Abstract
Although the rate of acylation of a penicillin-resistant form of Streptococcus pneumoniae penicillin-binding protein 2x (PBP2x) by ceftaroline is 80-fold lower than that of its penicillin-sensitive counterpart, it remains sufficiently high (k2/K = 12,600 M−1 s−1) to explain the sensitivity of the penicillin-resistant strain to this new cephalosporin. Surprisingly, the Actinomadura R39 DD-peptidase is not very sensitive to ceftaroline.
TEXT
Ceftaroline fosamil, the prodrug of ceftaroline, is a parenteral cephalosporin exhibiting broad-spectrum bactericidal in vitro activity against Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant Streptococcus pneumoniae, as well as a few common Gram-negative pathogens (1). Ceftaroline fosamil is approved in the United States for the treatment of acute bacterial skin and skin-structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP). In the European Union, ceftaroline fosamil is approved for the treatment of complicated skin and soft tissue infections (cSSTI) and community-acquired pneumonia (CAP). In S. pneumoniae, mutations affecting penicillin-binding protein 2x (PBP2x) appear to be particularly important in conferring β-lactam resistance (2–4). A study of pneumococcal penicillin-resistant strains (5) showed that they were sensitive to ceftaroline with MICs ranging from 0.015 to 2 mg/liter. Affinities of ceftaroline for S. pneumoniae PBPs were measured in binding studies, and 50% inhibitory concentration (IC50) values were determined for PBP2x of one penicillin-sensitive (IC50, 0.1 mg/liter) and three penicillin-resistant (IC50, 0.1 to 1.0 mg/liter) strains. However, with very sensitive PBPs, the IC50 values can represent just 50% of the PBP concentration in the assay. In the present study, we determined the second-order rate constants (k2/K) (see scheme I below) for acylation by ceftaroline of a sensitive PBP2x from strain R6 and a resistant form from strain 5204 (6). The k2/K value was also determined for the reference Actinomadura R39 DD-peptidase, which is usually highly sensitive to β-lactam antibiotics.
The proteins were purified as previously described (7, 8). In brief, PBP2x proteins from strains R6 and 5204 were expressed as fusion proteins to glutathione S-transferase with a thrombin-cleavable linker (pGEX-4T1 plasmid) in the host strain Escherichia coli MC1061. The cell lysate was loaded onto a glutathione-Sepharose 4B affinity column (GE Healthcare) equilibrated with 50 mM Tris HCl, pH 8, 100 mM NaCl, and 1 mM EDTA. Proteins were eluted by applying one hundred units of human thrombin (Sigma) onto the column. For R39 and R6 PBP2x, the rate of inactivation was measured by the reporter substrate method (9, 10) with thioester S2d in the presence of dithiodipyridine (11, 12). The interaction of PBPs with β-lactams follows a 3-step model, designated scheme I:
In this equation, E is the enzyme, I is the inhibitor, EI* is the acylenzyme, K is the dissociation constant, and k2 and k3 are first-order rate constants (see reference 9 for a complete kinetic analysis).
For R39, the pseudo-first-order rate constant ka increased linearly with the ceftaroline concentration (ka = k2 [I]/K), yielding a k2/K value of 3,400 ± 200 M−1 s−1 (K > 10 μM). For R6 PBP2x, the reaction was complete within 1 min with 0.1 μM PBP and 1 μM ceftaroline. Hydrolysis of the reporter substrate at a lower PBP concentration could not be recorded with adequate accuracy, so the experiment was performed with equimolar concentrations (0.1 μM) of ceftaroline and PBP. Under these conditions, the concentration of active enzyme ([E]) (measured via the rate of S2d thioester hydrolysis) varies with time (t) according to the equation 1/[E] = 1/E0 + (k2/K) × t, where E0 is the concentration of enzyme at t = 0.
Figure 1 shows a plot of 1/[E] versus time for R6 PBP2x. The slope of the line yields a k2/K value of 1 × 106 M−1 s−1 (± 10%).
Fig 1.
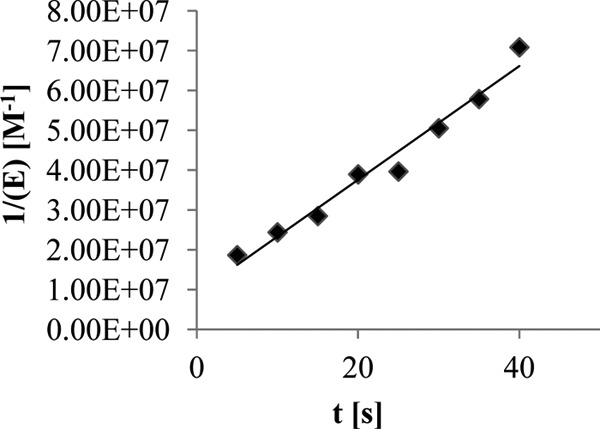
Plot of 1/[E] versus time for the interaction between 0.1 μM R6 PBP2x and 0.1 μM ceftaroline. Incubation was performed at 30°C in 10 mM sodium phosphate buffer, pH 7.0, in the presence of 1 mM S2d and 0.5 mM 4,4′-dithiodipyridine. The hydrolysis of S2d was monitored at 324 nm. The rate of spontaneous hydrolysis of S2d was subtracted.
Since S2d is not a sufficiently good substrate of 5204 PBP2x, the formation of EI* was followed by monitoring the quenching of the protein fluorescence at 340 nm as done previously (13, 14). With 0.5 μM PBP and 2.5 μM ceftaroline, the reaction also occurred too rapidly to allow a valid determination of k2/K and the reaction was again studied by using equimolar concentrations (0.5 μM) of the reagents. The slope of the line 1/[E] versus time yielded a k2/K value of 12,600 ± 1,000 M−1 s−1 (Fig. 2).
Fig 2.
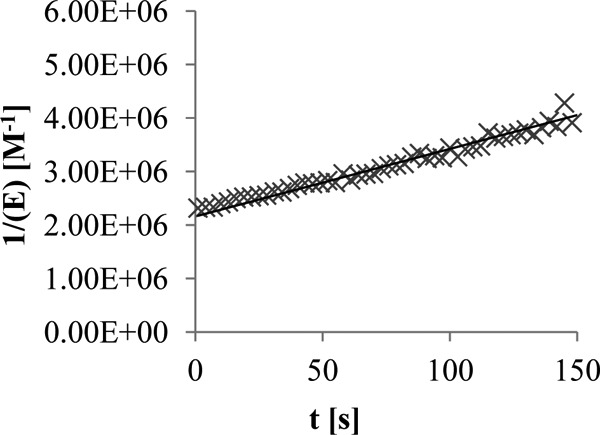
Plot of 1/[E] versus time for the interaction between 0.5 μM 5204 PBP2x and 0.5 μM ceftaroline. The formation of EI* was followed by monitoring the quenching of the protein fluorescence at 340 nm in 10 mM sodium phosphate buffer, pH 7.0, at 30°C.
The deacylation rate constants (k3) were determined at 25°C after complete inactivation of the PBPs with 100 μM ceftaroline. The residual free ceftaroline was hydrolyzed in less than 5 min by the VIM-4 β-lactamase (15), and the reactivation of PBPs was followed by measuring the recovered activity at different times up to about 150 min as previously described (16).
The k3 values of R39 and R6 PBP2x were negligible, since no significant reactivation of the enzymes was recorded over a 60-min period after eliminating excess ceftaroline (reactivation was <10% after 2 h; k3 < 1.5 × 10−5 s−1; half-life [t1/2], >12 h). The k3 value for 5204 PBP2x (t1/2 = 53 min) was 2.2 × 10−4 ± 0.4 × 10−4 s−1. However, the k3 value remained too low to result in a major error in the determination of the k2/K value and is unlikely to affect the efficiency of ceftaroline as an inactivator of the resistant PBP2x (PBP2xr). Indeed, a simple calculation indicates that at a 1 μM concentration of ceftaroline at steady state, the inactivated adduct represents 98% of the resistant PBP2x versus more than 99% of the sensitive one. Such an increase of the k3 value was already described (17) for the adduct formed between cefotaxime and PBP2xr from a resistant clinical isolate (CS109).
These results show that ceftaroline is a particularly efficient inactivator of the transpeptidase activity of R6 PBP2x. Indeed, a higher value of k2/K yields an increased rate of PBP inactivation and a decreased MIC value if the PBP is an important target in the strain (Table 1). Surprisingly, the second-order inactivation rate constant for R6 is 300-fold greater than that of R39, which is generally considered a highly β-lactam-sensitive PBP and was included in the present study for this reason.
Table 1.
MICs and k2/K second-order rate constants
Although it is 80-fold less than that of the sensitive PBP2x, the k2/K value of the penicillin-resistant 5204 PBP2x (12,600 M−1 s−1) remains quite high in comparison to the value obtained by Carapito (6) for cefotaxime and the same PBP (85 M−1 s−1). This nicely explains the much lower MIC of 5204 for ceftaroline compared to that for cefotaxime (Table 1).
In consequence, ceftaroline seems to be particularly well adapted for inactivating the transpeptidase activity of S. pneumoniae PBP2x, even for mutants that exhibit high levels of resistance to other β-lactams. Table 1 compares the kinetics of PBP2x inactivation with MIC values of the two strains for ceftaroline and two reference compounds. The general agreement is excellent.
Finally, on the basis of the very high k2/K value of R6 PBP2x, it can be concluded that the IC50 for ceftaroline measured after 10 min would probably correspond to 50% of the PBP concentration in the assay. Indeed, at the IC50 observed with penicillin-sensitive PBP2x (0.1 mg/liter, i.e., 0.17 μM) (5), the half-reaction time would be about 4 s. Even with a 10-fold-lower k2/K value, the half-reaction time would be 40 s, and after 10 min, the reaction should be complete. This shows that the IC50 values can underestimate the sensitivity of very sensitive PBPs.
In summary, there is a good correlation between PBP2x sensitivity and the MIC values for ceftaroline. The latter are quite low even for the β-lactam-resistant 5204 strain. Even for the 5204 PBP2x, a 1 μM ceftaroline concentration would result in a half-inactivation time of 69 s. This is certainly sufficiently rapid to kill the bacterium. A 1 μM concentration corresponds to 0.6 mg liter−1, which should easily be obtained after administration of the compound.
ACKNOWLEDGMENTS
This study was supported by Forest Laboratories, Inc. We also acknowledge the support of the Belgian Federal Government (IUAP program P6/19).
Forest Laboratories, Inc., was involved in the design and decision to present these results. Forest Laboratories, Inc., had no involvement in the collection, analysis, and interpretation of data. Scientific Therapeutics Information, Inc., provided editorial assistance, which was funded by Forest Research Institute, Inc.
Footnotes
Published ahead of print 12 November 2012
REFERENCES
- 1. Sader HS, Fritsche TR, Kaniga K, Ge Y, Jones RN. 2005. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob. Agents Chemother. 49:3501–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hakenbeck R, Balmelle N, Weber B, Gardes C, Keck W, de Saizieu A. 2001. Mosaic genes and mosaic chromosomes: intra- and interspecies genomic variation of Streptococcus pneumoniae. Infect. Immun. 69:2477–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laible G, Spratt BG, Hakenbeck R. 1991. Interspecies recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 5:1993–2002 [DOI] [PubMed] [Google Scholar]
- 4. Munoz R, Dowson CG, Daniels M, Coffey TJ, Martin C, Hakenbeck R, Spratt BG. 1992. Genetics of resistance to third-generation cephalosporins in clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 6:2461–2465 [DOI] [PubMed] [Google Scholar]
- 5. Kosowska-Shick K, McGhee PL, Appelbaum PC. 2010. Affinity of ceftaroline and other β-lactams for penicillin-binding proteins from Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob. Agents Chemother. 54:1670–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carapito R, Chesnel L, Vernet T, Zapun A. 2006. Pneumococcal β-lactam resistance due to a conformational change in penicillin-binding protein 2x. J. Biol. Chem. 281:1771–1777 [DOI] [PubMed] [Google Scholar]
- 7. Granier B, Jamin M, Adam M, Galleni M, Lakaye I, Zorzi W, Grandchamps J, Wilkin J-M, Fraipont C, Joris B, Duez C, Nguyen-Disteche M, Coyetteme J, Leyh-Bouille L, Dusart J, Christiaens L, Frere J-M, Ghuysen J-M. 1994. Serine-type d-Ala-d-Ala peptidases and penicillin-binding proteins. Methods Enzymol. 244:249–266 [DOI] [PubMed] [Google Scholar]
- 8. Mouz N, Gordon E, Di Guilmi AM, Petit I, Petillot Y, Dupont Y, Hakenbeck R, Vernet T, Dideberg O. 1998. Identification of a structural determinant for resistance to beta-lactam antibiotics in Gram-positive bacteria. Proc. Natl. Acad. Sci. U. S. A. 95:13403–13406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Meester F, Joris B, Reckinger G, Bellefroid-Bourguignon C, Frère J-M, Waley SG. 1987. Automated analysis of enzyme inactivation phenomena: application to β-lactamases and DD-peptidases. Biochem. Pharmacol. 36:2393–2403 [DOI] [PubMed] [Google Scholar]
- 10. Frère J-M, Nguyen-Disteche M, Coyette J, Joris B. 1992. Mode of action: interaction with penicillin binding proteins, p 148–195 In Page M. (ed), The chemistry of beta-lactams. Chapman and Hall, Glasgow, Scotland [Google Scholar]
- 11. Macheboeuf P, Fischer DS, Brown T, Jr, Zervosen A, Luxen A, Joris B, Dessen A, Schofield CJ. 2007. Structural and mechanistic basis of penicillin-binding protein inhibition by lactivicins. Nat. Chem. Biol. 3:565–569 [DOI] [PubMed] [Google Scholar]
- 12. Sauvage E, Zervosen A, Dive G, Herman R, Amoroso A, Joris B, Fonze E, Pratt RF, Luxen A, Charlier P, Kerff F. 2009. Structural basis of the inhibition of class A β-lactamases and penicillin-binding proteins by 6-β-iodopenicillanate. J. Am. Chem. Soc. 131:15262–15269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jamin M, Damblon C, Millier S, Hakenbeck R, Frere JM. 1993. Penicillin-binding protein 2x of Streptococcus pneumoniae: enzymic activities and interactions with beta-lactams. Biochem. J. 292(Part 3):735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jamin M, Hakenbeck R, Frere JM. 1993. Penicillin binding protein 2x as a major contributor to intrinsic beta-lactam resistance of Streptococcus pneumoniae. FEBS Lett. 331:101–104 [DOI] [PubMed] [Google Scholar]
- 15. Lassaux P, Traore DA, Loisel E, Favier A, Docquier JD, Sohier JS, Laurent C, Bebrone C, Frere JM, Ferrer JL, Galleni M. 2011. Biochemical and structural characterization of the subclass B1 metallo-β-lactamase VIM-4. Antimicrob. Agents Chemother. 55:1248–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zervosen A, Bouillez A, Herman A, Amoroso A, Joris B, Sauvage E, Charlier P, Luxen A. 2012. Synthesis and evaluation of boronic acids as inhibitors of penicillin binding proteins of classes A, B and C. Bioorg. Med. Chem. 20:3915–3924 [DOI] [PubMed] [Google Scholar]
- 17. Lu WP, Kincaid E, Sun Y, Bauer MD. 2001. Kinetics of beta-lactam interactions with penicillin-susceptible and -resistant penicillin-binding protein 2x proteins from Streptococcus pneumoniae. Involvement of acylation and deacylation in beta-lactam resistance. J. Biol. Chem. 276:31494–31501 [DOI] [PubMed] [Google Scholar]
- 18. Chesnel L, Carapito R, Croize J, Dideberg O, Vernet T, Zapun A. 2005. Identical penicillin-binding domains in penicillin-binding proteins of Streptococcus pneumoniae clinical isolates with different levels of beta-lactam resistance. Antimicrob. Agents Chemother. 49:2895–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]


