Abstract
The NS5A replication complex inhibitor daclatasvir (DCV; BMS-790052) inhibits hybrid replicons containing hepatitis C virus (HCV) genotype 3a (HCV3a) NS5A genes with 50% effective concentrations (EC50s) ranging from 120 to 870 pM. Selection studies with a hybrid HCV3a replicon identified NS5A residues 31 and 93 as sites for DCV-selected resistance. Our results support the potential use of DCV as a component in combination therapies for HCV3a chronic infection.
TEXT
Hepatitis C virus genotype 3 (HCV3) is distributed worldwide and is prevalent in India, Southeast Asia, and Australia (1). Daclatasvir (DCV) potently inhibits HCV RNA replication by targeting the essential replication factor NS5A (2). The antiviral activity of DCV has been confirmed in clinical trials with subjects chronically infected with HCV genotype 1 (HCV1) (3–6). In both in vitro and in vivo studies, mutations that confer resistance to DCV have mapped to the N-terminal 93 amino acids of NS5A, with residues 28, 30, 31, and 93 being particularly prominent resistance-associated sites (2, 6–9).
With the promise of all-oral, interferon-free HCV treatment regimens becoming an ever more attainable goal (3, 10), the ability of direct-acting antivirals (DAA) to exhibit broad HCV genotype coverage is increasingly important. We previously reported on the in vitro activity of DCV toward HCV genotypes 1, 2, and 4 (7, 11, 12). Here, we examined the effectiveness of DCV for hybrid replicons containing NS5A sequences derived from three HCV3a isolates. NS5A cDNA was isolated by reverse transcription-PCR (RT-PCR) from HCV3a-positive sera (lot numbers 10650533, 10650577, and 9990964; ProMedDx, Norton, MA) and inserted into BspEI sites in a JFH1 subgenomic replicon (11) such that the resulting replicons encoded hybrid NS5A proteins with amino acids 1 to 429 derived from the HCV3a isolates and amino acids 430 to 471 derived from the parental HCV2a JFH1 strain (Fig. 1). Since residues within the first 100 amino acids of NS5A are largely responsible for mediating sensitivity to DCV and related NS5A inhibitors (2, 13, 14), an alignment of this region of NS5A from the HCV3a isolates (HCV3a1, HCV3a2, and HCV3a4) and four HCV3a reference sequences (15–17) is shown in Fig. 1B. Over NS5A amino acids 1 to 429, HCV3a1, HCV3a2, and HCV3a4 were 94 to 96% identical to the four reference strains, indicating that they are representative HCV3a strains. In replicon transient assays (7), DCV inhibited the JFH-HCV3a hybrid replicons with 50% effective concentrations (EC50s) ranging from 120 to 870 pM (Table 1). Similar DCV potencies were also observed with replicon cell lines (EC50s = 0.14 to 1.25 nM) (Table 1) that were established by neomycin selection as previously described (18). Sequence analysis of NS5A cDNA isolated by RT-PCR from the established replicon cell lines revealed no changes within the NS5A coding region compared to input sequences, consistent with the ability of JFH1-based replicons to replicate efficiently without cell culture-adaptive mutations (19). To identify DCV resistance-associated mutations, the HCV3a1 replicon cell line was treated with 10 or 100 nM DCV until resistant cell populations emerged as previously described (7). At both selection concentrations, the selected replicon cells were highly resistant to DCV (EC50 > 1 μM) (Table 2) but still sensitive to other DAA (data not shown). NS5A cDNA, isolated by RT-PCR from resistant cells as previously described (7), revealed the presence of two amino acid changes (L31F and Y93H) that were not observed in cDNA isolated from dimethyl sulfoxide (DMSO)-treated control cells. Based on visual examination of the population sequence traces, we estimated that the L31F and Y93H amino acid substitutions were present in 30 to 40% and 60 to 70% of the DCV-selected replicon populations, respectively (Table 2). The L31F and Y93H amino acid substitutions were introduced into the parental HCV3a1 hybrid replicon by standard cloning techniques. DCV inhibited these mutant replicons with EC50s of 80 nM (L31F) and 688 nM (Y93H) in transient assays and 320 nM (L31F) and 1.4 μM (Y93H) in assays with neomycin-selected replicon cell lines (Table 3). The L31F mutant replicon replicated as well as or better than the parental replicon, but the replication capacity of the Y93H replicon was ∼29% of that of the parental replicon (Table 3).
Fig 1.
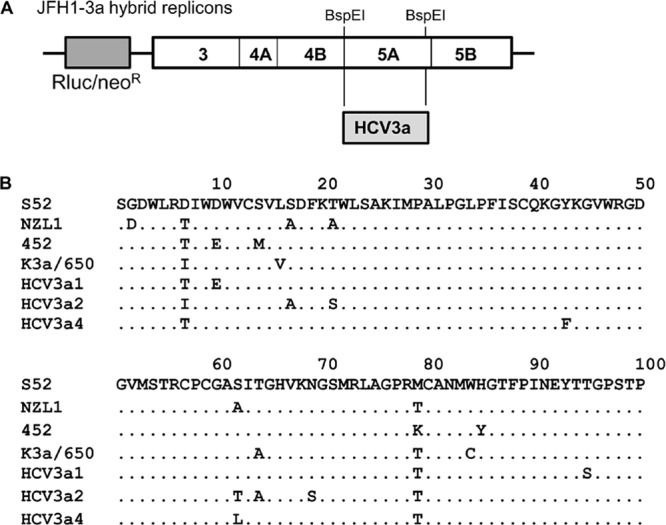
(A) Schematic diagram of a bicistronic JFH1 replicon with Renilla luciferase (Rluc) and neomycin resistance (neoR) genes. The 5′ untranslated region (UTR) is derived from the HCV1b Con1 strain, and the nonstructural genes and 3′ UTR are from the HCV2a JFH1 strain. Hybrid replicons were constructed by replacing the indicated BspEI restriction fragment with sequences from HCV3a1, HCV3a2, and HCV3a4 clinical isolates. (B) Alignment of the N-terminal 100 amino acids of NS5A from the HCV3a1, HCV3a2, and HCV3a4 isolates and four genotype 3a reference sequences (S52, GenBank accession number GU814263; NZL1, GenBank accession number D17763; 452, GenBank accession number DQ437509; K3a/650, GenBank accession number D28917). Amino acid identities are indicated with dots.
Table 1.
Susceptibility of HCV3a hybrid replicons to DCV
| Replicon | Replication capacitya | EC50 (nM)b |
|
|---|---|---|---|
| Transient assay | Replicon cells | ||
| JFH1 | 100 | 0.051 ± 0.025 | 0.021 ± 0.004 |
| HCV3a1 | 74 ± 44 | 0.25 ± 0.10 | 0.53 ± 0.15 |
| HCV3a2 | 19 ± 17 | 0.12 ± 0.09 | 0.14 ± 0.04 |
| HCV3a4 | 98 ± 52 | 0.87 ± 0.45 | 1.25 ± 0.5 |
Relative to the parental JFH1 replicon. Values are means and standard deviations.
Half-maximal effective concentrations. Values are means and standard deviations (n ≥ 3).
Table 2.
Amino acid substitutions in NS5A cDNA isolated from DCV-treated HCV3a1 replicon cells
| DCV selection conc (nM) | DCV EC50 (nM) toward selected cellsa | NS5A amino acid substitutionsb |
|---|---|---|
| 0c | 0.47 ± 0.08 | None |
| 10 | >1,000 | L31F (40%), Y93H (60%) |
| 100 | >1,000 | L31F (30%), Y93H (70%) |
Values are means and standard deviations (where applicable) (n ≥ 3).
Values in parentheses are estimates of likelihood deduced from bulk population sequence traces.
DMSO control.
Table 3.
Susceptibility of HCV3a1 variants to DCVa
| NS5A variant | Replication capacity | EC50 (nM) |
|
|---|---|---|---|
| Transient assay | Cell line | ||
| HCV3a1 | 100 | 0.25 ± 0.10 | 0.53 ± 0.15 |
| A30K | 13.8 ± 2.0 | 15.40 ± 7.3 | 29.6 ± 4.4 |
| A30T | 103.3 ± 4.5 | 0.11 ± 0.05 | 0.57 ± 0.013 |
| L31F | 152.8 ± 4.1 | 80.00 ± 35 | 320 ± 5.9 |
| S62L | 88.1 ± 3.1 | 0.53 ± 0.039 | 0.93 ± 0.17 |
| Y93H | 29.3 ± 5.3 | 688.00 ± 253 | 1,451 ± 344 |
Values are means and standard deviations (n ≥ 3).
The naturally occurring variability at NS5A positions 31 and 93 in HCV3a was assessed by examining sequences in the European HCV database (20) (Table 4). DCV resistance-associated NS5A residues (28, 30, 62, and 92) identified from previous studies with HCV1a and HCV2a were also included in this analysis (7, 8, 21). A leucine was present at NS5A position 31 in all 454 HCV3a sequences examined; however, 1.3% (6/454) of the sequences had a histidine at NS5A position 93. Variability was also observed at NS5A positions 30 and 62, while very little or no variability was observed at positions 28 and 92 (Table 4). At position 30, alanine was the predominant residue, but threonine and lysine residues were also commonly observed (5.5% and 2.6%, respectively). An A30T amino acid substitution in the HCV3a1 hybrid replicon did not negatively impact DCV sensitivity, but a replicon with an A30K substitution was ∼62-fold more resistant to DCV than was the parental replicon (Table 3). A lysine at NS5A position 30 (Q30K) was also previously found to be associated with DCV resistance in an HCV1a replicon (9). At position 62, most database sequences had a serine residue (67.6%), but threonine (18.9%) and leucine (10.6%) residues were also commonly observed. Each of these amino acids was represented in one of the HCV3a hybrid replicons described in this study (Fig. 1). DCV exhibited similar potencies toward these replicons (Table 1), suggesting that the variations at position 62 did not substantially impact DCV potency. In agreement with this assessment, the DCV potency toward an HCV3a1 hybrid replicon with an S62L amino acid substitution was only slightly less than that toward the parental replicon (∼2-fold less) (Table 3).
Table 4.
Variability at HCV3a NS5A amino acid positions associated with DCV resistance
| NS5A position | No. of sequences in the European HCV database with the indicated amino acid |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | V | I | A | T | K | L | S | P | E | Y | H | X | Other | |
| 28 | 451 | 2 | 1 | |||||||||||
| 30 | 1 | 414 | 25 | 12 | 2 | |||||||||
| 31 | 454 | |||||||||||||
| 62 | 5 | 86 | 48 | 307 | 3 | 5 | ||||||||
| 92 | 454 | |||||||||||||
| 93 | 447 | 6 | 1 | |||||||||||
In this report, we showed that DCV is a potent inhibitor of hybrid replicons with NS5A sequences derived from three HCV3a strains. Resistance selection and site-directed mutagenesis studies with one of the HCV3a hybrid replicons identified resistance-associated amino acid substitutions at positions 30 (A30K), 31 (L31F), and 93 (Y93H), residues also associated with DCV resistance in HCV1 (7). Previous studies have demonstrated a good correlation between DCV resistance-associated sites in vitro and in vivo (6, 8, 12, 21), indicating that A30, L31, and Y93 are probable sites for resistance development in DCV-treated HCV3a-infected subjects. Amino acid substitutions at these positions resulted in substantial resistance to DCV (Table 3), possibly indicating a low resistance barrier of DCV in HCV3a strains. On the whole, however, our results indicate that DCV is an effective HCV3a inhibitor with the potential to be a valuable component of future combination therapies.
Nucleotide sequence accession numbers.
HCV3a1, HCV3a2, and HCV3a4 NS5A sequences were deposited in GenBank under accession numbers JX944789, JX944790, and JX944791.
Footnotes
Published ahead of print 22 October 2012
REFERENCES
- 1. Kamal S. 2006. Genotypic variations around the world: is hepatitis C virus evolving? Curr. Hepat. Rep. 5:142–149 [Google Scholar]
- 2. Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, Serrano-Wu MH, Langley DR, Sun J-H, O'Boyle DR, II, Lemm JA, Wang C, Knipe JO, Chien C, Colonno RJ, Grasela DM, Meanwell NA, Hamann LG. 2010. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 465:96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, Watanabe H, McPhee F, Hughes E, Kumada H. 2012. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology 55:742–748 [DOI] [PubMed] [Google Scholar]
- 4. Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M, Persson A, Zhu K, Dimitrova DI, Eley T, Guo T, Grasela DM, Pasquinelli C. 2012. Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 366:216–224 [DOI] [PubMed] [Google Scholar]
- 5. Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, Goldwater R, DeMicco MP, Rodriguez-Torres M, Vutikullird A, Fuentes E, Lawitz E, Lopez-Talavera JC, Grasela DM. 2011. Multiple ascending dose study of BMS-790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology 54:1956–1965 [DOI] [PubMed] [Google Scholar]
- 6. Pol S, Ghalib RH, Rustgi VK, Martorell C, Everson GT, Tatum HA, Hézode C, Lim JK, Bronowicki J-P, Abrams GA, Bräu N, Morris DW, Thuluvath PJ, Reindollar RW, Yin PD, Diva U, Hindes R, McPhee F, Hernandez D, Wind-Rotolo M, Hughes EA, Schnittman S. 2012. Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. Lancet Infect. Dis. 12:671–677 [DOI] [PubMed] [Google Scholar]
- 7. Fridell RA, Qiu D, Wang C, Valera L, Gao M. 2010. Resistance analysis of the hepatitis C virus NS5A inhibitor BMS-790052 in an in vitro replicon system. Antimicrob. Agents Chemother. 54:3641–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fridell RA, Wang C, Sun J-H, O'Boyle DR, Nower P, Valera L, Qiu D, Roberts S, Huang X, Kienzle B, Bifano M, Nettles RE, Gao M. 2011. Genotypic and phenotypic analysis of variants resistant to hepatitis C virus nonstructural protein 5A replication complex inhibitor BMS-790052 in humans: in vitro and in vivo correlations. Hepatology 54:1924–1935 [DOI] [PubMed] [Google Scholar]
- 9. Wang C, Huang H, Valera L, Sun J- H, O'Boyle DR, Nower PT, Jia L, Qiu D, Huang X, Altaf A, Gao M, Fridell RA. 2012. Hepatitis C virus RNA elimination and development of resistance in replicon cells treated with BMS-790052. Antimicrob. Agents Chemother. 56:1350–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gane E. 2011. Future hepatitis C virus treatment: interferon-sparing combinations. Liver Int. 31:62–67 [DOI] [PubMed] [Google Scholar]
- 11. Fridell RA, Qiu D, Valera L, Wang C, Rose RE, Gao M. 2011. Distinct functions of NS5A in hepatitis C virus RNA replication uncovered by studies with the NS5A inhibitor BMS-790052. J. Virol. 85:7312–7320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang C, Jia L, Huang H, Qiu D, Valera L, Huang X, Sun J-H, Nower PT, O'Boyle DR, Gao M, Fridell RA. 2012. In vitro activity of BMS-790052 on hepatitis C virus genotype 4 NS5A. Antimicrob. Agents Chemother. 56:1588–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lemm JA, O'Boyle D, II, Liu M, Nower PT, Colonno R, Deshpande MS, Snyder LB, Martin SW, St Laurent DR, Serrano-Wu MH, Romine JL, Meanwell NA, Gao M. 2010. Identification of hepatitis C virus NS5A inhibitors. J. Virol. 84:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Targett-Adams P, Graham EJS, Middleton J, Palmer A, Shaw SM, Lavender H, Brain P, Tran TD, Jones LH, Wakenhut F, Stammen B, Pryde D, Pickford C, Westby M. 2011. Small molecules targeting hepatitis C virus-encoded NS5A cause subcellular redistribution of their target: insights into compound modes of action. J. Virol. 85:6353–6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gottwein JM, Scheel TKH, Callendret B, Li Y-P, Eccleston HB, Engle RE, Govindarajan S, Satterfield W, Purcell RH, Walker CM, Bukh J. 2010. Novel infectious cDNA clones of hepatitis C virus genotype 3a (strain S52) and 4a (strain ED43): genetic analyses and in vivo pathogenesis studies. J. Virol. 84:5277–5293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, Abid K, Negro F, Dreux M, Cosset F-L, Bartenschlager R. 2006. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc. Natl. Acad. Sci. U. S. A. 103:7408–7413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sakamoto M, Akahane Y, Tsuda F, Tanaka T, Woodfield DG, Okamoto H. 1994. Entire nucleotide sequence and characterization of a hepatitis C virus of genotype V/3a. J. Gen. Virol. 75:1761–1768 [DOI] [PubMed] [Google Scholar]
- 18. Lemm JA, Liu M, Rose RE, Fridell R, O'Boyle DR, II, Colonno R, Gao M. 2005. Replication-competent chimeric hepatitis C virus subgenomic replicons. Intervirology 48:183–191 [DOI] [PubMed] [Google Scholar]
- 19. Kato T, Date T, Miyamoto M, Furusaka A, Tokushige K, Mizokami M, Wakita T. 2003. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology 125:1808–1817 [DOI] [PubMed] [Google Scholar]
- 20. Combet C, Garnier N, Charavay C, Grando D, Crisan D, Lopez J, Dehne-Garcia A, Geourjon C, Bettler E, Hulo C, Mercier PL, Bartenschlager R, Diepolder H, Moradpour D, Pawlotsky J-M, Rice CM, Trepo C, Penin F, Deleage G. 2007. euHCVdb: the European hepatitis C virus database. Nucleic Acids Res. 35:D363–D366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun J-H, O'Boyle DR, II, Zhang Y, Wang C, Nower P, Valera L, Roberts S, Nettles RE, Fridell RA, Gao M. 2012. Impact of a baseline polymorphism on the emergence of resistance to the hepatitis C virus nonstructural protein 5a replication complex inhibitor, BMS-790052. Hepatology 55:1692–1699 [DOI] [PubMed] [Google Scholar]


