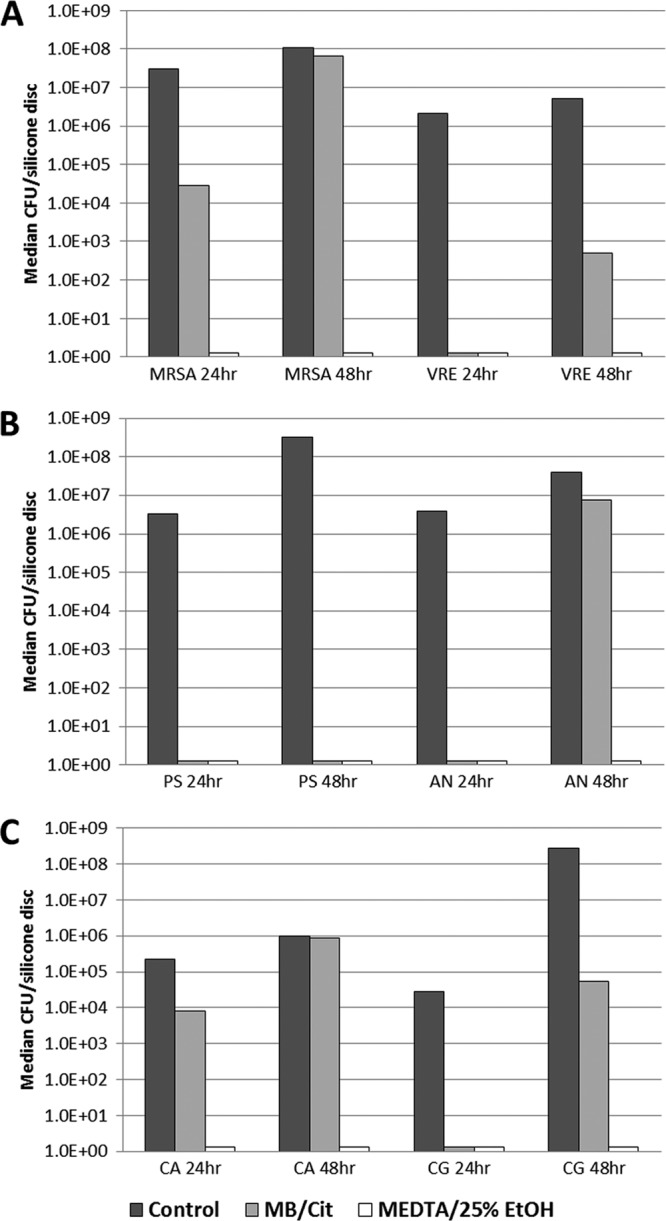
Median quantitative recovery of organisms from biofilm after 24 and 48 h of exposure to resistant Gram-positive organisms (A), Gram-negative organisms (B), and candida (C) followed by 2 h of exposure to control (broth), MB-CIT, and M-EDTA-25% ETOH lock solutions.
Our data show that M-EDTA-25% ETOH and MB-CIT were equally effective in eradicating VRE E. faecium and MDR P. aeruginosa within 2 h in immature biofilm. In addition, these two lock solutions were equally efficacious in rapidly eradicating Acinetobacter in immature biofilm. However, M-EDTA-25% ETOH was more effective than MB-CIT in eradicating MRSA, Candida albicans, and Candida glabrata in mature biofilm.
MB-CIT has been shown to have activity against organisms embedded in biofilm after 24 h of exposure, and Maki et al. have shown that this solution, when locked in for at least 48 h in hemodialysis patients, does significantly decrease the risk of CLABSI (8, 10). However, even in that hemodialysis clinical trial, it was shown that there were breakthrough infections with Staphylococcus aureus and Gram-negative Bacillus organisms in the arm that used MB-CIT, which is consistent with our data. Since staphylococci, Gram-negative organisms (such as Klebsiella spp., P. aeruginosa, Enterobacter spp., and Escherichia coli), and Candida species represent more than 70% of organisms causing CLABSI (13), MB-CIT might have a limited role in prevention. However, based on our data, MB-CIT might be useful as salvage therapy in CLABSI caused by VRE E. faecium or Pseudomonas aeruginosa.
On the other hand, M-EDTA-25% ETOH was highly effective in eradicating all biofilm-embedded organisms tested in this study within 2 h of exposure. Its broad spectrum and rapid cidal activity potentially make it a useful agent for prevention of CLABSI in high-risk patients that require heavy usage of the catheter and concurrent short lock time. In addition, M-EDTA-25% ETOH may be used in the salvage of indwelling CVC in the setting of CLABSI caused by any organism where there is a mature biofilm that has already been formed. Patients with CLABSI are often hospitalized and require various intravenous products, and in that setting, a daily hour-long catheter lock with M-EDTA-25% ETOH may provide a rapid and effective salvage solution to the indwelling CVC.
In conclusion, the two chelator-based lock solutions tested are highly effective in eradicating VRE E. faecium and P. aeruginosa embedded in immature and mature biofilm within 2 h. For eradication of the majority of organisms that cause CLABSI, such as Staphylococcus, Acinetobacter, and Candida species, through a short lock time period, the M-EDTA-25% ETOH lock was highly effective and should be studied further in clinical trials to verify its utility in the prevention and treatment of CLABSI. It also remains to be verified that the complete M-EDTA-25% ethanol lock composition does not impair the functional (mechanical) performance of polymethane catheters within which it will indwell.
Footnotes
Published ahead of print 15 October 2012
REFERENCES
- 1. Shanks RM, Donegan NP, Graber ML, Buckingham SE, Zegans ME, Cheung AL, O'Toole GA. 2005. Heparin stimulates Staphylococcus aureus biofilm formation. Infect. Immun. 73:4596–4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shanks RM, Sargent JL, Martinez RM, Graber ML, O'Toole GA. 2006. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol. Dial. Transplant. 21:2247–2255 [DOI] [PubMed] [Google Scholar]
- 3. Buturovic-Ponikvar J, Gubensek J, Ponikvar R. 2005. Citrate anticoagulation for single-needle hemodialysis: safety and efficacy. Ther. Apher. Dial. 9:237–240 [DOI] [PubMed] [Google Scholar]
- 4. Raad II, Fang X, Keutgen XM, Jiang Y, Sherertz R, Hachem R. 2008. The role of chelators in preventing biofilm formation and catheter-related bloodstream infections. Curr. Opin. Infect. Dis. 21:385–392 [DOI] [PubMed] [Google Scholar]
- 5. Reardon DM, Warner B, Trowbridge EA. 1991. EDTA, the traditional anticoagulant of haematology: with increased automation is it time for a review? Med. Lab. Sci. 48:72–75 [PubMed] [Google Scholar]
- 6. Bleyer AJ, Mason L, Russell G, Raad II, Sherertz RJ. 2005. A randomized, controlled trial of a new vascular catheter flush solution (minocycline-EDTA) in temporary hemodialysis access. Infect. Control Hosp. Epidemiol. 26:520–524 [DOI] [PubMed] [Google Scholar]
- 7. Chatzinikolaou I, Zipf TF, Hanna H, Umphrey J, Roberts WM, Sherertz R, Hachem R, Raad I. 2003. Minocycline-ethylenediaminetetraacetate lock solution for the prevention of implantable port infections in children with cancer. Clin. Infect. Dis. 36:116–119 [DOI] [PubMed] [Google Scholar]
- 8. Maki DG, Ash SR, Winger RK, Lavin P. 2011. A novel antimicrobial and antithrombotic lock solution for hemodialysis catheters: a multi-center, controlled, randomized trial. Crit. Care Med. 39:613–620 [DOI] [PubMed] [Google Scholar]
- 9. Raad I, Chatzinikolaou I, Chaiban G, Hanna H, Hachem R, Dvorak T, Cook G, Costerton W. 2003. In vitro and ex vivo activities of minocycline and EDTA against microorganisms embedded in biofilm on catheter surfaces. Antimicrob. Agents Chemother. 47:3580–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sauer K, Steczko J, Ash SR. 2009. Effect of a solution containing citrate/methylene blue/parabens on Staphylococcus aureus bacteria and biofilm, and comparison with various heparin solutions. J. Antimicrob. Chemother. 63:937–945 [DOI] [PubMed] [Google Scholar]
- 11. Raad I, Hanna H, Dvorak T, Chaiban G, Hachem R. 2007. Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob. Agents Chemother. 51:78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hidron AI, Patel EJJ, Horan TC, Sievert DM, Pollock DA, Fridkin SK, National Healthcare Safety Network Team, Participating National Healthcare Safety Network Facilities 2009. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control. Hosp. Epidemiol. 30:107. [DOI] [PubMed] [Google Scholar]


