Abstract
Invasive fungal infections are of great concern in pediatric hematopoietic stem cell transplantation (HSCT) recipients. Voriconazole is usually the drug of first choice for treating or preventing invasive aspergillosis. Optimum trough levels (Ctroughs) are between 1 and 5 mg/liter. It is unclear whether these levels are reached with currently advised pediatric dosing schedules. Between 2007 and 2011, 11 patients <2 years of age, 31 between 2 and 12 years, and 20 between 12 and 20 years were (prophylactically or therapeutically) treated with voriconazole in the HSCT unit of UMC Utrecht. For children <2 years of age, the dosage recommended for 2 to 12 years was used. In 34% of children who started with the recommended dose, an adequate Ctrough was reached irrespective of age or administration route. After therapeutic drug monitoring (TDM)-based dose adjustments, adequate Ctroughs were reached in 80% of the patients at median doses of 31.5 (age, <2 years), 16 (age, 2 to 12 years), and 9.4 mg/kg of body weight/day (age, >12 years) (P = 0.034). The intrapatient variability in Ctrough ranged between 1 and 238%. Voriconazole was discontinued in six patients due to toxicity. These patients had a median Ctrough of 0.5 mg/liter at the initial dose (ranging from 0.5 to 2.6 mg/liter), and a medium maximal concentration of 4 mg/liter was reached. Inter- and intrapatient variability is a major concern in voriconazole treatment and necessitates therapeutic drug monitoring of dosing, especially in young children.
INTRODUCTION
Invasive fungal infections are of great concern in pediatric hematopoietic stem cell transplantation (HSCT) recipients. Voriconazole is a broad-spectrum triazole antifungal used for the prophylaxis as well as for the treatment of invasive fungal infections in these patients. Voriconazole is usually the drug of first choice for treating invasive aspergillosis. However, incomplete response to therapy and toxicity can cause ineffective treatment of this and other fungal infections. Studies in adults have shown that host immunity, localization and extension of infection, early start of antifungal therapy, lack of response to previous therapy, surgical management, and in vitro antifungal susceptibility of the pathogen are key determinants for a successful response (1–3). However, data regarding the efficacy and safety of voriconazole in children are limited (4–6). Several studies show that the variability in pharmacokinetics (PK) of voriconazole between children is large (4, 5, 7, 8). Michael et al. showed a 73% interpatient variability in trough levels when the recommended pediatric dosing regimen was used (7). Karllson et al. observed 66% interpatient variability in clearance using a population approach (9). Shima et al. showed that the dose advised in the current summary of product characteristics (SPC) (or extrapolated from it) (10) leads to highly variable exposures, especially in children <3 years of age (11). This PK variability has major implications for outcomes of treatment: several independent studies have demonstrated a relationship between higher voriconazole serum concentrations and outcomes of voriconazole treatment (5, 12–14). These studies indicate that voriconazole trough levels of >1 mg/liter are associated with a higher rate of success, and trough concentrations (Ctrough) of >5 mg/liter are associated with an increased risk of (hepato)toxicity. However, a study of 74 immunocompromised children showed no correlation between Ctrough and laboratory/clinical adverse effects or treatment response (15). A recent study showed that a Ctrough/MIC between 2 and 5 is needed for an optimal response. Meletiadis et al. showed a cutoff MIC of <1 mg/liter for Aspergillus fumigatus, which is susceptible to voriconazole, whereas higher MICs were measured in Aspergillus species with reduced susceptibility to voriconazole (17). Two studies demonstrated that when using the recommended pediatric dose, only 55 to 75% of children would achieve a Ctrough of >1 mg/liter (4, 5). Dose targeting based on therapeutic drug monitoring (TDM) may therefore help to individualize the voriconazole doses and optimize treatment outcomes. The aim of this retrospective study was to assess the value of voriconazole TDM in pediatric HSCT. This was performed by assessing the use of voriconazole in these patients to test whether recommended dosing regimens in children and young adults (aged 0.3 to 20 years) leads to adequate therapeutic drug levels and to determine whether TDM was feasible and leads to stable and adequate plasma concentrations within a reasonable time period.
MATERIALS AND METHODS
Setting and study population.
This retrospective study was performed in the pediatric HSCT unit of the University Medical Center in Utrecht (UMC Utrecht) and was approved by the institutional ethical committee. All children and young adults who received an allogeneic HSCT and received voriconazole between 2007 and 2011 at this unit were included. Written informed consent was obtained from all participating patients or their legal representatives prior to HSCT. In this consent it was stated that their medical data may be used for research purposes. Voriconazole was, according to the hospital's standard operating procedures, prescribed as an antifungal prophylaxis for children at high risk for invasive fungal disease and was started during the conditioning phase until recovery (>300/μl) of CD3+ lymphocytes. Screening for Aspergillus infections using serum galactomannan was performed twice weekly during neutropenia and weekly until CD3+ cells exceeded 300/μl. In the case of a positive screening result for galactomannan or prolonged neutropenic fever (>72 h) despite broad-spectrum antibiotics without a focus, a high-resolution computed tomography (HRCT) of the lungs (with our without bronchoalveolar lavage) was performed. Voriconazole treatment was started empirically in this situation (if patients were not yet receiving voriconazole prophylaxis). Treatment was ended based on the clinical response and recovery of the (cellular) immunity (CD4+ cells of >200 cells/μl) and resolution of the aberrations on HRCT. Other reasons for discontinuations were based on the results of cultures or on toxicity. Initial dosing in children who were >2 years old was generally according to the SPC as shown in Table 1, based on the SPC of the European Medicines Agency (EMA) (10). For children <2 years of age, the recommended initial dose for children 2 to 12 years of age was used as guidance. To determine if a patient was given the recommended dose, a deviation of maximally 25% was allowed (based on changes in the proposed dosing schedules between 2007 and 2011).
Table 1.
Current recommendations of voriconazole dosing, derived from the summary of product characteristics (10)
| Patient age/wt | Recommended dose by treatment type |
|||
|---|---|---|---|---|
| Intravenous (mg/kg/12 h) |
Oral |
|||
| Loading | Maintenance | Loading (mg/12 h) | Maintenance | |
| <2 yra (1) | 9 | 8 | 9 mg/kg/12 h | |
| 2–12 yr or 12–14 yr/<50 kg | 9 | 8 | 9 mg/kg/12 h (max 350 mg/12 h) | |
| >14 yr/<40 kg | 6 | 4 | 200 | 100 mg/12 h |
| >12–14 yr/>40 kg | 6 | 4 | 400 | 200 mg/12 h |
For patients <2 years of age, the dose as recommended in the summary of product characteristics for patients 2 to 12 years of age was used.
Therapeutic drug monitoring.
Voriconazole Ctrough samples were drawn according to standard operating procedures of UMC Utrecht: 3 days after the start of therapy, once weekly until therapeutic concentrations are reached, and thereafter two times per month. In addition, patients using high doses or unstable patients were monitored once or twice weekly. Patients were considered unstable if concentrations between two subsequent measurements differed by at least 50% or dose adjustments of at least 50% were performed. The serum concentrations were determined in the pharmacy laboratory of UMC Utrecht twice weekly, and doses were adjusted based on the results. Ctroughs below 1.0 mg/liter or above 5.0 mg/liter were considered decision points for dose adjustment in all patients. Subsequent doses were linearly adjusted in order to reach an adequate Ctrough (in children near-linear pharmacokinetics was assumed). When doses of >40 mg/kg of body weight/day were used, doses were divided into three times per day, and the maximum concentration (Cmax) and concentration at 4 h after dosing were measured. A Cmax of <9 mg/liter was accepted.
A validated high-performance liquid chromatography (HPLC) method was used (Spherisorb ODS-2; 5-μm column; mobile phase included acetonitrile, methanol, and 0.01 M tetramethylethylenediamine, pH 7.4 [30:40:30]; flow rate, 1 ml/min) after liquid-liquid extraction. Ketoconazole was used as an internal standard. UV detection was set at 255 nm. Linearity of the calibration curve was found in the range of 0.5 to 10 mg/liter. The lower limit of quantification was 0.5 mg/liter. Analytical recovery of voriconazole and the internal standard were 104.4% ± 14.1% and 86.6% ± 10.2% (means ± standard deviations [SD]), respectively. Coefficients of variation for intra- and interday precision and accuracy all were <5% for both voriconazole and the internal standard.
Endpoints.
The main study endpoint to assess the value of voriconazole TDM in pediatric HSCT was the number of patients who reach an adequate Ctrough initially and after dose adjustments based on TDM. In addition, the time to adequate Ctrough was studied, and intraindividual variability (based on the range in concentrations reached and the variability of the Ctrough at constant dose) was considered the secondary endpoint.
Data collection.
Demographic data, including age, gender, and body weight, and clinical characteristics, such as underlying disease, donor type, and days of voriconazole use, were prospectively collected. For all children and young adults, the dosage at the start, the route of administration (intravenous or oral), and the first dosage at which an adequate Ctrough was reached were noted. The range between the lowest and highest concentrations measured per patient was registered.
The indication for voriconazole use and reason for discontinuing voriconazole were retrieved from patient charts. The use of voriconazole was registered by (i) the indication of voriconazole use (prophylactic, empirical, or therapeutic) and (ii) the reason for discontinuation. Prophylactic use of voriconazole was divided into primary and secondary prophylactic use. Empirical treatment was defined as the prescription of voriconazole for a patient with prolonged neutropenic fever despite broad-spectrum antibiotics without a focus. Therapeutic use of voriconazole was divided into treatment for aspergillosis and nonaspergillosis. For the indication of treatment of aspergillosis, we used the EORTC/MSG (European Organization for Research and Treatment of Cancer/Mycoses Study Group of the National Institute of Allergy and Infectious Diseases) definitions and made a distinction between possible, probable, and proven aspergillosis.
Data analysis.
Patients were divided in three age groups (0 to 2, 2 to 12, and >12 years of age) based on the dose recommendations (extrapolations) shown in Table 1. Differences in patient characteristics among the three age groups were assessed using Kruskal-Wallis tests for continuous data and chi-square tests for categorical data. The difference between the recommended dose and the dose used to reach an adequate Ctrough was tested using a paired t test. Intrapatient variability in Ctrough was analyzed in patients who had a minimum of three serum concentrations above the detection limit at the same dose.
RESULTS
Patients and voriconazole use.
Ninety-two patients between 0 and 20 years of age underwent HSCT in the study period. Sixty-one of these patients (66% of all patients who underwent HSCT) were treated with voriconazole, either prophylactically (n = 25), empirically (n = 9), or as treatment (n = 27). Forty-seven patients received voriconazole intravenously and 14 patients orally.
The demographic data of voriconazole users and their clinical characteristics are summarized in Table 2. Eleven patients were <2 years, 31 were between 2 and 12 years, and 20 were >12 years. Twenty-one of the 27 children treated therapeutically had aspergillosis (13 possible, 4 probable, 4 proven). Initial dosing was according to the SPC or extrapolated from the SPC in children <2 years of age in 41 patients (67%). Voriconazole was prescribed for a median period of 64 days (range, 6 to 415). The most common reason for discontinuation was immune recovery (29 patients) or clinical improvement (10 patients). In six patients (10%) the drug was discontinued due to toxicity (hepatotoxicity or urticaria). Discontinuation due to toxicity was not related to the age of the patient, dose given, or any of the parameters related to TDM of voriconazole (data not shown). The six patients had a median Ctrough of 0.5 mg/liter at the initial dose and a maximal Ctrough of 3.3 mg/liter measured throughout therapy.
Table 2.
Patient characteristicsa
| Parameter | Total | Result by age group |
P value | ||
|---|---|---|---|---|---|
| 0–2 yr | 2–12 yr | >12 yr | |||
| Total no. | 61 | 11 | 31 | 19 | |
| Age (yr; median [range]) | 7.3 (0.3–20) | ||||
| Body wt (median [range]) | 23 (3.7–65) | 9 (3.7–12) | 20 (11–50) | 50 (29–65) | <0.001 |
| Sex (no. [% male]) | 36 (59) | 64% | 58% | 58% | 0.942 |
| Match (10/10 or 6/6 [%]) | 37 (61) | 45% | 65% | 63% | 0.520 |
| Indication (no. [%]) | |||||
| Malignancies | 34 (56) | 3 | 17 | 14 | 0.039 |
| Metabolic disorders | 8 (13) | 3 | 3 | 2 | |
| Immune deficiencies | 14 (23) | 5 | 6 | 3 | |
| Bone marrow failure | 5 (8) | 0 | 5 | 0 | |
| Type of HSCT (no. [%) | |||||
| BM | 22 (36) | 3 | 10 | 9 | 0.742 |
| CB | 33 (54) | 7 | 18 | 8 | |
| CB-PBSC | 4 (7) | 1 | 2 | 1 | |
| Double-CB | 1 (2) | 0 | 0 | 1 | |
| PBSC | 1 (2) | 0 | 1 | 0 | |
| Start dose used (median [range]) | 61 | 12.7 (7–15) | 13.8 (6–20) | 8.1 (7–15) | 0.011 |
| Recommended dose (no. [%]) | 41 (67) | 7 | 22 | 12 | 0.793 |
| Administration (no. [%]) | |||||
| Intravenous | 47 (77) | 8 | 25 | 14 | 0.862 |
| Oral | 14 (23) | 3 | 6 | 5 | |
| Days of use (median [range]) | 39 (6–415) | 89 (8–415) | 36 (6–191) | 40 (10–210) | 0.134 |
| Prior antifungal use (no. [%]) | 4 (7) | 1 | 3 | 0 | 0.211 |
| Indication (no. [%]) | |||||
| Therapeutic; possible invasive aspergillosis | 13 (21) | 2 | 8 | 3 | 0.893 |
| Therapeutic; proven invasive aspergillosis | 4 (7) | 0 | 3 | 1 | |
| Therapeutic; probable invasive aspergillosis | 4 (7) | 0 | 2 | 2 | |
| Empirical | 9 (15) | 1 | 6 | 2 | |
| Therapeutic other fungal infection than aspergillus | 6 (10) | 2 | 2 | 2 | |
| Prophylactic (primary: received no antifungal agents in past) | 20 (33) | 5 | 8 | 7 | |
| Prophylactic (secondary: in past received other antifungal agent) | 5 (8) | 1 | 2 | 2 | |
| Reason for stop (no. [%]) | |||||
| Recovery of immune system | 29 (48) | 5 | 18 | 6 | 0.202 |
| Clinical improvement | 10 (17) | 1 | 4 | 5 | |
| Toxicity | 6 (10) | 1 | 3 | 2 | |
| Therapeutic failure | 6 (10) | 1 | 2 | 3 | |
| Death | 5 (8) | 1 | 2 | 2 | |
| Resistance | 2 (3) | 0 | 2 | 0 | |
| No. (%) of patients still on voriconazole at end of study | 2 (3) | 2 | 0 | 0 | |
Values are expressed as numbers unless specified otherwise. HSCT, hematopoietic stem cell transplantation; BM, bone marrow; CB, cord blood; PBSC, peripheral blood stem cell.
Therapeutic drug monitoring of voriconazole.
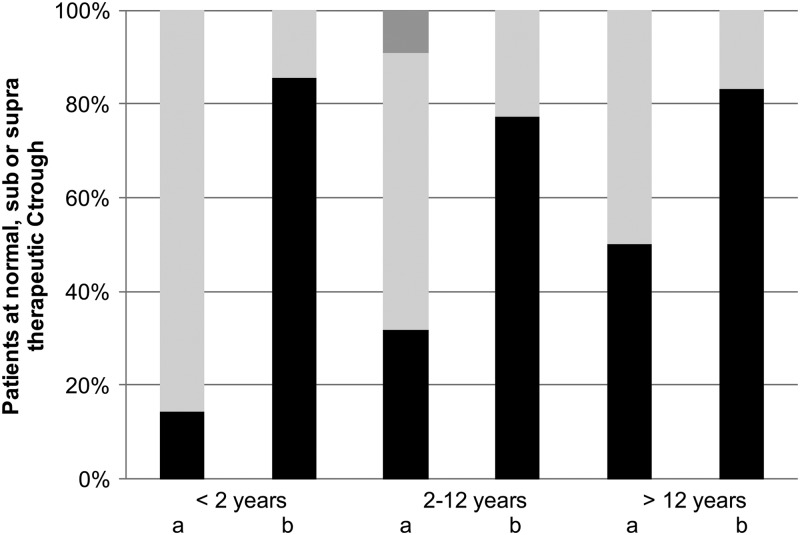
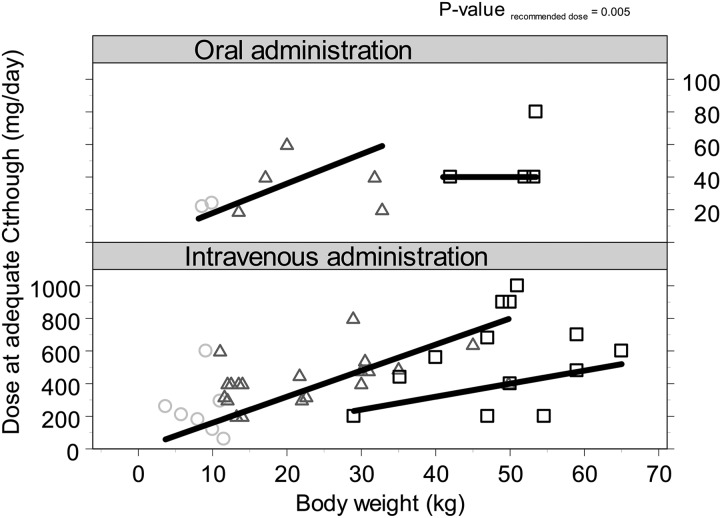
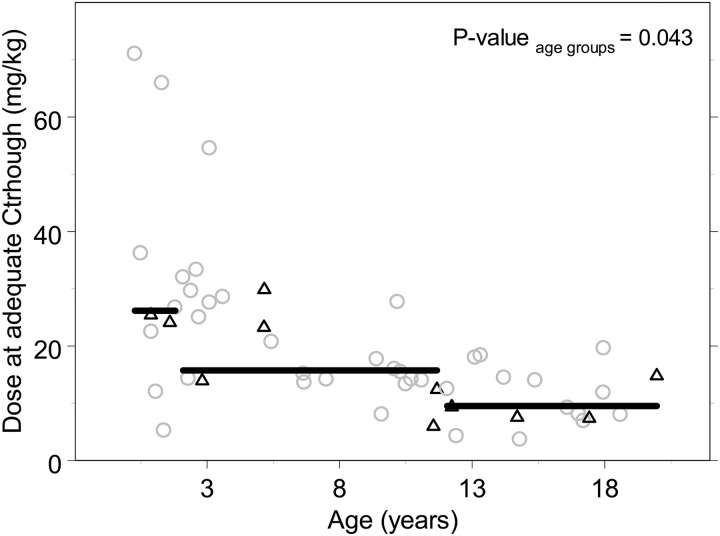
A total of 380 voriconazole Ctroughs were measured in all 61 patients treated with voriconazole (mean of 8 samples per patient). The first measurement of Ctrough was performed after a median of 4 days. The results of TDM in patients who received the recommended (or extrapolated) dosages (41 patients) are shown in Fig. 1 and Table 3. Thirty-four percent of these patients reached an adequate level at initial dosing: 14, 32, and 50% in the three age groups of <2, 2 to 12, and 12 to 20 years, respectively (P = 0.354). Sixty-one percent of patients had a subtherapeutic Ctrough, of which 56% had a voriconazole Ctrough below the limit of detection (<0.5 mg/liter), whereas 5% had an initial Ctrough of >5 mg/liter. After intravenous administration, 38% reached an adequate level initially versus 43% after oral administration (P = 0.800). Nine patients (20%) did not reach a therapeutic concentration throughout therapy. In these patients the Ctrough remained too low, even though TDM was performed. One patient had immune recovery before the Ctrough was within the therapeutic concentration. In two patients doses were not optimized since their clinical situation was fatal, and in another two patients the suspicion of Aspergillus remained low. In three patients the Ctrough remained highly variable after TDM-based dose adjustments. For the remaining patient no reasons were registered for why the dose was not raised high enough to reach an adequate Ctrough. The median time of use of voriconazole in these patients was 27 days (range, 7 to 46 days). In 80% of patients an adequate Ctrough was reached after TDM-based dose adjustments and 79% after intravenous and 85% after oral administration of voriconazole, after a median of 10 days (range, 3 to 41 days). In order to reach an adequate Ctrough, doses were increased in 57% of patients, unaltered in 12% of patients, and decreased in 30% of patients and were significantly different from the recommended doses, as shown in Fig. 2 (P = 0.005). The median daily dose to reach an adequate Ctrough in children of <2 years of age was 31.5 mg/kg (range 12 to 71 mg/kg). For the age group 2 to 12 years, adequate levels were reached with a median daily dose of 16 mg/kg (range, 13 to 55 mg/kg), and above 12 years this daily dose was 9.4 mg/kg (7.5 to 20 mg/kg) as shown in Fig. 3 (P = 0.034). Both figures show that the interpatient variability was high and was only partly related to age and/or body weight. Concentrations varied widely during the treatment; the lowest and highest concentrations reached within each patient could differ by a factor of 10 as a result of dose changes and intrapatient variability. No difference was seen in the highest concentrations reached between the patients who did and did not need a dose increase.
Fig 1.
Results of therapeutic drug monitoring of voriconazole. Shown is the percentage of children reaching normal, subnormal, or supranormal therapeutic Ctroughs (black, light gray, and dark gray boxes, respectively) after the initial recommended or extrapolated dose (a) and after TDM-based (recurrent) dose adjustments (b). The results are divided by age group (<2 years of age, 2 to 12 years of age, and >12 years of age).
Table 3.
Results of therapeutic drug monitoring of voriconazole in patients using the recommended dose as the first dosea
| TDM per age group | Total no. of subjects | Result by age group (median [range]) |
P value | ||
|---|---|---|---|---|---|
| 0–2 yr |
2–12 yr |
>12 yr |
|||
| Median (range) | Median (range) | Median (range) | |||
| No. of days between start and adequate Ctrough | 33 | 11.5 (8–21) | 6 (4–41) | 10.5 (3–19) | 0.127 |
| Dose at adequate Ctrough (mg/day) | 33 | 235 (120–600) | 400 (190–800) | 540 (400–1,000) | 0.234 |
| Dose at adequate Ctrough (mg/kg) | 33 | 31.5 (12–71) | 15.5 (13–55) | 9.4 (8–19.6) | 0.043 |
| Intravenous (mg/kg) | 27 | 31.5 (12–71) | 15.5 (13–55) | 11.9 (9–20) | 0.035 |
| Oral (mg/kg) | 6 | 22 (14–30) | 8.6 (8–15) | ||
| Maximum dose used (mg/day) | 40 | 300 (180–600) | 400 (190–800) | 600 (320–1,100) | 0.479 |
| Lowest Ctrough measured (mg/liter) | 41 | BLQ (BLQ-2.8) | BLQ (BLQ-7.2) | 0.6 (BLQ-1.7) | 0.264 |
| Highest Ctrough measured (mg/liter) | 41 | 8.5 (1.0–14) | 3.3 (1.0–10) | 2.8 (1–15.3) | 0.273 |
| Reached at first measurement | 14 | 13.5 | 2.6 (1.8–9.6) | 4.0 (2.6–7.7) | |
| Reached after dose increase | 25 | 7.8 (0.8–10.2) | 3.8 (BLQ-10.8) | 1.2 (BLQ-15.3) | |
For this analysis, patients were included only if they initially received the recommended daily dose as defined in Table 1. BLQ, below the limit of quantification.
Fig 2.
Daily voriconazole dose at adequate Ctrough versus body weight in relation to the recommended (or extrapolated) dose per age group. The gray circles show the patients of <2 years of age, black triangles show the data of patients between 2 and 12 years of age, and black squares show the data of patients who were >12 years of age. The plots are separated based on route of administration (oral or intravenous). The black straight lines show the recommended or extrapolated dose.
Fig 3.
Daily voriconazole dose, in mg/kg, at adequate Ctrough versus age. The black triangles show the data of patients receiving oral voriconazole, and gray circles show the patients receiving intravenous voriconazole. The black straight lines show the median value per predefined age group.
During therapy with identical daily doses in nine patients, the median intrapatient variability of Ctroughs was a factor of 2.9, and values ranged between 11 and 238% of the first Ctrough measured at the specific dose. In five patients Ctroughs decreased during the course of treatment (median 49%), in two patients the following Ctroughs increased (a median increase in Ctrough of 170% per patient), and in two patients Ctroughs varied (between 56 and 160% of the first Ctrough). The two patients under 7 years of age had the highest fluctuation of at least 2 mg/liter.
DISCUSSION
This retrospective study in children and young adults between 0.3 and 20 years of age was performed to assess the value of voriconazole TDM in pediatric HSCT. The recommended (or extrapolated) dose does not lead to adequate therapeutic drug levels in the majority of patients, especially in children <2 years of age. Only one-third of patients had initial voriconazole levels within the predefined, supposed therapeutic range of 1 to 5 mg/liter. In all patients using voriconazole, TDM was performed and TDM-based dose adjustments led to an adequate Ctrough in 80% of patients, showing that TDM was feasible and useful to achieve adequate plasma concentrations (as illustrated in Fig. 1). As a relationship between voriconazole serum concentrations and outcome of voriconazole treatment has been established in prior studies (5, 12–14), this study shows that dose targeting based on TDM is needed to individualize the voriconazole doses in pediatrics when aiming to optimize treatment outcomes.
The current observation that the recommended dose does not lead to adequate therapeutic drug levels in the majority of children under treatment is in line with prior studies. Neely et al. predicted that when using the recommended dose, around 34% of children >2 years of age receiving voriconazole would not achieve Ctroughs of >1 mg/liter (5). Brüggeman et al. showed that in 44% of 18 children and Spriet et al. in 31% of 16 children >2 years of age, the recommended dose resulted in a Ctrough below the target concentration of 1 mg/liter (4, 18). In comparison, our study showed that 60% of 41 patients had an initial Ctrough below this target concentration, and this data set included 11 patients <2 years of age. Based on simulations, Karlsson and Neely (5, 9) suggest that a dose of 7 mg/kg every 12 h is a good starting dose in children between 2 and 12 years of age, which is consistent with the median dose at adequate Ctroughs in our study in this age range. However, in neonates and toddlers, the dose should be increased, which was also suggested in a prior study of oral voriconazole by Shima et al. (11). The age-related cutoff values defined in the SPC do not seem to suffice, as the correlation between the dose of voriconazole in mg/kg and the age of the child appears to show a continuous relationship (Fig. 3). Analysis of the variability in pharmacokinetics (PK) and pharmacodynamics (PD) using population PK-PD modeling in combination with an identification of covariates that account for the large inter- and intrapatient variability is needed in order to define appropriate doses in children, especially in neonates and toddlers. Patient outcome may benefit from an optimal starting dose of voriconazole, which would lead to a more predictable exposure and efficacy/safety profile in these life-threatening fungal infections where the time to appropriate treatment is critical.
The influence of route of administration on the dose-concentration relationship was investigated. The dose to reach an adequate Ctrough was not significantly different after oral or intravenous administration in each of the age groups, suggesting that the same initial dose can be administered orally or intravenously. Data in the literature regarding the bioavailability in children differ between 45 and 80% (5, 8, 9). Absorption of voriconazole may differ between children and adults, as the SPC reports a bioavailability of 96% (10). In addition, the bioavailability of drugs may be impaired after HSCT due to mucositis, diarrhea, or other factors. Given the current uncertainty associated with the pharmacokinetics of enteral voriconazole in children, it may be appropriate to prolong intravenous therapy until there is clear evidence of sustained improvement.
Based on the prior observed relationships between voriconazole serum concentrations and outcome of voriconazole treatment (5, 12–14) and the observation regarding the large interpatient variability in PK of voriconazole, the use of TDM in pediatric patients on a routine basis to prevent suboptimal exposure when treating life-threatening fungal infections is warranted. The results of our study showed that TDM-guided voriconazole dosing was well tolerated, as only 6 patients (10%) were forced to stop treatment due to toxicity, even though doses as high as 70 mg/kg/day were given to patients based on TDM. In addition, in 65% of patients the treatment was considered effective due to recovery of the immune system or clinical improvement. TDM-based dose adjustments were performed very soon after the start of the treatment (median of 5 days). However, in 56% of patients it was difficult to identify an optimal dose based on the first measurement, as this measurement was below the detection limit. In some patients it took up to a month to find a dose leading to an adequate Ctrough. Due to inadequate dosing, these patients are more likely at risk of treatment failure. Therefore, we advocate to assess plasma concentrations before the start of treatment using a maximum, middle, and trough concentration (mini-area under the curve) to identify an adequate dose and adequate dosing interval. The large intrapatient variability in the Ctroughs suggests that Ctrough concentrations should be measured at least once weekly and should be continued throughout the treatment. In this study, the pharmacokinetics of voriconazole were assumed to be linear in children and dose adjustments were performed based on this assumption. However, recent studies show that when using doses of >7 mg/kg twice daily, the PK of voriconazole in children seems to be nonlinear (5, 8, 9). Therefore, dose increases based on TDM above 7 mg/kg should be performed with care, Ctroughs should be monitored even more frequently, and nonlinear PK models should be used when performing TDM in future patients.
The relatively high detection limit of the voriconazole assay was a limitation of the present study. In patients with a concentration below the detection limit, the actual concentration cannot be determined. The lack of a prospective study design with systematic voriconazole TDM at fixed time points limits the detection of correlations between the dose, Ctrough, and outcome of the treatment. Future studies should be performed prospectively to further assess the added value of TDM of voriconazole. Prior studies reported only small cohorts and no or limited numbers of patients of <2 years of age. The strength of this study is that it presents a large number of children and young adults treated with voriconazole, including a large number of neonates and toddlers. In conclusion, the present study shows that therapeutic drug monitoring is indispensable for correct dosage of voriconazole in pediatrics. Dosage recommendations for children need to be modified, especially in neonates and toddlers. Intrapatient variability is of major concern and necessitates continued Ctrough measurements. Patient characteristics that determine pharmacokinetic variability need to be identified in future studies.
ACKNOWLEDGMENTS
I. H. Bartelink performed and designed research, analyzed the data, and wrote the paper. T. Wolfs performed and designed research, collected data, and contributed to the writing of the paper. M. Jonker collected data, contributed in the study design, and contributed to the writing of the paper. M. de Waal collected data, contributed in the study design, and contributed to the writing of the paper. A. C. G. Egberts designed research and contributed to the writing of the paper. F. T. T. Ververs designed research and contributed to the writing of the paper. J. J. Boelens designed research and contributed to the writing of the paper. M. Bierings performed and designed research, analyzed the data, and contributed to the writing of the paper.
Footnotes
Published ahead of print 31 October 2012
REFERENCES
- 1. Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA, Schuler U, Lutsar I, Troke P, Thiel E. 2005. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 106:2641–2645 [DOI] [PubMed] [Google Scholar]
- 2. Schwartz S, Reisman A, Troke PF. 2011. The efficacy of voriconazole in the treatment of 192 fungal central nervous system infections: a retrospective analysis. Infection 39:201–210 [DOI] [PubMed] [Google Scholar]
- 3. Almyroudis NG, Kontoyiannis DP, Sepkowitz KA, DePauw BE, Walsh TJ, Segal BH. 2006. Issues related to the design and interpretation of clinical trials of salvage therapy for invasive mold infection. Clin. Infect. Dis. 43:1449–1455 [DOI] [PubMed] [Google Scholar]
- 4. Bruggemann RJ, van der Linden JW, Verweij PE, Burger DM, Warris A. 2011. Impact of therapeutic drug monitoring of voriconazole in a pediatric population. Pediatr. Infect. Dis. J. 30:533–534 [DOI] [PubMed] [Google Scholar]
- 5. Neely M, Rushing T, Kovacs A, Jelliffe R, Hoffman J. 2010. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin. Infect. Dis. 50:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walsh TJ, Lutsar I, Driscoll T, Dupont B, Roden M, Ghahramani P, Hodges M, Groll AH, Perfect JR. 2002. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr. Infect. Dis. J. 21:240–248 [DOI] [PubMed] [Google Scholar]
- 7. Michael C, Bierbach U, Frenzel K, Lange T, Basara N, Niederwieser D, Mauz-Körholz C, Preiss R. 2010. Voriconazole pharmacokinetics and safety in immunocompromised children compared to adult patients. Antimicrob. Agents Chemother. 54:3225–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walsh TJ, Driscoll T, Milligan PA, Wood ND, Schlamm H, Groll AH, Jafri H, Arrieta AC, Klein NJ, Lutsar I. 2010. Pharmacokinetics, safety, and tolerability of voriconazole in immunocompromised children. Antimicrob. Agents Chemother. 54:4116–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karlsson MO, Lutsar I, Milligan PA. 2009. Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob. Agents Chemother. 53:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pfizer Limited 2012. Vfend summary of product information. Pfizer Limited, London, United Kingdom [Google Scholar]
- 11. Shima H, Miharu M, Osumi T, Takahashi T, Shimada H. 2010. Differences in voriconazole trough plasma concentrations per oral dosages between children younger and older than 3 years of age. Pediatr. Blood Cancer 54:1050–1052 [DOI] [PubMed] [Google Scholar]
- 12. Bruggemann RJ, Donnelly JP, Aarnoutse RE, Warris A, Blijlevens NM, Mouton JW, Verweij PE, Burger DM. 2008. Therapeutic drug monitoring of voriconazole. Ther. Drug Monit. 30:403–411 [DOI] [PubMed] [Google Scholar]
- 13. Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201–211 [DOI] [PubMed] [Google Scholar]
- 14. Pasqualotto AC, Xavier MO, Andreolla HF, Linden R. 2010. Voriconazole therapeutic drug monitoring: focus on safety. Expert. Opin. Drug Saf. 9:125–137 [DOI] [PubMed] [Google Scholar]
- 15. Pieper S, Kolve H, Gumbinger HG, Goletz G, Würthwein G, Groll AH. 2012. Monitoring of voriconazole plasma concentrations in immunocompromised paediatric patients. J. Antimicrob. Chemother. 67:2717–2724 [DOI] [PubMed] [Google Scholar]
- 16. Troke PF, Hockey HP, Hope WW. 2011. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob. Agents Chemother. 55:4782–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meletiadis J, Mavridou E, Melchers WJ, Mouton JW, Verweij PE. 2012. Epidemiological cutoff values for azoles and Aspergillus fumigatus based on a novel mathematical approach incorporating cyp51A sequence analysis. Antimicrob. Agents Chemother. 56:2524–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spriet I, Cosaert K, Renard M, Uyttebroeck A, Meyts I, Proesmans M, Meyfroidt G, de Hoon J, Verbesselt R, Willems L. 2011. Voriconazole plasma levels in children are highly variable. Eur. J. Clin. Microbiol. Infect. Dis. 30:283–287 [DOI] [PubMed] [Google Scholar]