Abstract
With the exception of primaquine, tafenoquine, and atovaquone, there are very few antimalarials that target liver stage parasites. In this study, a transgenic Plasmodium berghei parasite (1052Cl1; PbGFP-Luccon) that expresses luciferase was used to assess the anti-liver stage parasite activity of ICI 56,780, a 7-(2-phenoxyethoxy)-4(1H)-quinolone (PEQ), as well as two 3-phenyl-4(1H)-quinolones (P4Q), P4Q-146 and P4Q-158, by using bioluminescent imaging (BLI). Results showed that all of the compounds were active against liver stage parasites; however, ICI 56,780 and P4Q-158 were the most active, with low nanomolar activity in vitro and causal prophylactic activity in vivo. This potent activity makes these compounds ideal candidates for advancement as novel antimalarials.
INTRODUCTION
Malaria kills more than one million people throughout the world annually, and with the increase in drug-resistant parasites, insecticide-resistant vectors, and the lack of a vaccine, new drugs are needed to treat this devastating disease (1). Infection in the mammalian host is initiated when a female Anopheles mosquito ingests a blood meal and injects sporozoites into the vertebrate host. The sporozoites migrate to the liver, invade hepatocytes, and undergo further development resulting in the release of merozoites into the bloodstream (2, 3). In the case of Plasmodium vivax, sporozoites form hypnozoites that can lie dormant in the liver for months or years before causing a relapse (4). The mechanism responsible for the formation and subsequent activation of hypnozoites is not clearly understood. Some suggest that the extended duration of infection may be evolutionarily advantageous, allowing for enhanced opportunities to transmit to new hosts (5). Relapses caused by hypnozoites of P. vivax make this species difficult to eradicate (5).
Currently, primaquine and atovaquone are the only commercially available antimalarials that target liver stage parasites. Primaquine and tafenoquine, both 8-aminoquinolines, are the only drugs shown to kill hypnozoites (6–8). However, due to the short half-life of primaquine in plasma, a lengthy treatment regimen of 14 days is required, which can make patient compliance difficult. Additionally, widespread use in areas of endemicity is limited because individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency are unable to take primaquine due to a high risk of severe hemolytic anemia (9, 10). Therefore, it has become necessary to develop novel compounds that target the liver stages of parasite development and are safe for use in individuals from regions of endemicity (8).
Endochin, a 4(1H)-quinolone compound, was identified in the 1940s as a potential antimalarial. Salzer et al. (11) found that endochin had liver and blood stage activity in avian malaria models; however, due to the lack of appropriate preclinical models, additional studies were not conducted (11). The compounds related to endochin also have activity against Plasmodium cynomolgi liver stages (12), and more recently, studies have shown these compounds to be potent in vitro and in vivo against Plasmodium falciparum and rodent malaria blood stage parasites (13–18) (R. M. Cross, D. L. Flanigan, A. N. LaCrue, T. S. Mutka, F. E. Saenz, K. O. Udenze, L. L. White, J. N. Burrows, S. A. Charman, D. E. Kyle, and R. Manetsch, unpublished data).
Over the years, assessment of the effects of drugs on liver stage development in vivo has involved laborious and often expensive techniques, such as quantitative real-time PCR (qRT-PCR), flow cytometry, and intravital imaging (19–22). These procedures require that animals be sacrificed for tissue collection or imaging and do not allow for real-time monitoring of parasite development following drug treatment. The indirect and most common method of studying liver stage activity requires assessment of the prepatent period (the time elapsed until parasites appear in the peripheral blood following sporozoite infection); however, this technique does not distinguish between liver and blood stage activity of compounds (23, 24).
Recent studies have shown that bioluminescent imaging (BLI), a noninvasive technique that works by capturing the light emitted from the reaction of luciferase and its substrate, can be used to study the development of malaria parasites, as well as the effects of commercially available antimalarials in vivo (25–27). This technique is beneficial because it allows for real-time monitoring of a single individual over time. Here, we describe the use of BLI to evaluate quinolone compounds for their ability to kill liver stage parasites. A transgenic Plasmodium berghei parasite line, PbGFP-Luccon, which expresses luciferase, was used to assess the effects of these novel quinolones on liver stage development. Results show that depending on the concentration, most of the novel quinolones prevent or delay development of liver stage parasites and subsequent blood stage infection. Ultimately, these compounds could be potential candidates for prophylaxis or single-exposure radical cure treatment.
MATERIALS AND METHODS
Drugs and chemicals.
The drugs atovaquone (ATOV), chloroquine (CQ), and primaquine (PQ) used in these studies were obtained from Sigma (St. Louis, MO). The novel compounds ICI 56,780 (14, 28), P4Q-146, and P4Q-158 were synthesized and purified by the Manetsch laboratory at the University of South Florida, Department of Chemistry (Cross et al., unpublished). Structures of the compounds are shown in Fig. 1. For the in vitro studies, compounds were dissolved in dimethyl sulfoxide (DMSO). For the in vivo studies, drugs given per os (p.o.) were reconstituted in polyethylene glycol 400 (PEG400) and administered with an oral gavage cannula at doses of 10, 50, and 100 mg/kg of body weight. Compounds administered subcutaneously (s.c.) were dissolved in 10% DMSO–0.5% Tween and given at 50 mg/kg. All orally administered compounds were sonicated for 1 h and then allowed to sit at room temperature overnight before use. d-Luciferin (Caliper Life Sciences, Hanover, MD), a substrate for luciferase, was dissolved in 1× phosphate-buffered saline (PBS) (Gibco, Grand Island, NY) and injected intraperitoneally (i.p.) at 100 mg/kg.
Fig 1.
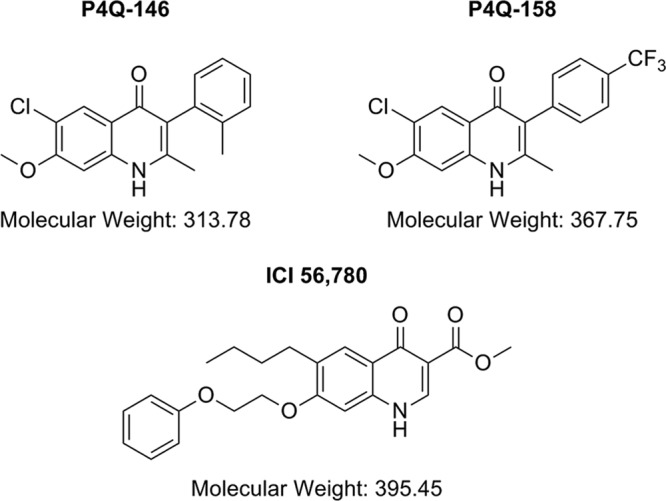
Structures of ICI 56,780, P4Q-146, and P4Q-158.
Animals and parasites.
All mice used in these experiments were female BALB/c mice (the average weight was approximately 18 g) obtained from Harlan (Frederick, MD). The transgenic P. berghei line 1052 Cl1 (Pb1052 Cl1) used in the liver stage studies was obtained from C. J. Janse at Leiden University. This line was generated from the reference clone of ANKA strain c115cy1 and was designed to express a green fluorescent protein (GFP) fusion protein and firefly luciferase (29, 30). Pb1052 Cl1 has two copies of GFP-luc integrated into its genome. One copy is under the control of a constitutively expressed eef1aα promoter, and the other is under the control of an ama1 promoter (29, 30). This study was conducted in compliance with the Guide for the Care and Use of Laboratory Animals of the National Research Council for the National Academies. The protocol was approved by the University of South Florida Institutional Animal Care and Use Committee.
Mosquito infections and sporozoite isolation.
Mice were infected following an i.p. injection of 1 × 106 parasites from cryopreservation as previously described (31). When a parasitemia of >3.0% was observed, the mice were anesthetized using a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) i.p. and placed on a carton containing 100 naive female 4- to 5-day-old Anopheles stephensi. Mosquitoes were allowed to feed for 20 min, after which they were maintained on 10% sucrose ad libitum at 26°C. On day 21 postexposure (PE), infected salivary glands were dissected and sporozoites were isolated and counted as previously described (32–34). Mice then were injected intravenously (i.v.) in the tail vein with 1 × 104 purified sporozoites.
Assessment of liver stage development in vitro.
HepG2 cells (75,000 per well) were seeded into collagen-coated, black 96-well plates with optically clear bottoms (Beckton Dickson, Franklin Lakes, NJ) for viewing on the IVIS spectrum system (Caliper Life Sciences, Hanover, MD) and white 96-well plates for analysis with the TopCount microplate luminometer (Packard, Meriden, CT). Cells were maintained at 37°C in 5% CO2 in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 1.0% penicillin-streptomycin (Sigma), and 1.0% l-glutamine. Mosquito salivary glands were dissected as described above, and 5,000 sporozoites were added per well. Plates were incubated at 37°C for 3 h and then washed three times with PBS. Serial dilutions of experimental compounds were prepared as previously described (35), added to parasite-infected HepG2 cells in triplicate, and incubated at 37°C for 44 h. Following the incubation, cells were washed once with PBS and then lysed with 10 μl of cell culture lysis reagent (Promega Luciferase Assay system kit; Promega, Madison, WI). Immediately after cell lysis, 100 μl of Luciferase Assay substrate was added, and then the parasite lysates were analyzed.
Drug treatment.
There were seven mice per treatment group. Experimental groups were treated with 50 mg/kg s.c. of ICI 56,780. P4Q-146 and P4Q-158 were administered at 50 mg/kg s.c. and 10, 50, and 100 mg/kg p.o. Mice were treated the day before infection (day −1), the day of infection (day 0), and the day after infection (day +1). Untreated, noninfected, and infected mice (infection controls) as well as PQ-treated (50 mg/kg) and ATOV-treated (3 mg/kg) mice (drug controls) were also included. This experiment was conducted in duplicate.
Assessment of liver and blood stage in vivo development.
In vivo imaging was performed 44 h postexposure to assess liver stage development and on days 6, 9, and 13 PE to monitor blood stage development using methods described previously (26). Briefly, the luciferase activities of whole animals and dissected livers were assessed using IVIS Spectrum (Caliper Life Sciences, Hanover, MD). Prior to analysis, the abdomens of all mice were shaved. Animals (n = 7) were injected i.p. with d-luciferin (100 mg/kg), anesthetized with isoflurane, and imaged 5 min postinjection. While animals were continuously exposed to isoflurane, images were acquired with a 23-cm field of view (FOV), medium binning factor, and an exposure time of 20 to 120 s. To assess luciferase activity in dissected livers, two of the seven mice for each treatment group were given a second i.p. injection of d-luciferin. Livers were dissected 2 to 3 min postinjection and placed in a petri dish, and images were acquired using a 10-cm FOV with the same binning and exposure times as above. Images were analyzed using the Living Image 3.0 software (Caliper Life Sciences, Hanover, MD).
For blood stage assessment, BLI and Giemsa-stained blood smears were performed on the five remaining mice per group on days 3, 9, and 13 PE. Only Giemsa-stained smears were prepared on days 21 and 30 (final day of experiment) PE.
Statistical analysis.
All statistical analyses were carried out using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA). All values were expressed as means and standard deviations (SD). A P value of ≤0.05 was considered statistically significant. Data were analyzed using one-way analysis of variance (ANOVA) with Dunnett's post hoc test when comparing treated group means to those of an untreated control.
RESULTS
In vitro liver assay.
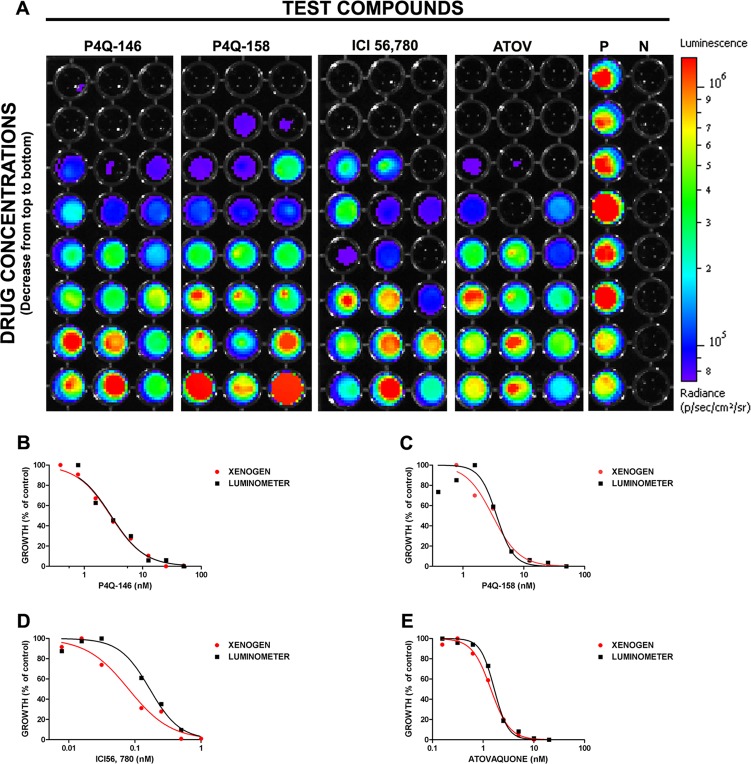
An in vitro liver stage assay was conducted to determine if three novel quinolone antimalarial compounds, ICI 56,780, P4Q-146, and P4Q-158, possess liver stage activity against P. berghei parasites. HepG2 cells (75,000 cells/well) were infected with 5,000 sporozoites per well, treated in duplicate with serial dilutions of experimental compounds, and visualized 44 h PE. ICI 56,780, P4Q-146, and P4Q-158 were active against liver stage parasites in vitro with 50% inhibitory concentrations (IC50s) of 0.08 nM, 2.82 nM, and 3.07 nM, respectively (Fig. 2A). As expected, ATOV (positive drug control) was active against liver stage parasites with an IC50 of 1.42 nM. In comparison, CQ (negative-control drug), which does not act on the liver stages, had an IC50 of >1.2 μM (data not shown). Dose-response curves (Fig. 2B to E) were used to obtain the IC50s for all of the compounds. Table 1 shows the relationship between the total flux obtained from the Xenogen IVIS and relative luminescence units (RLU) obtained from the luminometer. Although the IC50s are not identical, it is clear that the Xenogen IVIS and the luminometer show similar dose-response curves of drug activity, with ICI 56,780 being the most active and CQ being the least active.
Fig 2.
In vitro BLI analysis of the activity of ICI 56,780, P4Q-146, and P4Q-158 against Plasmodium berghei liver stage parasites. HepG2 cells (75,000 per well) were infected with Pb1052 Cl1 sporozoites (5,000 per well). The infected cells were treated with a series of eight 3-fold dilutions of each compound in triplicate. Concentrations started at 50 ng/ml for both P4Q-146 and P4Q-158, 1 ng/ml for ICI 56,780, and 20 ng/ml for atovaquone. At 44 h postexposure, plates were analyzed using the Xenogen IVIS spectrum. (A) All of the compounds with the exception of chloroquine (CQ) were active against Plasmodium berghei parasites in vitro. IC50s for ICI 56,780, P4Q-146, and P4Q-158 were 0.08, 2.82, and 3.07 nM, respectively. As expected, atovaquone (ATOV; positive drug control) was active against the liver stage parasites with an IC50 of 1.42 nM. Infected but untreated cells were used as a positive control (P), and noninfected HepG2 cells were used as a negative control (N). (B to E) Sigmoidal plots used to obtain IC50s from the Xenogen IVIS spectrum and luminometer data for atovaquone (B), ICI 56,780 (C), P4Q-146 (D), and P4Q-158 (E) are shown.
Table 1.
Comparison of IC50s from Xenogen IVIS and TopCount luminometera
| Treatment | Xenogen IC50 (nM) | R2 | TopCount IC50 (nM) | R2 |
|---|---|---|---|---|
| None (untreated) | NA | NA | NA | NA |
| Atovaquone | 1.42 ± 0.02 | 0.997 | 1.66 ± 0.02 | 0.996 |
| ICI 56,780 | 0.08 ± 0.07 | 0.971 | 0.17 ± 0.04 | 0.981 |
| P4Q-146 | 2.82 ± 0.03 | 0.992 | 2.88 ± 0.06 | 0.964 |
| P4Q-158 | 3.07 ± 0.04 | 0.978 | 3.58 ± 0.06 | 0.918 |
| CQ | ≥625 | NA | ≥625 | NA |
Our studies show that the Xenogen and TopCount results are reproducible and that the two methods show similar trends in compound activity. Data are given as means ± standard errors. NA, not applicable.
In vivo liver assays. (i) ICI 56,780.
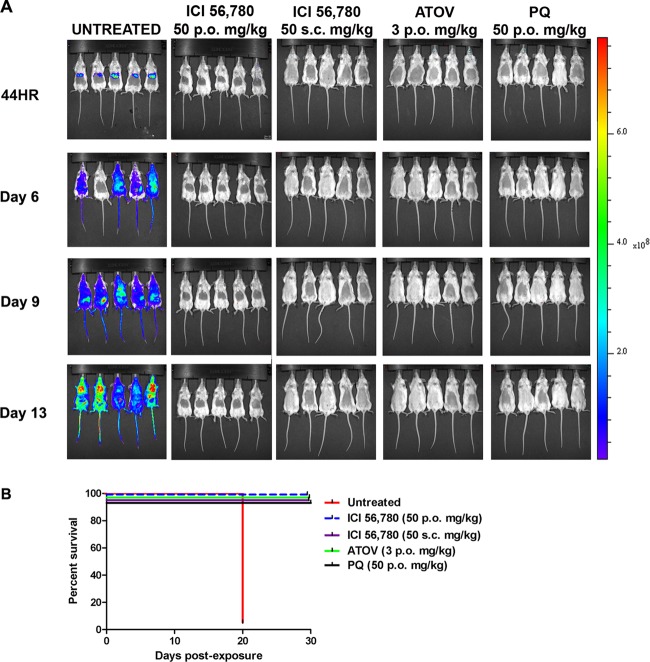
In this study, ICI 56,780 was administered at 50 mg/kg s.c. and p.o., and liver stage development was assessed at 44 h PE. Results show that at both drug administration routes ICI 56,780 prevented the development of liver stage parasites (Fig. 3A) and mice remained parasitemia free until the end of the experiment on day 30 PE (Fig. 3B). Mice treated with the control compounds atovaquone (3 mg/kg) and primaquine (50 mg/kg) also remained clear of parasitemia throughout the assay (Fig. 3B). Dissected livers showed absence of a luminescence signal (Fig. 4). The luminescence signals in the dissected untreated livers were lower than those observed in the intact mice; however, this is similar to what was observed in studies by Ploemen et al. (27).
Fig 3.
Analysis of in vivo liver stage activity of ICI 56,780 using bioluminescent imaging (BLI). Mice were treated the day before, the day of, and the day following infection with 10,000 Pb1052 Cl1 sporozoites. (A) At 44 h (44HR) postinfection, mice treated per os and subcutaneously with ICI 56,780 (50 mg/kg) did not develop a liver stage infection and remained free of blood stage infection until the final day of imaging (day 13 PI). As expected, the untreated mice showed heavy infections in the liver. (B) Survival curve analysis shows by comparison with the untreated control that ICI 56,780 is a causal prophylactic.
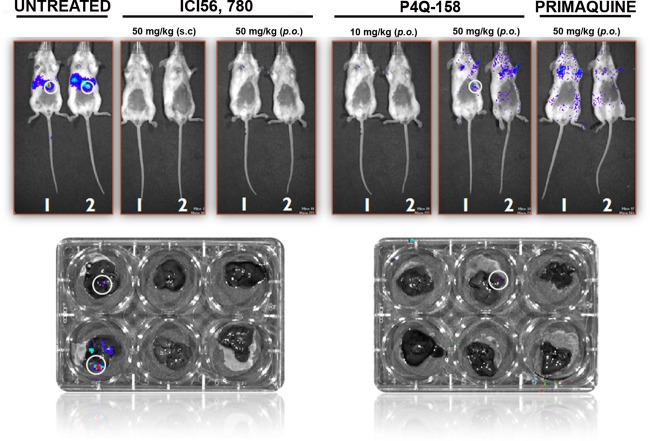
Fig 4.
Analysis of extracted livers via BLI. Livers from mice treated with ICI 56,780, P4Q-158, and primaquine were dissected to confirm that the luminescence signal was coming from the liver. Livers extracted from mice treated with ICI 56,780 did not have a signal, whereas one of the mice treated with P4Q-158 (50 mg/kg p.o.) did have a signal. As expected, mice treated with primaquine were parasitemia free.
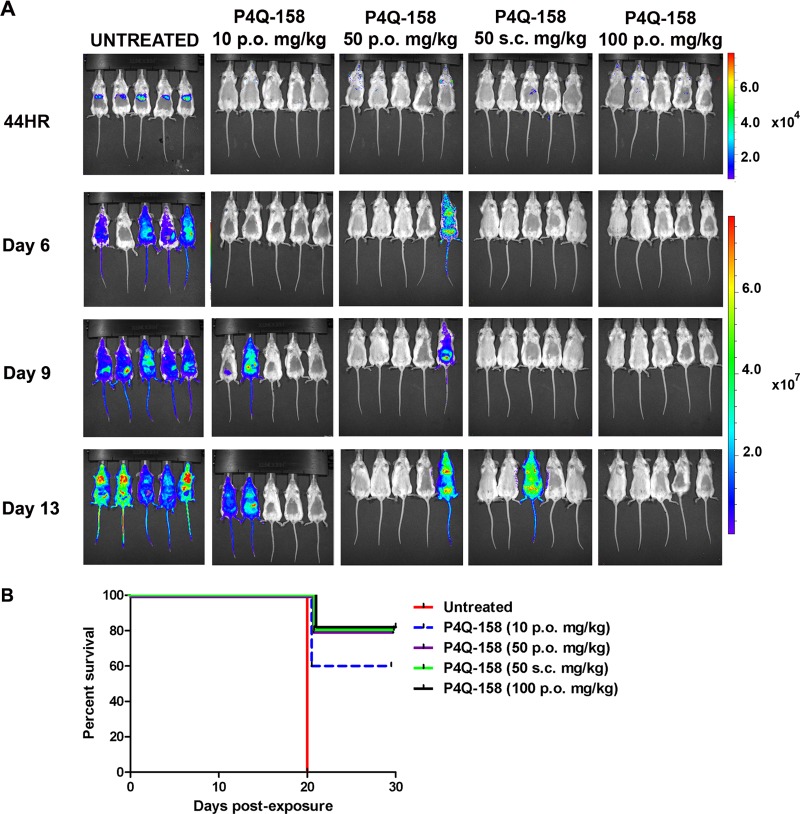
(ii) P4Q-158.
There was no detectable bioluminescent signal at 44 h PE in mice treated with P4Q-158; however, by day 13 PE, 2 of 5 mice in the 10-mg/kg group and 1 of 5 mice in both 50-mg/kg groups developed a blood stage infection (Fig. 5A). Mice treated with10 mg/kg had a survival rate of 60%, and those treated with 50 and 100 mg/kg had the highest survival rate, 80%, by the end of the study (day 30 PE). Compared to the untreated mice, all of the treatment groups had a significantly higher survival rate (Dunnett's multiple comparison test, P < 0.05; Fig. 5B). Dissection of livers from mice treated with P4Q-158 revealed one of the two mice had a liver infection when treated p.o. (50 mg/kg) (Fig. 4).
Fig 5.
Analysis of in vivo liver stage activity of P4Q-158 using bioluminescent imaging (BLI). Mice were treated the day before, the day of, and the day following infection with 10,000 Pb1052 Cl1 sporozoites. (A) At 44 h (44HR) postexposure, mice treated with P4Q-158 at 10, 50, and 100 mg/kg had significantly lower infection rates than the untreated mice (Dunnett's multiple-comparison test, P < 0.05). (B) Survival curve analysis showed that mice treated with P4Q-158 had a significantly higher survival rate than the untreated mice (Dunnett's multiple-comparison test, P < 0.05).
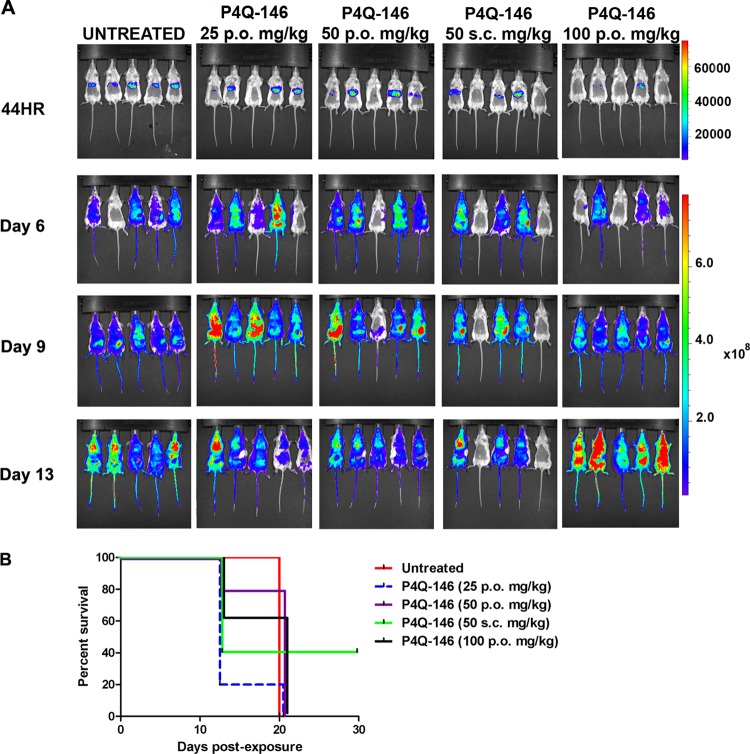
(iii) P4Q-146.
Mice treated with P4Q-146 had detectable parasitemia at 44 h PE (Fig. 6A). The most effective dose was 50 mg/kg s.c., with 2 of 5 mice (40%) surviving until the end of the assay on day 30 PE. However, the survival rate was not significantly different from that of the untreated group.
Fig 6.
Analysis of in vivo liver stage activity of P4Q-146 using bioluminescent imaging (BLI). Mice were treated the day before, the day of, and the day following infection with 10,000 Pb1052 Cl1 sporozoites. (A) At 44 h (44HR) postexposure, a majority of the mice treated with P4Q-146 had liver stage infections. (B) Survival curve analysis showed that mice treated with 50 mg/kg s.c. of P4Q-146 had the highest survival rate, 40%; however, this was not significantly different from the survival rate of the untreated mice.
DISCUSSION
To date, there is a limited arsenal of compounds that target malaria liver stage parasites. Both atovaquone and primaquine are active against liver stage parasites; however, primaquine is the only one that targets the dormant hypnozoite stages. Due to drug resistance and severe side effects, it has become necessary to develop new antimalarials to target this key developmental stage (36–38). Here, we have used bioluminescent imaging to evaluate novel quinolone compounds for activity against liver stage parasites in vitro and in vivo. Our studies show that in vitro, P4Q-146, P4Q-158, and ICI 56,780 all have potent liver stage activity; however, in vivo, only P4Q-158 and ICI 56,780 showed activity against the liver stages. For all of these compounds, it is still unknown whether or not they target the dormant hypnozoite stages.
ICI 56,780.
Previous studies have shown that ICI 56,780 is effective against P. cynomolgi and P. berghei liver stage parasites in vivo (12, 28). However, because this compound is also a potent blood schizonticide, the authors could not exclude a possible suppressive prophylactic activity of ICI 56,780 against the blood stages (28). Therefore, to confirm the in vivo liver stage activity of this compound, we used BLI. We found that not only is ICI 56,780 active against liver stage parasites in vitro, but it is also effective as a causal prophylactic in vivo when administered at 50 mg/kg p.o. or s.c. These data suggest ICI 56,780 kills growing liver stages in addition to its known radical curative activity against hypnozoites of P. cynomolgi (12).
P4Q-146 and P4Q-158.
Due to the remarkable antimalarial activity of 4(1H)-quinolones against erythrocytic and exoerythrocytic stages, the endochin series has recently been revived by several research teams (13, 14, 16–18). Using previously developed reaction conditions (39), our team synthesized and tested >100 3-phenyl-substituted P4Qs, of which P4Q-146 and P4Q-158 have been shown to have potent activity against P. falciparum blood stage parasites in vitro (14; Cross et al., unpublished). Here, we have shown that they are also very active against P. berghei liver stage parasites in vitro. When tested in vivo, P4Q-158 was highly potent against liver stage parasites, while P4Q-146 was unable to effectively prevent the development of liver stage parasites in vivo. The decreased in vivo activity of P4Q-146 over P4Q-158 may be due to a combination of potency differences against the parasite and suboptimal physicochemical properties of the test compound. In comparison to P4Q-158, P4Q-146 suffers from microsomal degradations and a shorter half-life decreasing the exposure (Cross et al., unpublished). Nevertheless, this set of experiments demonstrate the value and complementarity of the in vitro and in vivo assay conditions that allow for the assessment of anti-liver stage activity of potent test compounds as well as compounds with poor physicochemical properties and reduced bioavailabilities.
Summary.
Currently, the mechanism of action for ICI 56,780, P4Q-146, and P4Q-158 is unknown, but they are likely to target mitochondrial respiration. However, we can speculate that because of the structural differences, they do not work by the same mechanism as the 8-aminoquinoline primaquine. This would be ideal because the problems associated with hemolytic toxicity in G6PD deficiency patients should not be a concern.
Due to the limited arsenal of anti-liver stage compounds, it has become necessary to find novel antimalarials to target this key developmental stage. In these studies, we have shown that two novel quinolones have liver stage activity in vitro and in vivo against P. berghei parasites. Compounds with this type of activity could be beneficial for controlling the spread of disease in areas of malaria endemicity and for preventing disease in travelers to regions of endemicity. Further improvement of the physiochemical properties and bioavailability of quinolones could lead to a new generation of compounds for the radical cure of malaria.
ACKNOWLEDGMENTS
This work was supported by the National Institute of General Medical Sciences (R01 GM097118) and the Medicines for Malaria Venture.
We thank Anupam Pradhan, Anuradha Srivastava, Brenda T. Beerntsen, Renee Roberts, and Maggie Schlarman for proofreading the manuscript and providing valuable suggestions. We thank John Adams, Naresh Singh, and Sandra Kennedy for rearing the mosquitoes.
A.N.L., F.S., and D.E.K. conceived and designed the experiments; A.N.L., F.S., K.O.U., S.S., and T.S.M. performed the experiments; M.C., A.M., and R.M. synthesized the compounds; A.N.L., F.S., and D.E.K. analyzed the data; and A.N.L., F.S., R.M., and D.E.K. wrote the paper.
Footnotes
Published ahead of print 5 November 2012
REFERENCES
- 1. Murray CJL, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. 2012. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379:413–431 [DOI] [PubMed] [Google Scholar]
- 2. Shortt HE, Fairley NH, Covell G, Shute PG, Garnham PC. 1951. The pre-erythrocytic stage of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 44:405–419 [DOI] [PubMed] [Google Scholar]
- 3. Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT. 2006. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313:1287–1290 [DOI] [PubMed] [Google Scholar]
- 4. Alonso PL, Binka BQF, Brewer T, Chandra R, Culpepper J, Dinglasan R, Duncan K, Duparc S, Fukuda M, Laxminarayan R, MacArthur JR, Magill A, Marzetta C, Milman J, Mutabingwa T, Nosten F, Nwaka S, Nyunt M, Ohrt C, Plowe CV, Pottage J, Price R, Ringwald P, Serazin A, Shanks D, Sinden R, Tanner M, Vial H, Ward SA, Wellems TE, Wells T, White N, Wirth D, Yeung S, Yuthavong Y, Alonso PL, Djimde A, Magill A, Milman J, Nájera J, Plowe CV, Wells T, Yeung S, Kremsner P, Mueller I, Newman RD, Rabinovich R. 2011. A research agenda for malaria eradication: drugs. PLoS Med. 8:e1000402 doi:10.1371/journal.pmed.1000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dembele L, Gego A, Zeeman AM, Franetich JF, Silvie O, Rametti A, Le Grand R, Dereuddre-Bosquet N, Sauerwein R, van Gemert GJ, Vaillant JC, Thomas AW, Snounou G, Kocken CH, Mazier D. 2011. Towards an in vitro model of Plasmodium hypnozoites suitable for drug discovery. PLoS One 6:e18162 doi:10.1371/journal.pone.0018162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baird JK, Hoffman SL. 2004. Primaquine therapy for malaria. Clin. Infect. Dis. 39:1336–1345 [DOI] [PubMed] [Google Scholar]
- 7. Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. 2006. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am. J. Trop. Med. Hyg. 75:402–415 [PubMed] [Google Scholar]
- 8. Rodrigues T, Prudencio M, Moreira R, Mota MM, Lopes F. 2012. Targeting the liver stage of malaria parasites: a yet unmet goal. J. Med. Chem. 55:995–1012 [DOI] [PubMed] [Google Scholar]
- 9. Vale N, Moreira R, Gomes P. 2009. Primaquine revisited six decades after its discovery. Eur. J. Med. Chem. 44:937–953 [DOI] [PubMed] [Google Scholar]
- 10. Ziai M, Amirhakimi GH, Reinhold JG, Tabatabee M, Gettner ME, Bowman JE. 1967. Malaria prophylaxis and treatment in G-6-PD deficiency. An observation on the toxicity of primaquine and chloroquine. Clin. Pediatr. (Phila.) 6:242–243 [DOI] [PubMed] [Google Scholar]
- 11. Salzer W, Andersag THH. 1948. A new type of compounds active against avian malaria. Chem. Ber. 81:12–19 [Google Scholar]
- 12. Puri SK, Dutta GP. 1990. Quinoline esters as potential antimalarial drugs: effect on relapses of Plasmodium cynomolgi infections in monkeys. Trans. R. Soc. Trop. Med. Hyg. 84:759–760 [DOI] [PubMed] [Google Scholar]
- 13. Biagini GA, Fisher N, Shone AE, Mubaraki MA, Srivastava A, Hill A, Antoine T, Warman AJ, Davies J, Pidathala C, Amewu RK, Leung SC, Sharma R, Gibbons P, Hong DW, Pacorel B, Lawrenson AS, Charoensutthivarakul S, Taylor L, Berger O, Mbekeani A, Stocks PA, Nixon GL, Chadwick J, Hemingway J, Delves MJ, Sinden RE, Zeeman AM, Kocken CH, Berry NG, O'Neill PM, Ward SA. 2012. Generation of quinolone antimalarials targeting the Plasmodium falciparum mitochondrial respiratory chain for the treatment and prophylaxis of malaria. Proc. Natl. Acad. Sci. U. S. A. 109:8298–8303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cross RM, Monastyrskyi A, Mutka TS, Burrows JN, Kyle DE, Manetsch R. 2010. Endochin optimization: structure-activity and structure-property relationship studies of 3-substituted 2-methyl-4(1H)-quinolones with antimalarial activity. J. Med. Chem. 53:7076–7094 [DOI] [PubMed] [Google Scholar]
- 15. Cross RM, Namelikonda NK, Mutka TS, Luong L, Kyle DE, Manetsch R. 2011. Synthesis, antimalarial activity, and structure-activity relationship of 7-(2-phenoxyethoxy)-4(1H)-quinolones. J. Med. Chem. 54:8321–8327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pidathala C, Amewu R, Pacorel B, Nixon GL, Gibbons P, Hong WD, Leung SC, Berry NG, Sharma R, Stocks PA, Srivastava A, Shone AE, Charoensutthivarakul S, Taylor L, Berger O, Mbekeani A, Hill A, Fisher NE, Warman AJ, Biagini GA, Ward SA, O'Neill PM. 2012. Identification, design and biological evaluation of bisaryl quinolones targeting Plasmodium falciparum type II NADH:quinone oxidoreductase (PfNDH2). J. Med. Chem. 55:1831–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winter R, Kelly JX, Smilkstein MJ, Hinrichs D, Koop DR, Riscoe MK. 2011. Optimization of endochin-like quinolones for antimalarial activity. Exp. Parasitol. 127:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winter RW, Kelly JX, Smilkstein MJ, Dodean R, Hinrichs D, Riscoe MK. 2008. Antimalarial quinolones: synthesis, potency, and mechanistic studies. Exp. Parasitol. 118:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruna-Romero O, Hafalla JC, Gonzalez-Aseguinolaza G, Sano G, Tsuji M, Zavala F. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499–1502 [DOI] [PubMed] [Google Scholar]
- 20. Prudencio M, Mota MM, Mendes AM. 2011. A toolbox to study liver stage malaria. Trends Parasitol. 27:565–574 [DOI] [PubMed] [Google Scholar]
- 21. Prudencio M, Rodrigues CD, Ataide R, Mota MM. 2008. Dissecting in vitro host cell infection by Plasmodium sporozoites using flow cytometry. Cell. Microbiol. 10:218–224 [DOI] [PubMed] [Google Scholar]
- 22. Tarun AS, Baer K, Dumpit RF, Gray S, Lejarcegui N, Frevert U, Kappe SH. 2006. Quantitative isolation and in vivo imaging of malaria parasite liver stages. Int. J. Parasitol. 36:1283–1293 [DOI] [PubMed] [Google Scholar]
- 23. Coppi A, Cabinian M, Mirelman D, Sinnis P. 2006. Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrob. Agents Chemother. 50:1731–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panchal D, Bhanot P. 2010. Activity of a trisubstituted pyrrole in inhibiting sporozoite invasion and blocking malaria infection. Antimicrob. Agents Chemother. 54:4269–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meister S, Plouffe DM, Kuhen KL, Bonamy GM, Wu T, Barnes SW, Bopp SE, Borboa R, Bright AT, Che J, Cohen S, Dharia NV, Gagaring K, Gettayacamin M, Gordon P, Groessl T, Kato N, Lee MC, McNamara CW, Fidock DA, Nagle A, Nam TG, Richmond W, Roland J, Rottmann M, Zhou B, Froissard P, Glynne RJ, Mazier D, Sattabongkot J, Schultz PG, Tuntland T, Walker JR, Zhou Y, Chatterjee A, Diagana TT, Winzeler EA. 2011. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 334:1372–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mwakingwe A, Ting LM, Hochman S, Chen J, Sinnis P, Kim K. 2009. Noninvasive real-time monitoring of liver-stage development of bioluminescent Plasmodium parasites. J. Infect. Dis. 200:1470–1478 [DOI] [PubMed] [Google Scholar]
- 27. Ploemen IH, Prudencio M, Douradinha BG, Ramesar J, Fonager J, van Gemert GJ, Luty AJ, Hermsen CC, Sauerwein RW, Baptista FG, Mota MM, Waters AP, Que I, Lowik CW, Khan SM, Janse CJ, Franke-Fayard BM. 2009. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS One 4:e7881 doi:10.1371/journal.pone.0007881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ryley JF, Peters W. 1970. The antimalarial activity of some quinolone esters. Ann. Trop. Med. Parasitol. 64:209–222 [DOI] [PubMed] [Google Scholar]
- 29. Franke-Fayard B, Trueman H, Ramesar J, Mendoza J, van der Keur M, van der Linden R, Sinden RE, Waters AP, Janse CJ. 2004. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 137:23–33 [DOI] [PubMed] [Google Scholar]
- 30. Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, Engelmann S, Matuschewski K, van Gemert GJ, Sauerwein RW, Waters AP. 2006. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol. Biochem. Parasitol. 145:60–70 [DOI] [PubMed] [Google Scholar]
- 31. Lacrue AN, Scheel M, Kennedy K, Kumar N, Kyle DE. 2011. Effects of artesunate on parasite recrudescence and dormancy in the rodent malaria model Plasmodium vinckei. PLoS One 6:e26689 doi:10.1371/journal.pone.0026689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lacrue AN, James AA, Beerntsen BT. 2005. The novel Plasmodium gallinaceum sporozoite protein, Pg93, is preferentially expressed in the nucleus of oocyst sporozoites. Am. J. Trop. Med. Hyg. 73:634–643 [PubMed] [Google Scholar]
- 33. LaCrue AN, Sivaguru M, Walter MF, Fidock DA, James AA, Beerntsen BT. 2006. A ubiquitous Plasmodium protein displays a unique surface labeling pattern in sporozoites. Mol. Biochem. Parasitol. 148:199–209 [DOI] [PubMed] [Google Scholar]
- 34. Saenz FE, Balu B, Smith J, Mendonca SR, Adams JH. 2008. The transmembrane isoform of Plasmodium falciparum MAEBL is essential for the invasion of Anopheles salivary glands. PLoS One 3:e2287 doi:10.1371/journal.pone.0002287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cross RM, Maignan JR, Mutka TS, Luong L, Sargent J, Kyle DE, Manetsch R. 2011. Optimization of 1,2,3,4-tetrahydroacridin-9(10H)-ones as antimalarials utilizing structure-activity and structure-property relationships. J. Med. Chem. 54:4399–4426 [DOI] [PubMed] [Google Scholar]
- 36. Cohen RJ, Sachs JR, Wicker DJ, Conrad ME. 1968. Methemoglobinemia provoked by malarial chemoprophylaxis in Vietnam. N. Engl. J. Med. 279:1127–1131 [DOI] [PubMed] [Google Scholar]
- 37. Coleman MD, Coleman NA. 1996. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf. 14:394–405 [DOI] [PubMed] [Google Scholar]
- 38. Talisuna AO, Bloland P, D'Alessandro U. 2004. History, dynamics, and public health importance of malaria parasite resistance. Clin. Microbiol. Rev. 17:235–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cross RM, Manetsch R. 2010. Divergent route to access structurally diverse 4-quinolones via mono or sequential cross-couplings. J. Org. Chem. 75:8654–8657 [DOI] [PubMed] [Google Scholar]