Abstract
Three Candida tropicalis isolates were obtained from a patient with acute lymphoblastic leukemia. The first isolate was susceptible to all drug classes, while isolates 2 and 3, obtained after 8 and 8.5 weeks of caspofungin treatment, respectively, were resistant to the three echinocandins. Multilocus sequence genotyping suggested a clonal relation among all isolates. FKS1 sequencing revealed a stepwise development of a heterozygous and finally a homozygous mutation, leading to S80S/P and S80P amino acid substitutions.
TEXT
It is well recognized that long-term antifungal treatment entails a risk for in vivo selection of resistant fungi. Accordingly, an increasing number of reports demonstrate acquired echinocandin and azole resistance associated with both hetero- and homozygous mutations in the FKS and ERG11 genes, which encode antifungal target proteins in Candida (1–6). This is of clinical importance, as resistant Candida isolates are associated with breakthrough candidiasis, treatment failures, and increased mortality (7). Candida tropicalis is identified as one of the five most common pathogenic Candida species, with a geographically determined proportion ranging from 3 to 66% of candidemia cases (8–10). Unfortunately, acquired fluconazole resistance is increasing, with ranges from approximately 7% in Denmark (9) to 9% in a global study (11) and 40% in Japan (12). Based on such findings, echinocandins are increasingly being utilized in the management of candidiasis caused by C. tropicalis (10, 13–15).
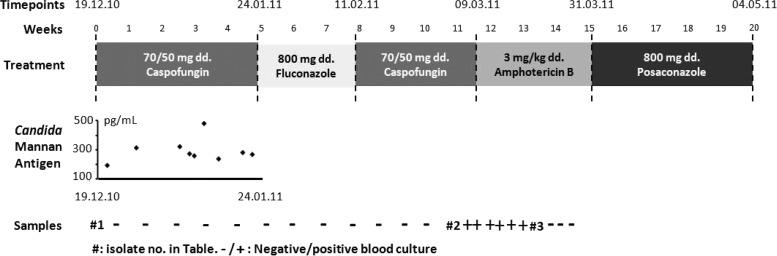
In this study, we analyzed three sequential C. tropicalis isolates (isolates 1, 2, and 3) obtained over a 4-month period from a patient with acute lymphoblastic leukemia who had been referred for allogeneic bone marrow transplantation. The patient was initially blood culture positive on 19 December 2010 for C. tropicalis (isolate 1) while receiving voriconazole prophylaxis. Caspofungin treatment was initiated (70/50 mg/day [70 mg on day 1 as a loading dose, followed by 50 mg daily thereafter]) (Fig. 1) and continued for a total of 8.5 weeks, interrupted by a 3-week fluconazole step-down treatment (Fig. 1). During the initial caspofungin treatment, nine serum samples tested positive for Candida mannan antigen, peaking at 479 pg/ml but stabilizing around 250 pg/ml on 20 January 2011 (Fig. 1). C. tropicalis was again detected in the blood on 5 March 2011 (isolate 2, after approximately 8 weeks of caspofungin treatment), and treatment was switched to amphotericin B (3 mg/kg/day) on 9 March. The patient was blood culture negative from 16 March, but the final C. tropicalis isolate (isolate 3, after approximately 8.5 weeks of caspofungin treatment) was obtained on 18 March from an oral swab, and treatment was changed to posaconazole (800 mg/day) on 31 March 2011. A Hickman catheter was kept in place, but sterilization was attempted with acid and fluconazole lock. Susceptibility testing was done according to EUCAST EDef 7.2 (azoles, anidulafungin, and micafungin) (16) and by Etest (amphotericin B and caspofungin). Etest was chosen for caspofungin susceptibility testing, as the biological potency of pure substance has been associated with an unacceptable lot-to-lot variation, with the most recently obtained powder giving rise to elevated MICs (17–19). Echinocandin susceptibility was evaluated using the EUCAST breakpoint for anidulafungin EUCAST MICs (susceptibility [S] ≤ 0.06 mg/liter) but adopting the revised CLSI breakpoint for interpretation of caspofungin Etest results (S ≤ 0.25 mg/liter) (20, 21). CLSI breakpoints were adopted for interpretation of the caspofungin Etest MICs as recommended by the manufacturer, as this has been found to be appropriate for Candida albicans and C. tropicalis (22). EUCAST breakpoints for micafungin have not yet been established, but 185 of 186 wild-type C. tropicalis isolates tested in our laboratory had a EUCAST MIC of ≤0.03 mg/liter, which was used to define susceptibility. Two unrelated isolates of C. tropicalis (REF-1 and REF-2) were included as wild-type FKS1 reference isolates. The FKS1 gene was amplified and sequenced using primers targeting hot spot 1 (FKS1-F, TCATTGCTGTGGCCACTTTAG; FKS1-R, TAGAATGAACGACCAATGGAGA) and hot spot 2 (FKS8-F, CTCCTGCCGTTGATTGGATTA; FKS8-R, ACCACCAACGGTCAAATCAG) and compared to the C. tropicalis FKS1 reference sequence (GenBank accession no. EU676168). Genetic relatedness was analyzed by multilocus sequence typing (MLST) based on polymorphisms in 6 sequenced housekeeping genes (ICL1, MDR1, SAPT2, SAPT4, XYR1, and ZWF1a) as described previously (23) by applying the PubMLST database, covering 205 diploid sequence types (http://pubmlst.org/ctropicalis).
Fig 1.
Systemic antifungal treatment of the leukemic patient illustrated in boxes with drugs administered as daily doses (dd.). Nine serum samples were positive for Candida mannan antigen during the first caspofungin treatment period, and subsequently, several positive blood cultures were obtained. Three isolates (isolates 1, 2, and 3) were chosen and sequenced for resistance mechanisms and genotyping.
Isolate 1 and the two reference isolates were susceptible to all tested antifungals, whereas isolates 2 and 3 were categorized as echinocandin resistant (Table 1). Isolate 1, REF-1, and REF-2 were wild type in both hot spot 1 and 2 in the FKS1 sequence, while isolate 2 harbored a heterozygous T238C mutation and isolate 3 a homozygous T238C mutation in hot spot 1 of FKS1, leading to S80S/P and S80P amino acid substitutions, respectively. The MLST data suggested that isolates 1, 2, and 3 were clonally related, since the diploid sequences in the 6 housekeeping genes were 100% identical (Table 1).
Table 1.
Origins, resistance mechanisms, genotypes, and susceptibility data for the three study and two control C. tropicalis isolates
| Isolate | Specimen origin | Collection date (day.mo.yr) | FKS1 resistance mechanism | Allelic profile according to PubMLST (ICL1-MDR1-SAPT2-SAPT4-XYR-ZWF) | MIC (μg/ml)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EUCAST (EDef 7.1) |
Etest |
|||||||||||
| ANI | MICA | POS | VOR | ITR | FLU | AMB | CAS | |||||
| 1 | BCb | 19.12.10 | Wild type | 16-20-4-10-25-5 | ≤0.03 | ≤0.008 | ≤0.03 | ≤0.03 | ≤0.03 | 1 | 0.5 | 0.25 |
| 2 | BC-CVCc | 05.03.11 | S80S/P | 16-20-4-10-25-5 | 0.25 | 1 | ≤0.03 | ≤0.03 | ≤0.03 | 0.5 | 0.5 | >32 |
| 3 | Cavum oris | 18.03.11 | S80P | 16-20-4-10-25-5 | 0.5 | >1 | ≤0.03 | 0.06 | ≤0.03 | 2 | 1 | >32 |
| REF-1d | BCb | 08.07.10 | Wild type | 1-7-4-6-2-4 | ≤0.03 | ≤0.008 | ≤0.03 | ≤0.03 | 0.03 | ≤0.125 | 1 | 0.25 |
| REF-2d | BCb | 23.01.11 | Wild type | 1-3-1-7-2 (99.7 %)-1 | ≤0.03 | ≤0.008 | ≤0.03 | ≤0.03 | 0.125 | 0.5 | 0.5 | 0.125 |
ANI, anidulafungin; MICA, micafungin; POS, posaconazole; VOR, voriconazole; ITR, itraconazole; FLU, fluconazole; AMB, amphotericin B; CAS, caspofungin.
Unspecified blood culture.
Blood culture obtained via the intravenous Hickman catheter.
Susceptible reference isolate from unrelated patients, used for comparison.
In vivo selection for echinocandin resistance has been demonstrated for several Candida species, including C. albicans (1, 24–27), C. glabrata (24, 28–31), C. krusei (5, 24, 32), and C. parapsilosis (33). However, to our knowledge, this is the first study to demonstrate the stepwise in vivo progression of a wild-type C. tropicalis strain to a homozygous fks1 mutant exhibiting echinocandin resistance. Even the heterozygous mutant isolate was classified as echinocandin resistant, with a significant ≥3 to 7 twofold-dilution step increase in echinocandin MICs, illustrating the significance of the S80 codon in FKS1 in C. tropicalis. Nevertheless, the MICs indicated that the homozygous mutant (isolate 3) may be slightly more resistant to echinocandins (at least 1 dilution step, as suggested by the increase in anidulafungin and micafungin MICs). Other homozygous mutations in C. tropicalis fks1 have been associated with elevated echinocandin MICs and amino acid substitutions, including L79W (4), F76S (6), and F76L (34). Moreover, heterozygous S80S/P mutants that display echinocandin resistance have been found (34, 35), but interestingly, the homozygous S80P mutation has not been described previously. This is in contrast to the findings for C. albicans, where a homozygous alteration at the corresponding codon (S645) has been detected in several resistant isolates (17, 36–38). Several factors may contribute to this difference. First, fitness cost when the second allele is mutated may vary, as supported by previous observations associating homozygous fks1 mutations in C. albicans with both fitness and virulence costs (39). Second, the resistance conferred by the heterozygous mutation may be sufficient to allow escape in S80S/P C. tropicalis during caspofungin treatment, whereas the homozygous variant may be required for high-level echinocandin resistance in C. albicans (37, 40).
Our and related studies contribute to the overall understanding of resistance development in vivo as a consequence of antifungal treatment, including understanding the duration of treatment and which compounds allow selection of resistant mutants. Finally, this study may assist in determining treatment guidelines for the management of C. tropicalis infections, as the development of echinocandin resistance should be acknowledged as a rising concern in the treatment of patients with long-term echinocandin exposure.
ACKNOWLEDGMENTS
We thank Birgit Brandt for her invaluable technical assistance in the laboratory.
R.H.J. has received a research grant from Gilead and travel grants from Astellas and MSD.
M.C.A. has received research grants from Astellas, Gilead, MSD, and Pfizer, been an advisor or consultant for Gilead, MSD, and Pfizer, and received a speaker's honorarium for talks from Astellas, Gilead, MSD, and Pfizer.
H.K.J. has nothing to declare.
Footnotes
Published ahead of print 22 October 2012
REFERENCES
- 1. Baixench M-T, Aoun N, Desnos-Ollivier M, Garcia-Hermoso D, Bretagne S, Ramires S, Piketty C, Dannaoui E. 2007. Acquired resistance to echinocandins in Candida albicans: case report and review. J. Antimicrob. Chemother. 59:1076–1083 [DOI] [PubMed] [Google Scholar]
- 2. Cleary JD, Garcia-Effron G, Chapman SW, Perlin DS. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob. Agents Chemother. 52:2263–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costa-de-Oliveira S, Marcos Miranda I, Silva RM, Pinto E Silva A, Rocha R, Amorim A, Gonçalves Rodrigues A, Pina-Vaz C. 2011. FKS2 mutations associated with decreased echinocandin susceptibility of Candida glabrata following anidulafungin therapy. Antimicrob. Agents Chemother. 55:1312–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desnos-Ollivier M, Bretagne S, Raoux D, Hoinard D, Dromer F, Dannaoui E. 2008. Mutations in the fks1 gene in Candida albicans, C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the method of the European Committee on Antibiotic Susceptibility Testing. Antimicrob. Agents Chemother. 52:3092–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durán-Valle MT, Gago S, Gómez-López A, Cuenca-Estrella M, Jiménez Díez-Canseco L, Gómez-Garcés JL, Zaragoza O. 2012. Recurrent episodes of candidemia due to Candida glabrata with a mutation in the hot spot 1 of the FKS2 gene developed after prolonged therapy with caspofungin. Antimicrob. Agents Chemother. 56:3417–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia-Effron G, Chua DJ, Tomada JR, DiPersio J, Perlin DS, Ghannoum M, Bonilla H. 2010. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrob. Agents Chemother. 54:2225–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun H-Y, Singh N. 2010. Characterisation of breakthrough invasive mycoses in echinocandin recipients: an evidence-based review. Int. J. Antimicrob. Agents 35:211–218 [DOI] [PubMed] [Google Scholar]
- 8. Arendrup MC, Fuursted K, Gahrn-Hansen B, Schønheyder HC, Knudsen JD, Jensen IM, Bruun B, Christensen JJ, Johansen HK. 2008. Semi-national surveillance of fungaemia in Denmark 2004–2006: increasing incidence of fungaemia and numbers of isolates with reduced azole susceptibility. Clin. Microbiol. Infect. 14:487–494 [DOI] [PubMed] [Google Scholar]
- 9. Arendrup MC, Bruun B, Christensen JJ, Fuursted K, Johansen HK, Kjaeldgaard P, Knudsen JD, Kristensen L, Møller J, Nielsen L, Rosenvinge FS, Røder B, Schønheyder HC, Thomsen MK, Truberg K. 2011. National surveillance of fungemia in Denmark (2004 to 2009). J. Clin. Microbiol. 49:325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chai LYA, Denning DW, Warn P. 2010. Candida tropicalis in human disease. Crit. Rev. Microbiol. 36:282–298 [DOI] [PubMed] [Google Scholar]
- 11. Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, Rodloff A, Fu W, Ling TA. 2010. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 48:1366–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chong Y, Shimoda S, Yakushiji H, Ito Y, Miyamoto T, Shimono N, Kamimura T, Akashi K. 2012. Fatal candidemia caused by azole-resistant Candida tropicalis in patients with hematological malignancies. J. Infect. Chemother. In press [DOI] [PubMed] [Google Scholar]
- 13. Kothavade RJ, Kura MM, Valand AG, Panthaki MH. 2010. Candida tropicalis: its prevalence, pathogenicity and increasing resistance to fluconazole. J. Med. Microbiol. 59:873–880 [DOI] [PubMed] [Google Scholar]
- 14. Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2009. Comparison of results of fluconazole and voriconazole disk diffusion testing for Candida spp. with results from a central reference laboratory in the ARTEMIS DISK Global Antifungal Surveillance Program. Diagn. Microbiol. Infect. Dis. 65:27–3419679232 [Google Scholar]
- 15. Yang Y-L, Lin C-C, Chang T-P, Lauderdale T-L, Chen H-T, Lee C-F, Hsieh C-W, Chen P-C, Lo H-J. 2012. Comparison of human and soil Candida tropicalis isolates with reduced susceptibility to fluconazole. PLoS One 7:e34609 doi:10.1371/journal.pone.0034609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope W. 2012. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 18:E246–247 [DOI] [PubMed] [Google Scholar]
- 17. Arendrup MC, Garcia-Effron G, Buzina W, Mortensen KL, Reiter N, Lundin C, Jensen HE, Lass-Flörl C, Perlin DS, Bruun B. 2009. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob. Agents Chemother. 53:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arendrup MC, Rodriguez-Tudela J-L, Park S, Garcia-Effron G, Delmas G, Cuenca-Estrella M, Gomez-Lopez A, Perlin DS. 2011. Echinocandin susceptibility testing of Candida spp. Using EUCAST EDef 7.1 and CLSI M27-A3 standard procedures: analysis of the influence of bovine serum albumin supplementation, storage time, and drug lots. Antimicrob. Agents Chemother. 55:1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shields RK, Nguyen MH, Press EG, Kwa AL, Cheng S, Du C, Clancy CJ. 2012. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob. Agents Chemother. 56:4862–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arendrup MC, Rodriguez-Tudela J-L, Lass-Flörl C, Cuenca-Estrella M, Donnelly JP, Hope W. 2011. EUCAST technical note on anidulafungin. Clin. Microbiol. Infect. 17:E18–20 [DOI] [PubMed] [Google Scholar]
- 21. Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS. 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat. 14:164–176 [DOI] [PubMed] [Google Scholar]
- 22. Arendrup MC, Pfaller MA. 2012. Caspofungin Etest susceptibility testing of Candida species: risk of misclassification of susceptible isolates of C. glabrata and C. krusei when adopting the revised CLSI caspofungin breakpoints. Antimicrob. Agents Chemother. 56:3965–3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tavanti A, Davidson AD, Johnson EM, Maiden MCJ, Shaw DJ, Gow NAR, Odds FC. 2005. Multilocus sequence typing for differentiation of strains of Candida tropicalis. J. Clin. Microbiol. 43:5593–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dannaoui E, Desnos-Ollivier M, Garcia-Hermoso D, Grenouillet F, Cassaing S, Baixench MT, Bretagne S, Dromer F, Lortholary O, French Mycoses Study Group 2012. Candida spp. with acquired echinocandin resistance, France, 2004–2010. Emerg. Infect. Dis. 18:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hernandez S, Lo L, Najvar LK, Mccarthy DI, Bocanegra R, Graybill JR. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 48:1382–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laverdière M, Lalonde RG, Baril J-G, Sheppard DC, Park S, Perlin DS. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57:705–708 [DOI] [PubMed] [Google Scholar]
- 27. Park S, Kelly R, Kahn JN, Robles J, Hsu M, Register E, Li W, Vyas V, Fan H, Abruzzo G, Flattery A, Gill C, Chrebet G, Parent SA, Kurtz M, Teppler H, Douglas CM, Perlin DS. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daneman N, Chan AK, Poutanen SM, Rennie R, Sand C, Porter S. 2006. The emergence of caspofungin resistance during treatment of recurrent Candida glabrata candidaemia. Clin. Microbiol. Infect. 12:P1204 [Google Scholar]
- 29. Dodgson KJ, Dodgson AR, Pujol C, Messer SA, Soll DR, Pfaller MA. 2005. Caspofungin resistant C. glabrata. Clin. Microbiol. Infect. 11:P1158 [Google Scholar]
- 30. Krogh-Madsen M, Arendrup MC, Heslet L, Knudsen JD. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938–944 [DOI] [PubMed] [Google Scholar]
- 31. Singh-Babak SD, Babak T, Diezmann S, Hill JA, Xie JL, Chen Y-L, Poutanen SM, Rennie RP, Heitman J, Cowen LE. 2012. Global analysis of the evolution and mechanism of echinocandin resistance in Candida glabrata. PLoS Pathog. 8:e1002718 doi:10.1371/journal.ppat.1002718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hakki M, Staab JF, Marr KA. 2006. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 50:2522–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moudgal V, Little T, Boikov D, Vazquez JA. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother. 49:767–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garcia-Effron G, Kontoyiannis DP, Lewis RE, Perlin DS. 2008. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob. Agents Chemother. 52:4181–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Axner-Elings M, Botero-Kleiven S, Jensen RH, Arendrup MC. 2011. Echinocandin susceptibility testing of Candida isolates collected during a 1-year period in Sweden. J. Clin. Microbiol. 49:2516–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Balashov SV, Park S, Perlin DS. 2006. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob. Agents Chemother. 50:2058–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garcia-Effron G, Park S, Perlin DS. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ben-Ami R, Garcia-Effron G, Lewis RE, Gamarra S, Leventakos K, Perlin DS, Kontoyiannis DP. 2011. Fitness and virulence costs of Candida albicans FKS1 hot spot mutations associated with echinocandin resistance. J. Infect. Dis. 204:626–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Niimi K, Monk BC, a Hirai Hatakenaka K, Umeyama T, Lamping E, Maki K, Tanabe K, Kamimura T, Ikeda F, Uehara Y, Kano R, a Hasegawa Cannon RD, Niimi M. 2010. Clinically significant micafungin resistance in Candida albicans involves modification of a glucan synthase catalytic subunit GSC1 (FKS1) allele followed by loss of heterozygosity. J. Antimicrob. Chemother. 65:842–852 [DOI] [PubMed] [Google Scholar]