Abstract
Candida albicans, an opportunistic fungus, and Staphylococcus aureus, a bacterial pathogen, are two clinically relevant biofilm-forming microbes responsible for a majority of catheter-related infections, with such infections often resulting in catheter loss and removal. Not only do these pathogens cause a substantial number of nosocomial infections independently, but also they are frequently found coexisting as polymicrobial biofilms on host and environmental surfaces. Antimicrobial lock therapy is a current strategy to sterilize infected catheters. However, the robustness of this technique against polymicrobial biofilms has remained largely untested. Due to its antimicrobial activity, safety, stability, and affordability, we tested the hypothesis that ethanol (EtOH) could serve as a potentially efficacious catheter lock solution against C. albicans and S. aureus biofilms. Therefore, we optimized the dose and time necessary to achieve killing of both monomicrobial and polymicrobial biofilms formed on polystyrene and silicone surfaces in a static microplate lock therapy model. Treatment with 30% EtOH for a minimum of 4 h was inhibitory for monomicrobial and polymicrobial biofilms, as evidenced by XTT {sodium 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide inner salt} metabolic activity assays and confocal microscopy. Experiments to determine the regrowth of microorganisms on silicone after EtOH treatment were also performed. Importantly, incubation with 30% EtOH for 4 h was sufficient to kill and inhibit the growth of C. albicans, while 50% EtOH was needed to completely inhibit the regrowth of S. aureus. In summary, we have systematically defined the dose and duration of EtOH treatment that are effective against and prevent regrowth of C. albicans and S. aureus monomicrobial and polymicrobial biofilms in an in vitro lock therapy model.
INTRODUCTION
In the United States alone, severe sepsis accounts for over 750,000 cases, results in 215,000 deaths, and generates roughly $16 billion in health care costs annually. Alarmingly, each year severe sepsis kills more individuals than breast, kidney, colon, rectal, pancreatic, and prostate cancer combined (1–3). Major risk factors for sepsis are surgical procedures or the use of invasive medical devices, such as catheters (4). Seeding of initially sterile catheter surfaces by microorganisms, followed by development into mature biofilms, poses a significant obstacle in the clinic, as biofilm-embedded organisms are recalcitrant to clearance by the host and inherently resistant to standard antimicrobial therapies (5). Therefore, effective broad-spectrum approaches to prevent and resolve catheter-mediated biofilm infections are urgently needed.
Further complicating the biofilm phenotype's elusiveness to orthodox treatment is the fact that many biofilms are polymicrobial in nature, which often renders the true identification of the etiological agent(s) and treatment of the infection more difficult (6). Not only may the inherent virulence of the organisms be modified considerably during polymicrobial growth, but also standard treatments normally effective against monomicrobial species infections may also be rendered futile. To further underscore the gravity of such infections, clinical surveys have demonstrated that polymicrobe-associated bacteremias account for 10 to 30% of all bloodstream infections and in certain cases have been linked to higher incidences of infection relapse, increased hospital stays, earlier catheter exchange, worse patient outcomes, and increased mortality (7–11). Two phylogenetically diverse microbes known for their prevalence as associated organisms in polymicrobial biofilms are the polymorphic opportunistic fungus Candida albicans and the bacterial pathogen Staphylococcus aureus. Despite causing a number of infections independently, C. albicans and S. aureus can also be coisolated from several diseases and from the surfaces of various biomaterials, including dentures, voice prostheses, implants, endotracheal tubes, feeding tubes, and, most commonly, catheters (12).
Aside from septic complications, failure to prevent or eradicate microbial contaminants from catheter surfaces may result in phlebitis, skin infections, and/or catheter loss and removal (13). Current Infectious Diseases Society of America (IDSA) clinical guidelines recommend removal of infected central venous catheters from neutropenic patients (and nonneutropenic patients, if possible) when C. albicans or S. aureus is suspected as the etiological agent (14). However, catheter removal may not be a viable option if thrombocytopenia, coagulopathies, a lack of alternative venous access, or critical illness is a confounding issue. The clinical need for catheters in some patients, including for parenteral nutrition and intravenous drips, further complicates the decision of catheter removal. Therefore, alternative strategies are needed to prevent contamination or permit salvage of catheters in these clinical situations.
One current approach is the use of antimicrobial catheter lock solutions, which are formulated to sterilize the lumenal catheter surface or prevent biofilm growth and subsequent septic infection (15). Significant study-to-study variability in catheter lock composition, model system, dosing, and duration of treatment has complicated the establishment of therapy standards. For example, Schinabeck et al. demonstrated that treatment of rabbits with 3 mg of liposomal amphotericin B for a lock duration of 7 days was required to sterilize venous catheter surfaces after infection by C. albicans (16). Treatment of S. aureus-infected catheters with 5 mg/ml daptomycin lock solution and systemic daptomycin administration for 2 days resulted in significantly reduced colonization of catheters (but not sterile catheters) in a rat central venous catheter model (17). Chelating agents, such as trisodium citrate and sodium-EDTA, have also been demonstrated to have antimicrobial activity against an array of pathogens (18, 19). While these treatments and others have demonstrated efficacy as potential lock therapies both in vitro and in vivo, the lack of standardized dosing and duration, potential problems of acquired antimicrobial resistance, side effects of therapy, physical insult to catheter materials, and failure to assess the regrowth of microorganisms after lock treatment remain critical barriers for routine clinical use. Furthermore, although numerous studies have evaluated the effect of antimicrobial lock therapy (ALT) against monomicrobial species biofilms, limited data exist on the effectiveness of ALT against fungal or polymicrobial biofilms.
Considering these issues, ethanol (EtOH) was recently demonstrated to be an efficacious antifungal agent against C. albicans biofilms in vitro (20, 21). The stability, availability, and cost-effectiveness of ethanol uniquely make it an attractive option for antimicrobial lock therapy. In light of our previous data and the frequency of polymicrobial biofilm-mediated catheter-related infections, we hypothesized that ethanol would be an excellent candidate for a catheter lock solution effective against fungal-bacterial polymicrobial biofilms. In fact, to our knowledge, this is the first systematic study in which optimized doses and the duration of ALT for EtOH were assessed in vitro against polymicrobial biofilms.
MATERIALS AND METHODS
Strains and growth conditions.
Biofilm-forming C. albicans prototypical isolate SC5314 and S. aureus strain M2 were used in this study and are as described previously (22, 23); both organisms were maintained as frozen stocks at −80°C. Prior to use, C. albicans was subcultured onto yeast-peptone-dextrose (YPD) agar at 30°C. A single colony was cultured in YPD liquid medium at 30°C for 24 h. S. aureus was subcultured onto Trypticase soy broth (TSB) agar, and a single colony was inoculated into TSB liquid medium for 24 h at 37°C. A 1:100 dilution of the overnight culture was made into fresh TSB and allowed to propagate at 37°C until mid-log phase was reached (approximately 3 h). Following growth, microbes were washed in phosphate-buffered saline (PBS) by centrifugation, counted on a hemocytometer, and adjusted to 2 × 107 CFU/ml in 0.6× TSB containing 0.2% glucose (TSB-g). TSB-g was previously determined to be an optimal medium for supporting both candidal and staphylococcal biofilm growth (18, 24).
Biofilm growth on polystyrene and silicone.
For monomicrobial biofilms, 50 μl of adjusted C. albicans or S. aureus culture was added to each well of a 96-well cell culture-treated polystyrene microtiter plate (1 × 106 CFU per well); to this, 50 μl of sterile TSB-g was added. For polymicrobial biofilms, 50 μl of each organism was added per well (1 × 106 CFU of each organism per well). Therefore, total volumes were 100 μl for both growth conditions. Plates were incubated for 24 h at 37°C to induce biofilm formation. For experiments assessing growth on silicone to mimic catheter surfaces, the experimental setup was exactly the same as that described above, except that each well contained a disk made from sterilized, medical-grade silicone sheeting (Invotek International) as described previously (21).
Crystal violet assay.
In order to quantify biomass during monomicrobial and polymicrobial growth, biofilms were grown as described above and processed for crystal violet staining as previously described (25), with some modifications. Briefly, wells were extensively washed in PBS to remove nonadherent cells, stained with 0.1% crystal violet, and repeatedly washed in distilled H2O. Bound crystal violet was resolubilized in 95% ethanol, and the absorbance was read at 590 nm on a VersaMax microplate reader (Molecular Devices) (25).
Ethanol treatment.
Following mature biofilm growth, plates or silicone disks were washed 3 times with 200 μl sterile saline and then 100 μl TSB-g containing EtOH at various concentrations (5%, 10%, 15%, 20%, 30%, 40%, 50%) was added. EtOH-free TBS-g was added to wells with and without microbes to serve as positive and negative controls, respectively. Plates were returned to a 37°C incubator for various times (0.5 h, 2 h, 4 h, 24 h). In some experiments, initial biofilm inocula were diluted directly into EtOH at various concentrations to determine the effects of EtOH on biofilm inhibition. After incubation with EtOH, plates were washed once with PBS and processed for the XTT {sodium 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide inner salt} metabolic activity assay or microscopic analyses.
XTT assay.
The XTT reduction assay was used to determine the metabolic activity of biofilms after EtOH treatment (26). Wells were washed once in PBS, and plates or silicone disks were incubated at 37°C for 2 h with 200 μl XTT working reagent (0.5 mg/ml XTT and 1 μM menadione); conversion of the XTT substrate to a soluble colored formazan product correlates with cell viability. The resulting absorbance was read at 490 nm, and the antimicrobial activity of each EtOH treatment was expressed as a percentage relative to the value for the EtOH-free controls.
Confocal microscopy.
Cells were grown on silicone disks or in 8-well Permanox Lab-Tek chamber slides (Nunc) as described above. After EtOH treatment, biofilms were washed once in PBS and stained with dyes from a BacLight Live/Dead staining kit (Invitrogen) (27). Live/Dead contains two dyes that differentially stain cells on the basis of their viability. Syto 9 is a cell-permeant DNA stain and stains all cells (green), while propidium iodide (PI) is impermeant and can stain only cells (red) with compromised membranes, indicative of death or lysis. All images were captured on an Olympus Fluoview 1000 confocal microscope using a fluorescein isothiocyanate/Texas Red filter set.
SEM.
Biofilms were grown on silicone disks as described above. Samples were initially fixed in 2.5% glutaraldehyde for 2 h and then slowly dehydrated in a series of ethanol washes (35%, 50%, 70%, 95%, 100%) for 10 min. The disks were then dried in 100% hexamethyldisilazane for 20 min. Disks were affixed to scanning electron microscopy (SEM) stubs with double-sided carbon tape and sputter coated. Biofilms were imaged with an Hitachi S-2700 scanning electron microscope (LSUHSC Core Imaging) with the voltage set to 15 kV. Images were captured at ×100 to ×1,000 magnifications.
Enumeration of CFU on silicone disks.
Biofilms were inoculated on silicone disks as described above. After 24 h of growth, disks were aseptically transferred to tubes containing 1 ml PBS and gently sonicated in a Branson 1510 water bath sonicator at 40 kHz for 5 min to dislodge biofilm-embedded microbes. Serial dilutions were made in sterile PBS and plated onto YPD agar supplemented with 2 μg/ml vancomycin (for C. albicans enumeration) or TSB agar containing 2.5 μg/ml amphotericin B (for S. aureus enumeration) using the drop plate method; plates were then incubated at 37°C (28). Following 24 h of growth, colonies were counted and expressed as the number of CFU/ml.
Regrowth experiments.
Regrowth of biofilms after treatment with EtOH was tested as previously described with adaptations (29). Biofilms were grown on silicone disks as described above. Various concentrations of EtOH (0%, 10%, 30%, 50%) were added to each disk, and the disks were incubated for 4 h at 37°C. After washing in PBS, disks were transferred to sterile 50-ml conical tubes containing 5 ml of YPD supplemented with 2 μg/ml vancomycin (for disks harboring C. albicans biofilms) or TSB containing 2.5 μg/ml amphotericin B (for disks harboring S. aureus biofilms). In order to obtain both C. albicans and S. aureus counts individually, polymicrobial biofilms were grown in parallel, so that one biofilm disk was regrown in YPD containing vancomycin and the other was regrown in TSB containing amphotericin B. These antimicrobials were chosen to inhibit the growth of either organism in the nonselective medium because overgrowth of the nontargeted microbe may have confounded the results. Samples were sonicated, and the tubes were returned to 30°C (C. albicans) or 37°C (S. aureus) and incubated with shaking at 200 rpm for 20 h. After incubation, 100-μl aliquots were placed into a 96-well microtiter plate and the optical density was recorded at 600 nm (OD600). The numbers of CFU were counted on selective media as described above; the upper limit of detection was approximately 108 CFU/ml.
Statistics.
All experiments were performed in biological triplicate and repeated a minimum of three times. Biomasses from crystal violet staining and CFU counts from silicone disks were compared using Student's t test. The metabolic activities of the treatment groups were compared to the controls using one- or two-way analyses of variance (ANOVAs) and Dunnett's multiple-comparison posttest. Differences were considered significant at a P value of <0.05. All statistical analyses were performed and graphs were composed with GraphPad Prism (version 5) or Microsoft Excel software.
RESULTS
Polymicrobial growth synergistically increases biomass.
The crystal violet assay was used to measure the biomass of monomicrobial and polymicrobial biofilms grown on polystyrene plates. As expected, the biomasses of polymicrobial biofilms were greater than the biomass of either C. albicans or S. aureus independently grown as a biofilm. Interestingly, the biomasses of polymicrobial biofilms were also significantly higher than the additive masses of the monomicrobial biofilms (Fig. 1). The increased biomass in polymicrobial biofilms may enhance resistance to catheter lock therapeutic agents, necessitating an increased dosing or duration of treatment; therefore, we wished to test this hypothesis further.
Fig 1.
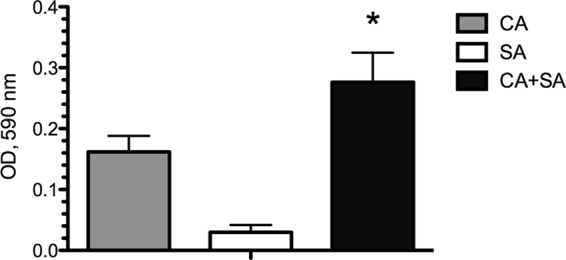
Polymicrobial biofilm formation leads to synergistic increases in biomass. Monomicrobial and polymicrobial C. albicans (CA) and/or S. aureus (SA) biofilms were grown on polystyrene plates for 24 h. Biomass was quantified by the crystal violet method. The biomass of the polymicrobial biofilm was significantly higher than the additive biomasses of the monomicrobial biofilms. *, P < 0.05, using Student's t test.
Ethanol activity against mono- and dual-species biofilms.
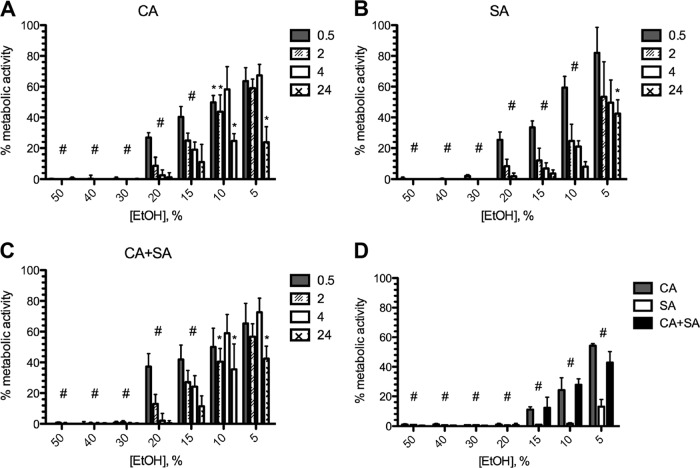
The XTT metabolic activity assay was used to measure the effectiveness of EtOH against monomicrobial and polymicrobial biofilms over a range of concentrations (5 to 50% EtOH) and endpoints (0.5 to 24 h) (Fig. 2). Both C. albicans (Fig. 2A) and S. aureus (Fig. 2B) demonstrated similar patterns of killing in concentration- and time-dependent manners, with increasing concentrations and incubation times resulting in enhanced inhibition of metabolic activity. Despite demonstrating increased biomass, treatment of polymicrobial biofilms with the same concentrations of EtOH resulted in killing profiles similar to those achieved for monomicrobial biofilms (Fig. 2C). Significant metabolic inhibition of both mono- and dual-species biofilms compared to the growth of the controls was demonstrated at nearly all time points and with nearly all concentrations, except for the lowest EtOH concentration (5%) at the minimum incubation time tested (0.5 h). The effectiveness of ethanol on growth inhibition of biofilms was also assessed at 24 h of incubation (Fig. 2D). EtOH significantly inhibited the formation of monomicrobial and polymicrobial biofilms, demonstrating enhanced efficacy against S. aureus even at low concentrations. Collectively, these data show that killing of biofilms can be achieved with ≥20% EtOH and complete metabolic inhibition of mature C. albicans and S. aureus monomicrobial or polymicrobial biofilms can be achieved with ≥30% EtOH within a 4-h exposure time.
Fig 2.
Optimized dosing and time course of activity of ethanol against monomicrobial and polymicrobial biofilms grown on polystyrene. Various concentrations of EtOH were added to preformed monomicrobial (A, B) and polymicrobial (C) biofilms of C. albicans and/or S. aureus for various incubation times. (D) Initial biofilm inocula were treated with various concentrations of EtOH for 24 h to inhibit growth. Metabolic activity was assessed using the XTT assay, and data are expressed as the percentage of the value for the untreated controls. Error bars represent SDs. * and # (entire group), P < 0.05, using one-way ANOVA and Dunnett's posttest.
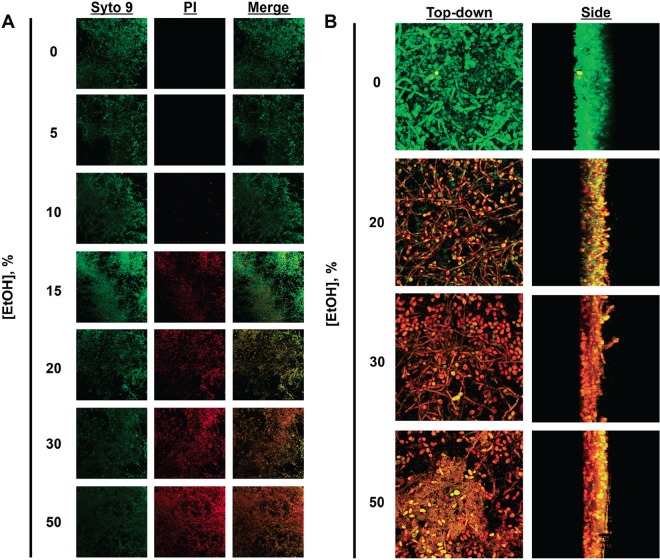
In order to qualitatively assess killing of biofilms, confocal microscopy and Live/Dead staining were utilized. Polymicrobial biofilms were subjected to a series of EtOH concentrations for 2 h (data not shown) and 4 h of incubation, stained, and examined by fluorescence microscopy. Similar to the XTT assay data, increased EtOH concentrations at 4 h of incubation correlated with increased killing by evidence of PI uptake, indicative of membrane damage (Fig. 3A). A mix of living and dead cells was found when biofilms were treated with 20% EtOH, but nearly all cells were positive for PI staining at concentrations of ≥30%. Zoomed images and confocal stacks revealed significant killing of C. albicans with 20% EtOH treatment for 4 h (red), while S. aureus appeared to be unaffected (green) by EtOH at the same concentration (Fig. 3B). Interestingly, S. aureus could be found enmeshed within the biofilm of C. albicans and attached to the hyphal filaments, as has been reported previously (30). Again, EtOH concentrations of 30% or greater appeared to completely kill both microbes.
Fig 3.
Efficacy of ethanol against polymicrobial biofilms. (A) C. albicans-S. aureus polymicrobial biofilms were treated with various concentrations of EtOH for 4 h and stained with Live/Dead reagent. All cells stain green, but only those with compromised membrane integrity stain red. Magnification, ×20. (B) Zoomed images demonstrate the susceptibility of C. albicans to 20% EtOH (red), while S. aureus remains unaffected (green). However, at 30% EtOH, significant killing of both species is demonstrated. Magnification, ×63.
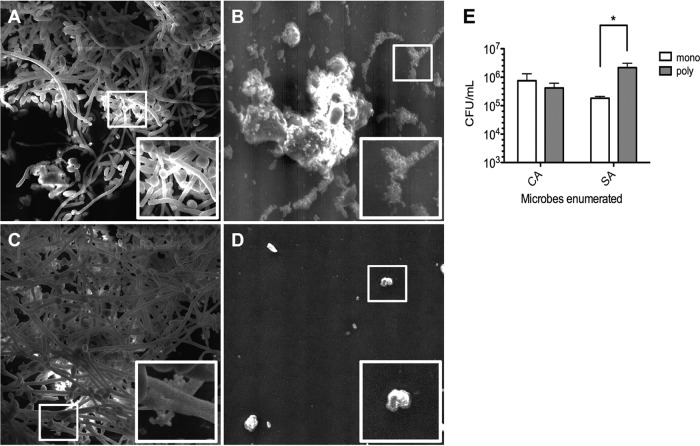
We also wished to assess biofilm formation on medical-grade silicone, the same material used for catheter fabrication. Scanning electron microscopy demonstrated attachment of both C. albicans (Fig. 4A) and S. aureus (Fig. 4B) to silicone surfaces, with elaboration of extracellular matrix material being indicative of biofilm formation. C. albicans biofilms covered a large area of the disk surface, while S. aureus attached in sporadic small microcolonies. Images of the polymicrobial biofilm revealed numerous staphylococci attached to the silicone-bound hyphal filaments of C. albicans (Fig. 4C). Differential CFU counts revealed a significant increase in recovery of S. aureus from polymicrobial biofilms compared to that from monomicrobial biofilms, while C. albicans counts remained similar under both growth conditions (Fig. 4E). An uninoculated silicone disk surface devoid of microbial growth is shown as a reference in Fig. 4D.
Fig 4.
SEM images of monomicrobial and polymicrobial biofilms on silicone disks. Analysis of silicone disk colonization by SEM demonstrated significant surface attachment by C. albicans (A) and S. aureus (B). (C) The polymicrobial biofilm showed numerous staphylococci attached to the biofilm components (hyphae and/or extracellular matrix) of C. albicans. (D) An uninoculated control is shown for reference. White boxes, zoomed areas of interest. Magnification, ×1,000. (E) Analysis of the numbers of CFU demonstrated that significantly more S. aureus cells could be recovered from silicone disks harboring polymicrobial biofilms (poly) than monomicrobial biofilms (mono). Error bars represent SDs. *, P < 0.05, using Student's t test.
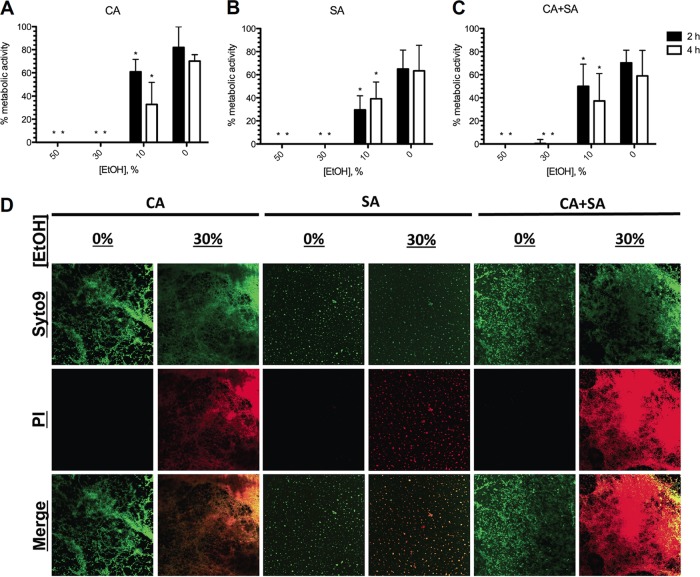
Because the physical properties and surface structure of polystyrene differ from those of medical-grade silicone catheters, which may alter biofilm formation efficiency and the subsequent treatment strategy, metabolic inhibition by EtOH of monomicrobial and polymicrobial biofilms grown on silicone disks was also assessed. The metabolism of C. albicans (Fig. 5A) and S. aureus (Fig. 5B) monomicrobial biofilms was completely inhibited by 30% EtOH at 2 and 4 h of incubation. Again, polymicrobial biofilms (Fig. 5C) were inhibited in a strikingly similar manner. Confocal microscopy images of biofilms grown on silicone disks were similar to those of biofilms grown in Permanox slides, demonstrating increased killing and PI uptake (red) concordant with EtOH treatment compared to those for the EtOH-free control (green) biofilms (Fig. 5D). Therefore, similar to our results from the polystyrene-grown biofilms, complete killing and metabolic inhibition of mature C. albicans and S. aureus monomicrobial and polymicrobial biofilms grown on silicone were achieved with ≥30% EtOH within a 4-h exposure time.
Fig 5.
Efficacy of ethanol against biofilms grown on silicone disks. Various concentrations of EtOH were added to preformed monomicrobial C. albicans (A), monomicrobial S. aureus (B), or polymicrobial (C) biofilms grown on silicone disks for 2 or 4 h. Metabolic activity was assessed using the XTT assay. (D) Disks colonized with mature biofilms were treated with EtOH for 4 h and then processed and stained with Live/Dead reagent. Treatment with 30% EtOH appeared to effectively kill all the microorganisms (red), whereas the EtOH-free controls (green) were not killed. Magnification, ×20. Error bars represent SDs. *, P < 0.05, using one-way ANOVA and Dunnett's posttest.
Prevention of regrowth by ethanol.
Finally, we designed experiments to test the regrowth of C. albicans and S. aureus recovered from monomicrobial or polymicrobial biofilms after treatment with EtOH to optimize the exposure regimen that would effectively sterilize contaminated catheter surfaces. Mature biofilms were grown on silicone disks, treated with EtOH at various concentrations (0, 10, 30, 50%) for 4 h, and then planktonically cultured in fresh medium containing selective antimicrobials; EtOH-free controls were also included for comparison. After 24 h of incubation, culture optical densities were measured and selective microbiological plating was performed to assess the ability of biofilms to recover from EtOH treatment. A summary of the data can be found in Table 1. C. albicans recovered from monomicrobial or polymicrobial biofilms demonstrated complete sterilization when treated with EtOH at concentrations of ≥30%. These findings were supportive of those of the XTT assay and the confocal microscopy data reported above. Surprisingly, S. aureus required treatment with EtOH at concentrations of 50% to completely sterilize it and prevent regrowth. XTT viability assays or Live/Dead staining performed as described above would not have predicted this outcome. While the difference was not statistically significant (P = 0.15), there was a trend toward increased regrowth of S. aureus from polymicrobial biofilms compared to that from monomicrobial biofilms. In summary, on the basis of these findings, treatment with 50% EtOH for a minimum of 4 h would be an efficacious lock therapy to inhibit and prevent regrowth of both C. albicans and S. aureus monomicrobial or polymicrobial biofilms.
Table 1.
Regrowth of biofilms after treatment with ethanola
| EtOH concn (%) | Monomicrobial |
Polymicrobial |
||||||
|---|---|---|---|---|---|---|---|---|
|
C. albicans |
S. aureus |
C. albicans |
S. aureus |
|||||
| OD600 | No. of CFU/ml | OD600 | No. of CFU/ml | OD600 | No. of CFU/ml | OD600 | No. of CFU/ml | |
| 0 | 1.182 | > 108 | 0.266 | > 108 | 1.187 | > 108 | 0.204 | > 108 |
| 10 | 0.961 | > 108 | 0.355 | > 108 | 1.006 | > 108 | 0.169 | 2.6 × 107 |
| 30 | 0.000 | 0 | 0.005 | 1.2 × 106 | 0.000 | 0 | 0.010 | 3.1 × 106 |
| 50 | 0.000 | 0 | 0.000 | 0 | 0.000 | 0 | 0.000 | 0 |
Mature biofilms were allowed to form on silicone disks overnight. On the following day, disks were treated with EtOH at various concentrations for 4 h. Immediately following EtOH treatment, the disks were briefly sonicated and cultured in selective liquid medium for 24 h. Optical densities were recorded, and serial dilutions were plated onto selective medium to quantify microbial regrowth.
DISCUSSION
The use of preservative, antimicrobial, and/or antiseptic agents distilled in the lumen of catheters for extended dwell periods to maintain sterilization is commonly referred to as antimicrobial lock therapy (ALT) (31). Although the use of ALT as a prophylactic against catheter-related infections has recently been advocated, it is routinely employed only for the immunocompromised patient population (32). Prevention of catheter contamination or salvage of infected catheters could significantly improve sepsis rates associated with catheter use, increase catheter life span, and reduce catheter turnover and reinstallation (33); ALT is especially beneficial for patients who have underlying comorbidities complicating catheter removal or are catheter dependent. Current guidelines by IDSA suggest the potential use of ALT for treatment if infection by less virulent organisms (including coagulase-negative staphylococci) is suspected (14). However, a paucity of information and strategies exists for the clinical use of ALT against fungal or polymicrobial catheter-related infections.
Various in vitro, in vivo, and clinical studies have assessed the use of ALT as a viable option to potentially sterilize catheter surfaces. Similar to this study, we have recently shown that treatment of mature C. albicans biofilms in vitro with 35% EtOH for 4 h is sufficient to inhibit metabolic activity and prevent fungal regrowth (21). Recent findings by Balestrino et al. demonstrated that eradication of S. aureus in vitro biofilms with an ethanol-based lock solution required concentrations of >60% for a minimum of 30 min of incubation; however, this study did not address the regrowth of staphylococci after ethanol treatment (34). In vivo studies utilizing a rabbit model of catheter-mediated biofilm infection performed by Mukherjee et al. showed the susceptibility of C. albicans to ethanol lock therapy, while staphylococci (including S. aureus) were inherently resistant to EtOH at doses of 50% (35). It is important to note that while ALT lasted for 7 days postinfection, the EtOH dwell time was permitted to be only 30 min per day. Collectively, these studies further highlight the need for the standardization of the use of ALT against a variety of pathogens.
The identification of a polymicrobial etiology may carry important clinical implications for the empirical treatment of infections. Therefore, we wished to assess the efficacy of ethanol-based ALT to inhibit and prevent the regrowth of clinically relevant polymicrobial biofilms composed of C. albicans and S. aureus. We determined that the metabolism of both monomicrobial and polymicrobial biofilms grown on polystyrene or catheter analogs could be significantly inhibited with as little as 10% EtOH but that concentrations of >20% for at least 2 h were needed to eliminate detectable metabolic activity (Fig. 2A and B). To our surprise, despite demonstrating synergistic increases in biomass (Fig. 1), similar patterns of metabolic inhibition emerged with respect to EtOH dose and duration, when comparing polymicrobial and monomicrobial biofilms (Fig. 2C). This finding was unexpected, because limited studies have shown that bacterial cells within fungal-bacterial biofilms tend to display enhanced drug resistance (24, 36). In fact, several studies have evaluated the use of antimicrobial chemotherapeutic agents (e.g., vancomycin, gentamicin, cefazolin) as efficacious ALT (37–39). While potentially useful, the frequency of use and the prolonged instillation of chemotherapeutic-based catheter lock solutions may lead to increased resistance to these agents by nosocomial pathogens (40). Because ethanol's proposed mechanism of action involves rapid disruption of membrane function, as evidenced by Live/Dead staining (Fig. 3), increased antimicrobial resistance by modulation of drug efflux pumps or via repeated exposure is likely of little concern (41). Coupled with its stability, availability, safety, and low cost, these attributes make ethanol-based lock solutions an extremely attractive option for ALT.
We have previously identified the hyphal surface of C. albicans to be a suitable substratum for the attachment of S. aureus (30). This phenomenon was further emphasized by confocal microscopy (Fig. 3) and scanning electron microscopy (Fig. 4). Monomicrobial biofilms of S. aureus sporadically colonized the surface of silicone disks (Fig. 4B); however, numerous clusters of cocci could be found associated with the hyphae of C. albicans (Fig. 4C). In fact, the numbers of S. aureus organisms recovered from polymicrobial biofilms increased approximately 10-fold compared to the numbers recovered from monomicrobial biofilms, while C. albicans growth remained nearly identical (Fig. 4E). These findings were strikingly similar to the polymicrobial biofilm growth on polystyrene previously reported by Harriott and Noverr (36). Importantly, the authors of that study demonstrated that S. aureus became encased in the fungal biofilm matrix during polymicrobial growth, rendering it completely resistant to treatment with vancomycin. Similar findings have also been reported for Staphylococcus epidermidis (24). Collectively, these findings hold important clinical implications, in that C. albicans may serve as a nidus for enhanced colonization of staphylococci on catheter surfaces. In fact, a retrospective survey of tertiary-care hospitals conducted by Klotz et al. reported that up to 20% of C. albicans fungemias result in synchronous or asynchronous bloodstream infection by staphylococci (42). Therefore, increased clinical surveillance for staphylococci or other bacterial contaminants may be warranted if catheter contamination by C. albicans is suspected.
The most striking results generated from this study came from experiments assessing the regrowth of microorganisms after EtOH treatment (Table 1). We specifically chose to assess regrowth after 4 h of EtOH treatment, as this would be a suitable time point for the practical clinical use of ALT in standard 8-h nursing shifts. Data from the XTT assay and Live/Dead staining on silicone disks suggested that 4 h of exposure to 30% EtOH should be satisfactory for eradicating biofilm growth (Fig. 5). However, in order to completely inhibit regrowth from monomicrobial or polymicrobial biofilms, we found that 30% EtOH was required for C. albicans and, to our surprise, 50% EtOH was required to inhibit S. aureus. These dwell times determined and EtOH concentrations identified are within acceptable guidelines for clinical ALT use in humans, pose little risk for silicone catheter deterioration, and avoid potential problems of occlusive catheter precipitate (43–47).
Although initially unexpected, the disparity between the various metabolic and viability measures used in this study is not uncommon. XTT assay readings are dependent on cell density, so cells metabolically active at levels below the threshold of detection will likely repopulate once the antimicrobial pressure is removed (48). Furthermore, oxygen-deprived biofilm-embedded organisms located at the basal layer and persisters (metabolically inert yet viable cells) exhibit inherently reduced or absent levels of metabolic activity and therefore may not be detectable by the XTT assay (49–51). Similarly, while it is a good predictor of cell death, Live/Dead staining truly assesses only membrane damage. Therefore, cells able to recover from the ethanol insult will also regrow in the absence of antimicrobial activity. Findings from the regrowth studies revealed that (i) decreased metabolic activity is a good indicator of microbicidal activity but does not replace the findings of true growth susceptibility studies and (ii) failure to completely sterilize the catheter surface will likely result in persistence or relapse of infection.
In summary, ethanol-based lock techniques are an attractive option for the routine prevention of bloodstream infections and resolution of infectious complications of catheter use. This study has defined optimized ethanol concentrations and incubation times effective against C. albicans and S. aureus monomicrobial and polymicrobial biofilms to inhibit their metabolic activity and prevent regrowth in vitro. Importantly, we have demonstrated that resistance to ethanol is not altered in polymicrobial biofilms but that the composition of the microbial consortium dictates resistance to treatment. Crucially, ethanol lock therapy offers hope for the salvage of infected catheters in critically ill, catheter-dependent patients. Future in vivo studies are warranted to test the efficacy of these treatments for their potential use in ALT in the clinical setting.
ACKNOWLEDGMENTS
We kindly thank Clorinda Johnson for providing expertise with the scanning electron microscope.
This work was supported by funding from the U.S. Department of Veterans Affairs (Merit Award to S.A.L.), the Biomedical Research Institute of New Mexico (to S.A.L.), the Louisiana Vaccine Center (to R.M.W. and M.C.N.), and the National Institutes of Health, NIAID grant R01-A172406 (to M.C.N.).
Footnotes
Published ahead of print 15 October 2012
REFERENCES
- 1. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 2. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. 2001. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 345:1368–1377 [DOI] [PubMed] [Google Scholar]
- 3. Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. 2005. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 45:524–528 [DOI] [PubMed] [Google Scholar]
- 4. Kallen AJ, Patel PR, O'Grady NP. 2010. Preventing catheter-related bloodstream infections outside the intensive care unit: expanding prevention to new settings. Clin. Infect. Dis. 51:335–341 [DOI] [PubMed] [Google Scholar]
- 5. Davies D. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2:114–122 [DOI] [PubMed] [Google Scholar]
- 6. Peters BM, Jabra-Rizk MA, O'May GA, Costerton JW, Shirtliff ME. 2012. Polymicrobial interactions: impact on pathogenesis and human disease. Clin. Microbiol. Rev. 25:193–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beathard GA. 1999. Management of bacteremia associated with tunneled-cuffed hemodialysis catheters. J. Am. Soc. Nephrol. 10:1045–1049 [DOI] [PubMed] [Google Scholar]
- 8. Lin JN, Lai CH, Chen YH, Chang LL, Lu PL, Tsai SS, Lin HL, Lin HH. 2010. Characteristics and outcomes of polymicrobial bloodstream infections in the emergency department: a matched case-control study. Acad. Emerg. Med. 17:1072–1079 [DOI] [PubMed] [Google Scholar]
- 9. Onland W, Pajkrt D, Shin C, Fustar S, Rushing T, Wong WY.2011. Pediatric patients with intravascular devices: polymicrobial bloodstream infections and risk factors. J. Pathog. 2011:826169 doi:10.4061/2011/826169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sutter D, Stagliano D, Braun L, Williams F, Arnold J, Ottolini M, Epstein J. 2008. Polymicrobial bloodstream infection in pediatric patients: risk factors, microbiology, and antimicrobial management. Pediatr. Infect. Dis. J. 27:400–405 [DOI] [PubMed] [Google Scholar]
- 11. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317 [DOI] [PubMed] [Google Scholar]
- 12. Shirtliff ME, Peters BM, Jabra-Rizk MA. 2009. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 299:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakazawa N. 2010. Infectious and thrombotic complications of central venous catheters. Semin. Oncol. Nurs. 26:121–131 [DOI] [PubMed] [Google Scholar]
- 14. Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49:1–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toltzis P. 2006. Antibiotic lock technique to reduce central venous catheter-related bacteremia. Pediatr. Infect. Dis. J. 25:449–450 [DOI] [PubMed] [Google Scholar]
- 16. Schinabeck MK, Long LA, Hossain MA, Chandra J, Mukherjee PK, Mohamed S, Ghannoum MA. 2004. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob. Agents Chemother. 48:1727–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Praagh AD, LI T, Zhang S, Arya A, Chen L, Zhang XX, Bertolami S, Mortin LI. 2011. Daptomycin antibiotic lock therapy in a rat model of staphylococcal central venous catheter biofilm infections. Antimicrob. Agents Chemother. 55:4081–4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shanks RM, Sargent JL, Martinez RM, Graber ML, O'Toole GA. 2006. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol. Dial. Transplant. 21:2247–2255 [DOI] [PubMed] [Google Scholar]
- 19. Weijmer MC, Debets-Ossenkopp YJ, Van De Vondervoort FJ, ter Wee PM. 2002. Superior antimicrobial activity of trisodium citrate over heparin for catheter locking. Nephrol. Dial. Transplant. 17:2189–2195 [DOI] [PubMed] [Google Scholar]
- 20. Chambers ST, Peddie B, Pithie A. 2006. Ethanol disinfection of plastic-adherent micro-organisms. J. Hosp. Infect. 63:193–196 [DOI] [PubMed] [Google Scholar]
- 21. Rane HS, Bernardo SM, Walraven CJ, Lee SA. 2012. In vitro analyses of ethanol activity against Candida albicans biofilms. Antimicrob. Agents Chemother. 56:4487–4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME. 2006. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect. Immun. 74:3415–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179–182 [DOI] [PubMed] [Google Scholar]
- 24. Adam B, Baillie GS, Douglas LJ. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 51:344–349 [DOI] [PubMed] [Google Scholar]
- 25. Croes S, Deurenberg RH, Boumans ML, Beisser PS, Neef C, Stobberingh EE. 2009. Staphylococcus aureus biofilm formation at the physiologic glucose concentration depends on the S. aureus lineage. BMC Microbiol. 9:229 doi:10.1186/1471-2180-9-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pierce CG, Uppuluri P, Tummala S, Lopez-Ribot JL. 2010. A 96 well microtiter plate-based method for monitoring formation and antifungal susceptibility testing of Candida albicans biofilms. J. Vis. Exp. 44:pii=2287. doi:10.3791/2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jurcisek JA, Dickson AC, Bruggeman ME, Bakaletz LO. 2011. In vitro biofilm formation in an 8-well chamber slide. J. Vis. Exp. 47:pii=2481. doi:10.3791/2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donegan K, Matyac C, Seidler R, Porteous A. 1991. Evaluation of methods for sampling, recovery, and enumeration of bacteria applied to the phylloplane. Appl. Environ. Microbiol. 57:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raad I, Hanna H, Dvorak T, Chaiban G, Hachem R. 2007. Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob. Agents Chemother. 51:78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peters BM, Jabra-Rizk MA, Scheper MA, Leid JG, Costerton JW, Shirtliff ME. 2010. Microbial interactions and differential protein expression in Staphylococcus aureus-Candida albicans dual-species biofilms. FEMS Immunol. Med. Microbiol. 59:493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Segarra-Newnham M, Martin-Cooper EM. 2005. Antibiotic lock technique: a review of the literature. Ann. Pharmacother. 39:311–318 [DOI] [PubMed] [Google Scholar]
- 32. Bleyer AJ. 2007. Use of antimicrobial catheter lock solutions to prevent catheter-related bacteremia. Clin. J. Am. Soc. Nephrol. 2:1073–1078 [DOI] [PubMed] [Google Scholar]
- 33. Berrington A, Gould FK. 2001. Use of antibiotic locks to treat colonized central venous catheters. J. Antimicrob. Chemother. 48:597–603 [DOI] [PubMed] [Google Scholar]
- 34. Balestrino D, Souweine B, Charbonnel N, Lautrette A, Aumeran C, Traore O, Forestier C. 2009. Eradication of microorganisms embedded in biofilm by an ethanol-based catheter lock solution. Nephrol. Dial. Transplant. 24:3204–3209 [DOI] [PubMed] [Google Scholar]
- 35. Mukherjee PK, Mohamed S, Chandra J, Kuhn D, Liu S, Antar OS, Munyon R, Mitchell AP, Andes D, Chance MR, Rouabhia M, Ghannoum MA. 2006. Alcohol dehydrogenase restricts the ability of the pathogen Candida albicans to form a biofilm on catheter surfaces through an ethanol-based mechanism. Infect. Immun. 74:3804–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harriott MM, Noverr MC. 2009. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob. Agents Chemother. 53:3914–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chiou PF, Chang CC, Wen YK, Yang Y. 2006. Antibiotic lock technique reduces the incidence of temporary catheter-related infections. Clin. Nephrol. 65:419–422 [DOI] [PubMed] [Google Scholar]
- 38. McIntyre CW, Hulme LJ, Taal M, Fluck RJ. 2004. Locking of tunneled hemodialysis catheters with gentamicin and heparin. Kidney Int. 66:801–805 [DOI] [PubMed] [Google Scholar]
- 39. Poole CV, Carlton D, Bimbo L, Allon M. 2004. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol. Dial. Transplant. 19:1237–1244 [DOI] [PubMed] [Google Scholar]
- 40. Dixon JJ, Steele M, Makanjuola AD. 2012. Anti-microbial locks increase the incidence of Staphylococcus aureus and antibiotic-resistant Enterobacter: observational retrospective cohort study. Nephrol. Dial. Transplant. 27:3575–3581 [DOI] [PubMed] [Google Scholar]
- 41. McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klotz SA, Chasin BS, Powell B, Gaur NK, Lipke PN. 2007. Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn. Microbiol. Infect. Dis. 59:401–406 [DOI] [PubMed] [Google Scholar]
- 43. Blackwood RA, Klein KC, Micel LN, Willers ML, Mody RJ, Teitelbaum DH, Cober MP. 2011. Ethanol locks therapy for resolution of fungal catheter infections. Pediatr. Infect. Dis. J. 30:1105–1107 [DOI] [PubMed] [Google Scholar]
- 44. Broom J, Woods M, Allworth A, McCarthy J, Faoagali J, Macdonald S, Pithie A. 2008. Ethanol lock therapy to treat tunnelled central venous catheter-associated blood stream infections: results from a prospective trial. Scand. J. Infect. Dis. 40:399–406 [DOI] [PubMed] [Google Scholar]
- 45. Crnich CJ, Halfmann JA, Crone WC, Maki DG. 2005. The effects of prolonged ethanol exposure on the mechanical properties of polyurethane and silicone catheters used for intravascular access. Infect. Control Hosp. Epidemiol. 26:708–714 [DOI] [PubMed] [Google Scholar]
- 46. Dannenberg C, Bierbach U, Rothe A, Beer J, Korholz D. 2003. Ethanol-lock technique in the treatment of bloodstream infections in pediatric oncology patients with Broviac catheter. J. Pediatr. Hematol. Oncol. 25:616–621 [DOI] [PubMed] [Google Scholar]
- 47. Oliveira C, Nasr A, Brindle M, Wales PW. 2012. Ethanol locks to prevent catheter-related bloodstream infections in parenteral nutrition: a meta-analysis. Pediatrics 129:318–329 [DOI] [PubMed] [Google Scholar]
- 48. Hawser S. 1996. Adhesion of different Candida spp. to plastic: XTT formazan determinations. J. Med. Vet. Mycol. 34:407–410 [PubMed] [Google Scholar]
- 49. Borriello G, Werner E, Roe F, Kim AM, Ehrlich GD, Stewart PS. 2004. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 48:2659–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. 2004. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 230:13–18 [DOI] [PubMed] [Google Scholar]
- 51. LaFleur MD, Kumamoto CA, Lewis K. 2006. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 50:3839–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]