Abstract
This report describes the pharmacokinetics of colistin methanesulfonate (CMS) and colistin in five intensive care unit patients receiving continuous venovenous hemodiafiltration. For CMS, the mean maximum concentration of drug in plasma (Cmax) after the fourth dose was 6.92 mg/liter and total clearance (CL) 8.23 liters/h. For colistin, the mean concentration was 0.92 mg/liter and CL/metabolized fraction (fm) 18.91 liters/h. Colistin concentrations were below the current MIC breakpoints, and the area under the concentration-time curve for the free, unbound fraction of the drug over 24 h in the steady state divided by the MIC (fAUC/MIC) was lower than recommended, suggesting that a dosage regimen of 160 mg CMS every 8 h (q8h) is inadequate.
TEXT
The use of colistin, an old antibiotic, has recently reemerged due to the increased prevalence of infections caused by multidrug-resistant Gram-negative bacteria (MDR-GNB), especially in critically ill patients (1, 2). Such patients often suffer from acute renal impairment necessitating renal replacement therapy (3). However, there are only scarce data on the effect of renal replacement methods on the pharmacokinetics of colistin (4–7). The present study was undertaken to study the elimination of colistin methanesulfonate (CMS) and colistin in patients undergoing continuous venovenous hemodiafiltration (CVVHDF) to contribute to the knowledge regarding the pharmacokinetics of colistin in this population.
Patients admitted to the Critical Care Unit of University General Hospital Attikon in Athens, Greece, were eligible for the study if they fulfilled the following inclusion criteria: (i) they were 18 years of age or older; (ii) they were receiving colistin treatment as part of their standard care due to probable or documented infection by MDR-GNB; and (iii) they were receiving CVVHDF treatment as renal replacement therapy. For each patient, the following were recorded: age, body weight, serum creatinine (s-Cr), creatine clearance (CrCL), serum albumin (s-Alb), hemoglobin (Hb), hematocrit (Hct), and Acute Physiology and Chronic Health Evaluation II (APACHE II) score on the first day of colistin administration.
The study was approved by the Ethics Committee of the Hospital (registration no. 3/30-3-07). Informed consent was provided by the patients or nearest relatives.
CMS (colistin methanesulfonate [Colistin/Norma]; Norma Hellas, Athens, Greece) was administered every 8 h (q8h) by intravenous infusion over 15 min according to local practice at a dose of 2 million units (MU), equivalent to 160 mg CMS, dissolved in 100 ml of normal saline solution.
Vascular access was obtained by insertion of a double lumen dialysis catheter. Each patient was dialyzed using the Prisma system (Hospal-Gambro). Continuous venovenous hemodiafiltration (CVVHDF) was performed using an acrylonitrile and sodium methallyl sulfonate copolymer hollow-fiber high-flux hemofilter (AN69 HF) with a membrane surface area of 0.9 m2 (M100 predilution filter set; Hospal). Hemosol B0 solution (Gambro) was infused as a substitution and dialysate fluid. The blood flow rate was set at 120 to 150 ml/min, the filtration rate at 0.6 to 0.9 liters/h, and the dialysate fluid rate at 1.5 to 2.5 liters/h.
Venous blood was collected immediately before the first and fourth infusion and at 15, 30, 60, 90, 120, 180, 240, and 360 min after the end of the first and fourth infusions. Prefilter and postfilter blood samples were also collected immediately before and at 60, 120, and 360 min after the end of the fourth infusion. All blood samples were immediately refrigerated and centrifuged, and plasma was collected and stored at −70°C until assayed.
Concentrations of colistin A and B in plasma were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (8, 9). Plasma concentrations of CMS were determined by hydrolysis of CMS to colistin using the method described by Li et al. (10) with modifications (9). The interday CV and accuracy for colistin were <4.4% and ±<1.9%, and the interday CV and accuracy for CMS were <8.2% and ±<3.2%.
Total clearance was calculated, based on plasma concentrations, as CLtotal = dose/AUC0–8, where AUC0–8 represents the area under the concentration-time curve from 0 to 8 h, assuming that the steady state was achieved, and extrapolation to 8 h from the three last observations, after omission of apparent outliers. For colistin, the metabolized fraction (fm) was undefined in this study, and clearance is therefore expressed as CLtotal/fm (CLtotal/fm = dose CMS/AUC0–8, colistin in molar terms). The dialyzer extraction ratio was calculated as E = (Cin − Cout)/Cin, where Cin and Cout are the total plasma concentrations before and after filtering, and dialyzer clearance as CLHDF = E × Q × (1 − Hct), where Q represents the blood flow rate through the filter. Creatinine clearance was estimated with the Cockcroft-Gault equation.
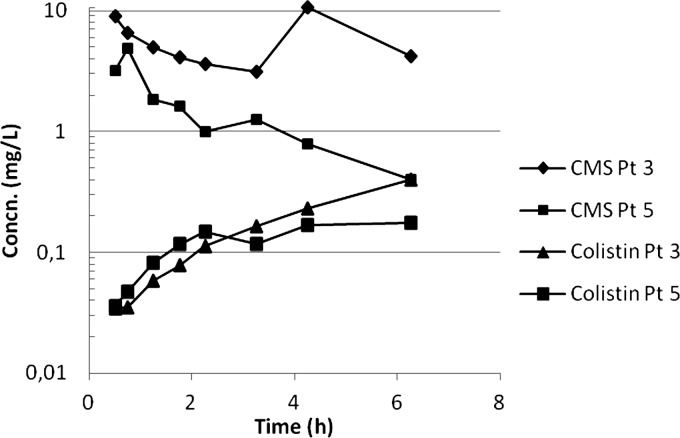
Five patients (3 female) of age 57 to 83 years with a mean Apache II score of 18 (range, 15 to 24) were included (Table 1). Concentration data from the first dose were available only from two patients for whom the CMS Cmax values were 4.89 and 8.90 mg/liter and time to maximum concentration of drug in serum (Tmax) values were 0.75 and 0.5 h, respectively. The colistin Cmax values were 0.18 and 0.40 mg/liter, and the Tmax was the last observed time point, 6 h (Fig. 1).
Table 1.
Patient characteristicsa
| Patient or value category | Gender | Age (yr) | Weight (kg) | s-Cr (mg/dl) | CrCL (ml/min) | s-Alb (g/dl) | Hb (g/dl) | Hct (%) | APACHE II score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 83 | 85 | 0.6 | 95.3 | 3.3 | 22.7 | 6.8 | 15 |
| 2 | M | 62 | 95 | 8.7 | 27.8 | ||||
| 3 | F | 65 | 70 | 1.5 | 41.3 | 2.9 | 9.4 | 29.1 | 18 |
| 4 | M | 57 | 70 | 1.6 | 50.4 | 2.2 | 6.9 | 22.3 | 24 |
| 5 | F | 62 | 70 | 0.5 | 128.9 | 3.1 | 25.0 | 15 | |
| Mean | 67 | 78 | 1.1 | 79.0 | 2.9 | 11.9 | 25.1 |
M, male; F, female.
Fig 1.
Concentrations of CMS and colistin after first dose in two patients.
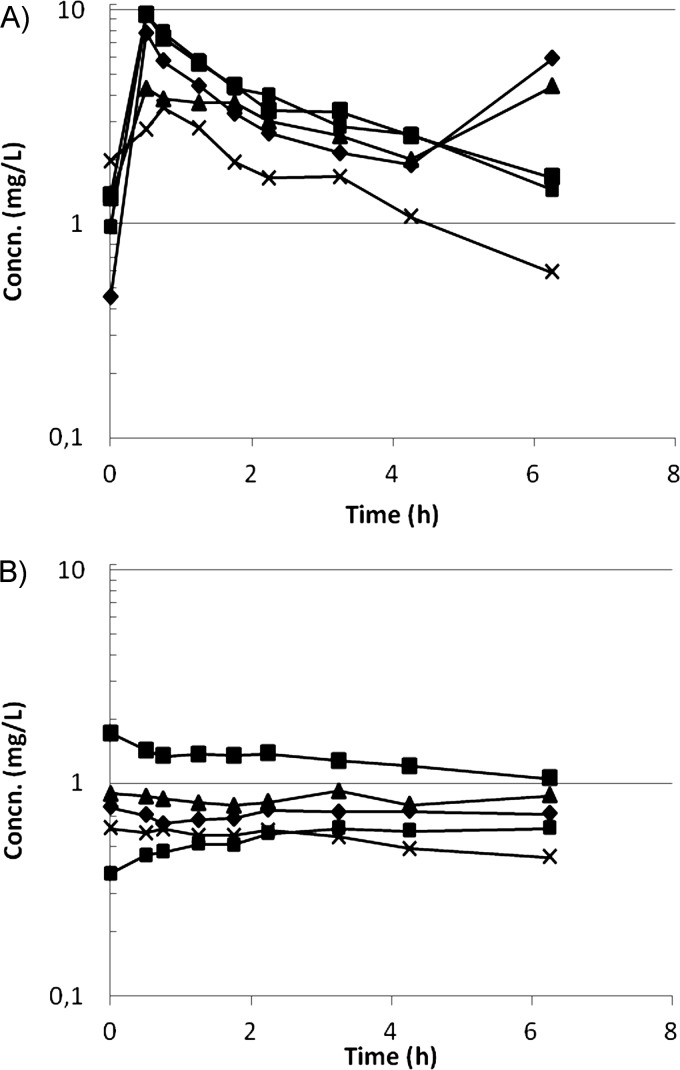
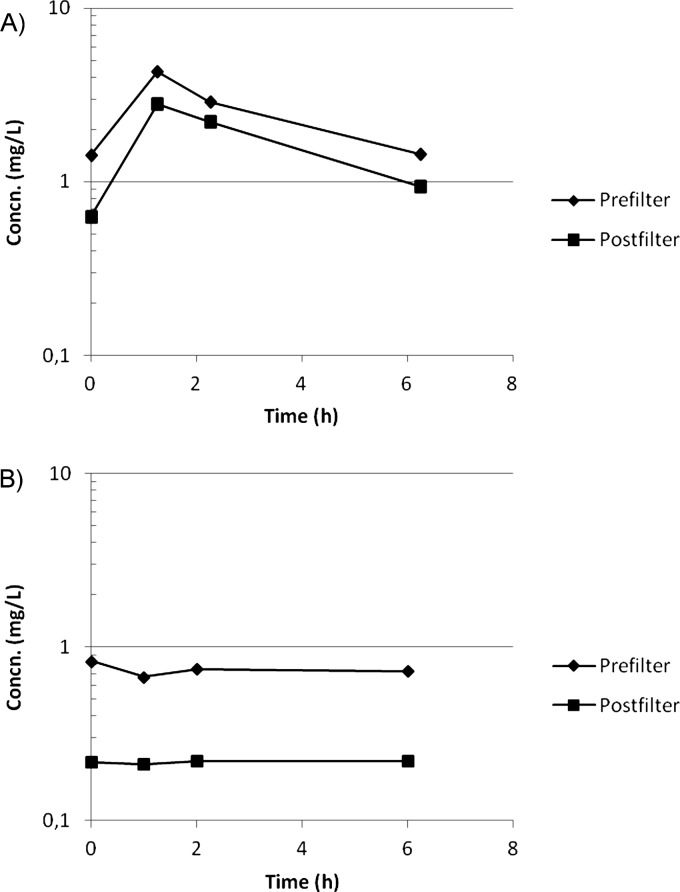
After the fourth dose, Cmax for CMS was 6.92 ± 2.83 mg/liter (mean ± standard deviation [SD]), Tmax was 0.5 h, and Cmin was 1.51 ± 0.56 mg/liter (Fig. 2A). CLtotal was 8.23 ± 3.07 liters/h, and the terminal half-life was 3.3 h. E was 0.30 ± 0.13 (mean ± SD) (Fig. 3A), and CLHDF was 1.94 ± 0.80 liters/h.
Fig 2.
Concentrations of CMS (A) and colistin (B) after the fourth dose.
Fig 3.
Median prefilter (Cin) and postfilter (Cout) concentrations of CMS (A) and colistin (B) after the fourth dose.
For colistin, concentrations remained at a plateau over the dosage interval, with a concentration of 0.92 ± 0.46 mg/liter (mean ± SD) (Fig. 2B). CLtotal/fm was 18.91 ± 5.95 liters/h. E was 0.68 ± 0.08 (mean ± SD) (Fig. 3B), and the corresponding CLHDF was 4.33 ± 1.31 liters/h.
The present report describes the pharmacokinetics of CMS and its active metabolite colistin in critically ill patients receiving CVVHDF. The results are on par with previously published results of studies by other investigators (4), showing that both CMS and colistin are eliminated by this technique. The dialyzer clearance was similar to that determined for the four patients on continuous renal replacement therapy reported by Garonzik et al. (4), whereas Marchand et al. (6) presented similar dialyzer extraction ratios. Unfortunately, colistin is notorious for its ability to be adsorbed to many different materials, such as laboratory utensils (11) as well as HDF filters (7). Therefore, we were not able to reliably measure the concentrations of CMS and colistin in the combined dialysate/ultrafiltrate, and thus sieving was not possible to calculate.
The current opinion on the pharmacokinetics of colistin is that a main fraction (fe) of the inactive CMS is mainly cleared renally, with a fraction being metabolized into the active substance colistin (fm) (2). In healthy volunteers, fe has been determined to be 70%, and assuming that fm = 1 − fe, fm is 30% (12). Colistin in turn is not eliminated renally but is probably eliminated by hydrolysis, although the mechanism of hydrolysis is not known. In patients with renal failure, it is hypothesized that, as the renal elimination of CMS is slower, fm increases and thus the accumulation of colistin increases. This in turn suggests that total daily doses should be lowered in these patients. However, when renal replacement therapy is introduced, it should theoretically compensate for the decrease in renal clearance and thus fm should revert to normal values. However, if the renal replacement therapy also eliminates colistin, as this and other studies (4–7) have indicated, the achieved concentrations of colistin would be lowered. In accordance with this, we found that the total clearance of CMS in our patients was similar to what has been found in other reports (4, 12), except the studies by Plachouras et al. (9) and Mohamed et al. (13), where the observed CL of CMS was higher. However, the pharmacokinetic analyses in these two studies were performed using molar units, i.e., one molecule of administered CMS can form one molecule of colistin. If the difference in molecular weight between CMS and colistin had not been considered, CL values for CMS similar to those estimated by Couet et al. (12) and Garonzik et al. (4) would have been obtained. Assuming that fm is 30% in all subjects, both patients and healthy volunteers, total colistin CL values would be comparable between studies, with the exception of the current study, where the total CL of colistin was calculated to be approximately double those values (5.7 liters/h).
Comparing the found concentrations of colistin with the susceptibility breakpoints defined by the European Committee on Antimicrobial Susceptibility Testing (14), the plasma concentrations were roughly half of the breakpoint concentration for Acinetobacter spp. (S ≤ 2 mg/liter) and one-fourth of the breakpoint concentration for Pseudomonas spp. (S ≤ 4 mg/liter). In addition, Bergen et al. (15) concluded that, in order to reach a 2 log10 killing target in animal models, the fAUC/MIC ratio of colistin should be in a range of 27.6 to 45.9. In this study, assuming that the unbound fraction (fu) is 34% (13), the resulting mean fAUC/MIC ratios for susceptible Acinetobacter and Pseudomonas species at the breakpoint MICs were 3.1 and 1.6, respectively. Conversely, the exposure profile from this study would exceed the recommended target only for bacteria with drug MICs equal to or lower than 0.12 mg/liter.
In conclusion, both CMS and colistin are cleared by CVVHDF, although the clearance of CMS is not restored to the values found in related studies (9, 13), and the data from this study corroborate findings in previous studies (4–7). The dosage regimen of 160 mg CMS q8h that was used has been motivated by the risk of accumulation of drug and concomitantly increased risk of toxicity. However, this regimen, together with the increased clearance of colistin, results in colistin concentrations that are approximately half of the corresponding concentrations in patients not undergoing CVVHDF given 240 mg CMS q8h (9). The resulting drug exposure raises serious concerns regarding the optimal dosage and the need of dosage adjustment in patients receiving CVVHDF. On the basis of this study, we recommend that the CMS dosage should not be reduced for patients undergoing CVVHDF but rather should be equal to or even higher than the daily dose in patients with normal renal function.
ACKNOWLEDGMENT
We thank Britt Jansson for skillful technical assistance.
Footnotes
Published ahead of print 12 November 2012
REFERENCES
- 1. Evans ME, Feola DJ, Rapp RP. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 33:960–967 [DOI] [PubMed] [Google Scholar]
- 2. Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589–601 [DOI] [PubMed] [Google Scholar]
- 3. Rahman TM, Treacher D. 2002. Management of acute renal failure on the intensive care unit. Clin. Med. 2:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55:3284–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, Rayner CR, Nation RL, Deans R, Boots R, Widdecombe N, Douglas A, Lipman J. 2005. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob. Agents Chemother. 49:4814–4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marchand S, Frat JP, Petitpas F, Lemaitre F, Gobin P, Robert R, Mimoz O, Couet W. 2010. Removal of colistin during intermittent haemodialysis in two critically ill patients. J. Antimicrob. Chemother. 65:1836–1837 [DOI] [PubMed] [Google Scholar]
- 7. Markou N, Fousteri M, Markantonis SL, Zidianakis B, Hroni D, Boutzouka E, Baltopoulos G. 2012. Colistin pharmacokinetics in intensive care unit patients on continuous venovenous haemodiafiltration: an observational study. J. Antimicrob. Chemother. 67:2459–2462 [DOI] [PubMed] [Google Scholar]
- 8. Jansson B, Karvanen M, Cars O, Plachouras D, Friberg LE. 2009. Quantitative analysis of colistin A and colistin B in plasma and culture medium using a simple precipitation step followed by LC/MS/MS. J. Pharm. Biomed. Anal. 49:760–767 [DOI] [PubMed] [Google Scholar]
- 9. Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob. Agents Chemother. 53:3430–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Valentine J. 2002. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob. Agents Chemother. 46:3304–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karvanen M, Malmberg C, Mohamed A, Lagerbäck P, Friberg LE, O. Cars O. 2011. Abstr. 51st Annu. Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, poster D-690 [Google Scholar]
- 12. Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, Mimoz O. 2011. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin. Pharmacol. Ther. 89:875–879 [DOI] [PubMed] [Google Scholar]
- 13. Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. 2012. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob. Agents Chemother. 56:4241–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Committee on Antimicrobial Susceptibility Testing 2010. Breakpoint tables for interpretation of MICs and zone diameters, version 1.1. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden [Google Scholar]
- 15. Bergen PJ, Li J, Nation RL. 2011. Dosing of colistin—back to basic PK/PD. Curr. Opin. Pharmacol. 11:464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]