Abstract
The efficacies of tigecycline and ceftazidime against fatal pneumonia in rats caused by an extended-spectrum β-lactamase (ESBL)-positive Klebsiella pneumoniae strain or its wild-type (WT) progenitor were compared. Ceftazidime at 12.5 or 50 mg/kg of body weight twice daily (b.i.d.) was effective (50% or 100% rat survival) in pneumonia caused by the WT isolate but unsuccessful (100% rat mortality) in pneumonia caused by the ESBL-positive variant. In contrast, tigecycline at 6.25, 12.5, or 25 mg/kg b.i.d. showed dosage-dependent efficacy up to 100% rat survival irrespective of the ESBL character of the infecting organism.
TEXT
In Gram-negative pathogens, β-lactamase production remains the most important factor contributing to β-lactam resistance. Extended-spectrum β-lactamases (ESBLs) are among the most important acquired resistance determinants emerging worldwide in members of the Enterobacteriaceae (1, 2). CTX-M enzymes are the most frequently encountered ESBL enzymes in Escherichia coli, followed by SHV-5 and SHV-2 in Klebsiella pneumoniae (3). Although ESBLs confer resistance to penicillins, cephalosporins, and aztreonam only, due to additional genes, encoding resistance to aminoglycosides and/or fluoroquinolones, the armamentarium for treatment of ESBL producers is limited (1, 2). Treatment with carbapenems is often the only alternative; however, increasing numbers of carbapenemase-producing organisms are also reported. Alternative antibiotics like tigecycline are therefore increasingly used to treat patients infected with multidrug-resistant Gram-negative pathogens (4, 5). Tigecycline is a new drug belonging to the new class of glycylcyclines which has been shown to have similar MICs for strains of E. coli and Klebsiella species with and without ESBLs (6).
Therefore, the objective of the present study is to compare the efficacies of ceftazidime and tigecycline therapy in experimental pneumonia caused by a wild-type (WT) K. pneumoniae strain and an ESBL-positive variant of this strain. As a parameter for therapeutic effect, animal survival was monitored. A decrease in bacterial load (CFU counts) in infected lung was not used as an outcome parameter because the relationship between CFU count and animal survival has not been clearly established for tigecycline. In addition, animal survival as an endpoint allowed us to determine the therapeutic efficacy of tigecycline during treatment and posttreatment (7). An ESBL-positive variant of a WT Klebsiella pneumoniae strain (ATCC 43816, capsular serotype 2) was constructed by transconjugating the plasmid containing the ESBL SHV-5 gene (8). The MICs for ceftazidime as determined by macrodilution were 0.5 μg/ml for the WT isolate and >256 μg/ml for the ESBL-positive variant. The tigecycline MICs obtained were similar for both strains, at 1.0 μg/ml. Both isolates are categorized as tigecycline susceptible according to CLSI as well as EUCAST guidelines. Left-sided pneumonia was induced in male specified-pathogen-free RP/AEur/RijHsd albino rats, as described in details elsewhere (9). The administration of the antibiotics was started 24 h after bacterial inoculation of the left lung, when the bacterial load in the lung was increased 103-fold. Rats were treated with either 12.5 or 50 mg/kg of body weight of ceftazidime twice daily (b.i.d.) for 18 days (10 to 12 rats per treatment group). The animal survival rate was monitored until 10 days after termination of treatment. In the tigecycline arm, rats (12 per treatment group) were treated with 6.25, 12.5, or 25 mg/kg b.i.d. for 10 days and the animal survival rate was monitored until 10 days after termination of treatment. Tigecycline concentrations in plasma were determined by high-performance liquid chromatography coupled with mass spectrometry (AB Sciex API 5000 triple quadrupole mass spectrometer fitted with a Turbo IonSpray interface, both from AB Sciex [Concord, Ontario, Canada]). The method was validated according to the international guidelines of the FDA and European Medicines Agency (EMA). The animal experimental protocols adhere to the rules laid down in the Dutch Animal Experimentation Act (10) and the published Guidelines on the Protection of Experimental Animals by the Council of the European Union (11). The study protocols (117-10-02) are approved by the Institutional Animal Care and Use Committee of the Erasmus University Medical Center Rotterdam.
Infected animals developed pneumonia as well as septicemia and pleuritis (data not shown) and eventually died if untreated between day 3 and day 10 after infection (Fig. 1 and 2). Significant differences in the mortality rate of the untreated animals infected with the WT isolate or the ESBL-positive variant were not observed.
Fig 1.
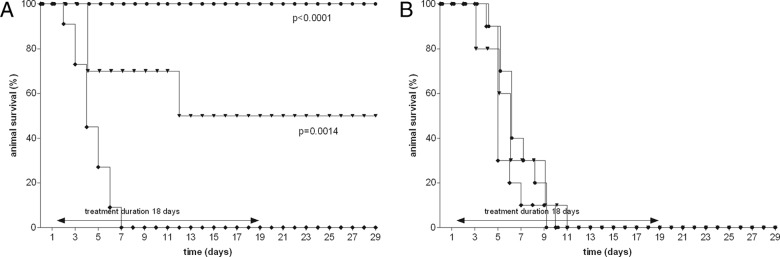
Kaplan-Meyer curves in rats during the course of left-sided pneumonia caused by the WT K. pneumoniae strain (A) or the ESBL-positive K. pneumoniae strain (B). Rats (10 to 12 per group) were treated with ceftazidime starting 24 h after bacterial inoculation of the left lung. Ceftazidime was administered intramuscularly every 12 h with doses of 12.5 mg/kg (▼) or 50 mg/kg (●) for 18 days. Untreated rats (⧫) are also shown. Rat survival rates were monitored daily until 10 days posttreatment.
Fig 2.
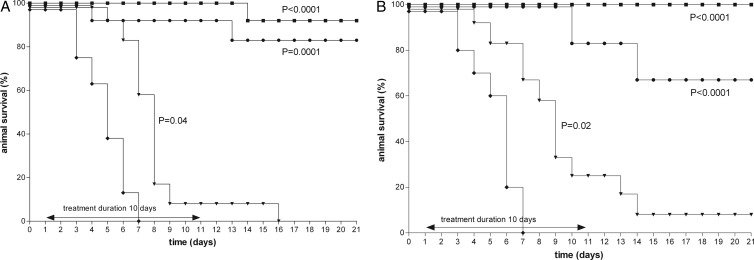
Kaplan-Meyer curves in rats during the course of left-sided pneumonia caused by the WT K. pneumoniae strain (A) or the ESBL-positive K. pneumoniae strain (B). Rats (12 per group) were treated with tigecycline starting 24 h after bacterial inoculation of the left lung. Tigecycline was administered intraperitoneally every 12 h with doses of 6.25 (▼), 12.5 (●), or 25 (■) mg/kg for 10 days. Untreated rats (⧫) are also shown. Rat survival rates were monitored daily until 10 days posttreatment.
Based upon previous observations (7), the rats infected with the WT isolate were treated with a dose of 12.5 or 50 mg/kg of ceftazidime b.i.d. Whereas all untreated rats died, the survival rate of the rats infected with the WT isolate were significantly increased, being 50% and 100% for the 12.5- and 50-mg/kg b.i.d. treatment regimens, respectively, at day 21 and at day 29 (Fig. 1). In the animals infected with the ESBL-positive variant, ceftazidime administered at the maximum dose of 50 mg/kg b.i.d. was not successful and resulted in 100% mortality. To compare the efficacies of tigecycline and ceftazidime, the efficacy of tigecycline administered in three different doses, 6.25, 12.5, or 25 mg/kg b.i.d., was investigated for both infections. The tigecycline doses administered were chosen based upon pilot experiments in order to be able to investigate a tigecycline dose-effect relationship ranging from 0% to 100% animal survival. Tigecycline treatment of the animals infected with the WT isolate or the ESBL-positive variant resulted in a dose-dependent therapeutic effect (Fig. 2). There was no significant difference in animal survival rate between the WT isolate and the ESBL-positive variant at any dosage using Kaplan-Meyer analysis (Prism 5.0; GraphPad Inc., San Diego, CA). All dosing regimens used showed a significant effect on rat survival compared to that of the untreated controls. Efficacy of tigecycline increased with increasing doses from 6.25 mg/kg b.i.d. to 25 mg/kg b.i.d., and animal survival was more than 90% at the highest dose. These results demonstrate that the efficacy of tigecycline is not influenced by the ESBL character of the infecting strain, in contrast to the efficacy of ceftazidime.
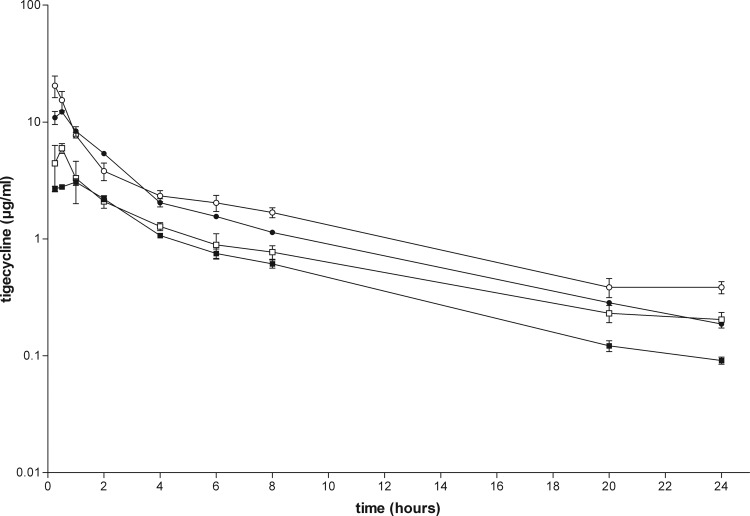
The pharmacokinetic profile obtained with the different dosing regimens of tigecycline is displayed in Fig. 3. The plasma concentration-time curves indicate a two-compartment disposition. Pharmacokinetic parameter estimates after single and multiple dosing of tigecycline are displayed in Table 1. The elimination half-life of tigecycline is approximately 6.5 h, and there is neither a significant difference between single and multiple dosing nor a dose-dependent effect. Five consecutive doses of 6.25 mg/kg and 12.5 mg/kg result in areas under the plasma concentration-time curves (AUCs) of 16.8 μg · h/ml and 34.3 μg · h/ml, respectively. The AUC during multiple dosing is slightly higher than that during single dosing due to slight accumulation, as expected (12).
Fig 3.
Plasma concentrations of tigecycline in rats treated with a single dose (filled symbols) or 5 consecutive doses (open symbols) of 6.25 mg/kg (squares) or 12.5 mg/kg (circles). Blood samples of rats were taken at 0, 15, and 30 min and 1, 2, 4, 8, 12, and 24 h after administration of tigecycline. The concentration at each time point was determined in triplicate. Concentrations are expressed as means ± standard deviations (SDs). The limit of quantification of tigecycline in rat plasma samples was 0.0114 μg/ml. The response from calibration standards was linear from 0.0114 to 15.0 μg/ml, and the coefficient of correlation for all measured sequences was at least 0.995. The intraday precision and analytical recovery of the tigecycline assay during study sample analysis ranged from 2.9 to 7.7% and from 100.8 to 104.5%, respectively. Concentration-time curves were analyzed using WinNonlin employing a two-compartment model.
Table 1.
Pharmacokinetic parameters after single doses and 5 consecutive doses of tigecycline in ratsa
| Treatment regimen | AUC (mg · h/liter) |
V/F (liters/kg) |
CL/F (liters/h) |
t1/2α (h) |
t1/2β (h) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| 6.25 mg/kg 1× | 15.71 | 0.44 | 1.54 | 0.18 | 0.40 | 0.01 | 1.19 | 0.36 | 6.05 | 0.54 |
| 6.25 mg/kg 5× | 16.82 | 0.94 | 0.65 | 0.24 | 0.37 | 0.02 | 0.44 | 0.19 | 7.06 | 0.62 |
| 12.5 mg/kg 1× | 38.37 | 0.94 | 0.78 | 0.08 | 0.33 | 0.01 | 0.76 | 0.12 | 6.14 | 0.29 |
| 12.5 mg/kg 5× | 34.29 | 2.15 | 0.58 | 0.15 | 0.36 | 0.02 | 0.40 | 0.15 | 6.52 | 0.60 |
Serum tigecycline levels were determined after administration of tigecycline at 6.25 mg/kg and 12.5 mg/kg in either a single dose (1×) or 5 consecutive doses (5×). Samples for pharmacokinetic analysis were taken (steady state) at 0.25, 0.5, 1, 2, 4, 6, 8, 20, and 24 h after the single administration or after the fifth dose of tigecycline. Concentration-time curves were analyzed using WinNonlin employing a two-compartment model. V, volume of distribution; CL, clearance; t1/2α, half-life at α phase; t1/2β, half-life at β phase; SE, standard error.
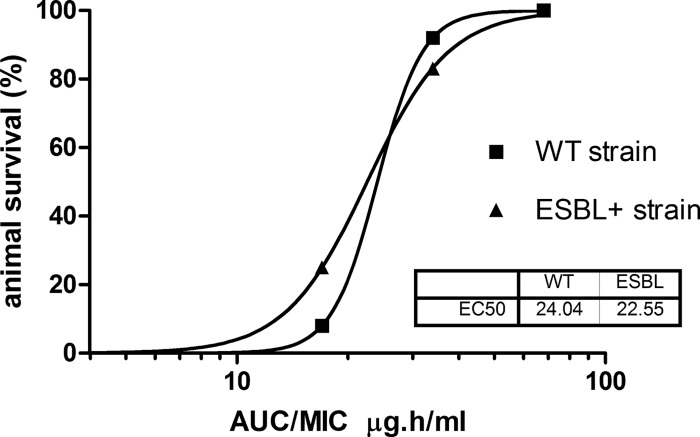
Subsequently, the exposure-response relationship between AUCs and animal survival was determined and shows a sigmoid relationship (Fig. 4). The values for the mean effective exposure index at 50% (EI50) for the AUC for the free, unbound fraction of the drug (fAUC) were 24 and 23 μg · h/ml for the WT isolate and ESBL-positive variant, respectively, and appear to be not significantly different. Because of the low number of data points, a meaningful 95% confidence interval (CI) was not able to be determined. The survival rates and the AUC/MIC ratio are correlated to efficacy, showing no significant differences between the WT isolate and the ESBL-positive variant.
Fig 4.
AUC-response relationship of tigecycline in rats infected with WT or ESBL-positive K. pneumoniae at the end of the 10-day treatment. Each of the AUC/MIC ratios has been calculated for all three dosing regimens and depicted against the animal survival.
Although we used a limited number of different antibiotic doses and a twice-daily dosing regimen for ceftazidime as well as tigecycline, the data obtained fit a sigmoid exposure-response curve for both the WT isolate and the ESBL-positive variant. Preclinical and clinical data reported earlier have shown that the AUC/MIC ratio is the pharmacodynamic index that is correlated to the efficacy of tigecycline (13, 14). Although we did not assess the degree of protein binding of tigecycline in this study, data from Chen et al. (15) show that in heparinized blood, the unbound fraction of tigecycline is 15% in rats and 16% in humans. Due to this similarity in protein binding of tigecycline in rats and humans, we therefore refer to AUC in the following discussion, as these are the values reported for most clinical studies.
The EI50 obtained for the tigecycline AUC/MIC ratio found in this study is significantly higher than the threshold value of 6.96 obtained in the clinical study of Pasarell et al. (14) using tigecycline for intra-abdominal infections. Likewise, the study of Meagher et al. (13) using tigecycline in complicated skin and skin structure infections also showed a lower threshold value derived from a classification and regression tree (CART) analysis. In the present study, the deduced AUC/MIC ratio for tigecycline at the lowest dose was 8.5, but despite the statistically significant increase in rat survival, the 21-day survival rate of the rats treated with a 6.25-mg/kg b.i.d. dose is 0% in the animals with pneumonia caused by the WT isolate and 10% in pneumonia caused by the ESBL-positive variant. Despite the significant effect of the lower dose (and exposure) and the AUC/MIC ratio being in the same range as the CART-derived values in the clinical studies, it is not the dose resulting in an optimal effect in our rat model, because in our model of pneumonia which is fatal if left untreated, we used animal survival as an endpoint. This severe disease condition requires a higher exposure, resulting in a higher AUC/MIC ratio to obtain efficacy in our rat model. This finding helps to explain why and corresponds well to the observation that a lower response was obtained for tigecycline than for the imipenem-treated group of ventilated patients in the clinical study of Freire et al. (16). They also found that a dose of 50 mg resulted in a significantly decreased AUC, i.e., a 15% decrease in ventilator-associated pneumonia (VAP) patients compared to the non-VAP patients. The exposure of tigecycline in patients with severe pulmonary disease is probably too low to result in an optimal therapeutic effect.
We conclude that efficacy of tigecycline is dose dependent, irrespective of the presence of an ESBL-positive infecting organism. For the treatment of severe disease such as pneumonia, higher AUC/MIC ratios—and, therefore, doses—may be required for optimal therapeutic effect in patients infected with organisms having tigecycline MICs close to the current clinical breakpoint of 1 μg/ml.
ACKNOWLEDGMENT
This work was supported by research grants from the company of Pfizer. We have no other funding issues to declare.
Footnotes
Published ahead of print 5 November 2012
REFERENCES
- 1. Jacoby GA, Munoz-Price LS. 2005. The new β-lactamases. N. Engl. J. Med. 352:380–391 [DOI] [PubMed] [Google Scholar]
- 2. Paterson DL, Bonomo RA. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Potz NAC, Hope R, Warner M, Johnson AP, Livermore DM, London & South East ESBL Project Group 2006. Prevalence and mechanisms of cephalosporin resistance in Enterobacteriaceae in London and South-East England. J. Antimicrob. Chemother. 58:320–326 [DOI] [PubMed] [Google Scholar]
- 4. Chen PL, Yan JJ, Wu CJ, Lee HC, Chang CM, Lee NY, Ko NY, Wang LR, Shih HI, Lee CC, Ko WC. 2010. Salvage therapy with tigecycline for recurrent infection caused by ertapenem-resistant extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 68:312–314 [DOI] [PubMed] [Google Scholar]
- 5. Poulakou G, Kontopidou FV, Paramythiotou E, Kompoti M, Katsiai M, Mainas E, Nicolaou C, Yphantis D, Antoniadou A, Trikka-Graphakos E, Roussou Z, Clouva P, Maguina N, Kanellakopoulou K, Armaganidis A, Giamarellou H. 2009. Tigecycline in the treatment of infections from multi-drug resistant Gram-negative pathogens. J. Infect. 58:273–284 [DOI] [PubMed] [Google Scholar]
- 6. Garau J. 2008. Other antimicrobials of interest in the era of extended-spectrum β-lactamases: fosfomycin, nitrofurantoin and tigecycline. Clin. Microbiol. Infect. 14(Suppl 1):198–202 [DOI] [PubMed] [Google Scholar]
- 7. Bakker-Woudenberg IAJM, ten Kate MT, Goessens WHF, Mouton JW. 2006. Effect of treatment duration on pharmacokinetic/pharmacodynamic indices correlating with therapeutic efficacy of ceftazidime in experimental Klebsiella pneumoniae lung infection. Antimicrob. Agents Chemother. 50:2919–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gruteke P, Goessens WHF, van Gils J, Peerbooms P, Lemmens-den Toom N, van Santen-Verheuvel M, van Belkum A, Verbrugh HA. 2003. Patterns of resistance associated with integrons, the extended-spectrum β-lactamase SHV-5 gene, and a multidrug efflux pump of Klebsiella pneumoniae causing a nosocomial outbreak. J. Clin. Microbiol. 41:1161–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bakker-Woudenberg IAJM, van den Berg JC, Michel MF. 1982. Therapeutic activities of cefazolin, cefotaxime, and ceftazidime against experimentally induced Klebsiella pneumoniae pneumonia in rats. Antimicrob. Agents Chemother. 22:1042–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ministry of Welfare, Public Health and Cultural Affairs 1977. Dutch animal experimentation act. Ministry of Welfare, Health and Cultural Affairs, The Netherlands [Google Scholar]
- 11. Council of the European Union 1986. Guidelines on the protection of experimental animals. Council of the European Union, Brussels, Belgium [Google Scholar]
- 12. Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 49:220–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. 2005. The pharmacokinetic and pharmacodynamics profile of tigecycline. Clin. Infect. Dis. 41(Suppl 5):S333–S340 [DOI] [PubMed] [Google Scholar]
- 14. Passarell JA, Meagher AK, Liolios K, Cirincione BB, van Wart SA, Babinchak T, Ellis-Grosse EJ, Ambrose PG. 2008. Exposure-response analyses of tigecycline efficacy in patients with complicated intra-abdominal infections. Antimicrob. Agents Chemother. 52:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Q, Tung EC, Cicotto SL, Strauss JR, Ortiga R, Ramsay KA, Tang W. 2008. Effect of anticoagulant ethylenediamine tetra-acetic acid (EDTA) on the estimation of pharmacokinetic parameters: a case study with tigecycline and ciprofloxacin. Xenobiotica 38:76–86 [DOI] [PubMed] [Google Scholar]
- 16. Freire AT, Melnyk V, Kim MJ, Datsenko O, Dzyublik O, Glumcher F, Chuang YC, Maroko RT, Dukart G, Cooper CA, Korth-Bradley JM, Dartois N, Gandjini H, 311 Study Group 2010. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn. Microbiol. Infect. Dis. 68:140–151 [DOI] [PubMed] [Google Scholar]