Summary
Oral bisphosphonates comprise the most widely prescribed class of antiosteoporotic drugs. Recent reports, however, suggest a link between prolonged bisphosphonate use and atypical low-energy, subtrochanteric fractures. We describe the clinical course of two patient treated for a long term with different bisphosphonates who developed subtrochanteric atypical fractures. They were treated initially with intramedullary rodding without pain disappearance or healing of the fracture. Strontium ranelate, a new orally administered agent for the treatment of osteoporosis, was given to these patients with complete closure of the fracture and pain disappearance after a few months. We conclude that based on the chronology of fracture healing and pain disappearance of our patients and published evidence that strontium ranelate can accelerate fracture healing in a rat model, that strontium ranelate had a positive anabolic effect that contributed to fracture healing that produced the secondary disappearance of pain.
Keywords: bisphosphonate, atypical fractures, delayed healing, strontium ranelate
Introduction
Bisphosphonates are the primary therapy for postmenopausal and glucocorticoid-induced osteoporosis worldwide. Several large, randomized controlled trials have shown that bisphosphonates are effective in improving bone mineral density and reducing osteoporotic fractures in postmenopausal women (1, 2). However, recent studies report a possible link between prolonged bisphosphonate therapy and atypical, subtrochanteric low-energy fractures of the femoral shaft (3–7). A task force from the American Society for Bone and Mineral Research has defined major and minor features for atypical femoral fractures. They recommend that all major features, including their location in the subtrochanteric region and femoral shaft, transverse or short oblique orientation, minimal or no associated trauma, a medial spike when the fracture is complete, and absence of comminution, be present to designate a femoral fracture as atypical. The incidence of these atypical subtrochanteric femoral fractures in bisphosphonate users is unknown, but considering the large number of patients taking this class of medication, it appears to be low (8).
There have been reports of treatment of atypical subtrochanteric femoral fractures related to long-term bisphosphonate therapy that healed after treatment with a bone forming agent as teriparatide (9, 10). Recently Carvalho et al. have shown a similar finding with short term strontium ranelate treatment (10). Although these beneficial effects are consistent with the efficacy of anabolic therapy in the healing of these types of fractures, more evidence is needed.
In this report we describe the cases of two postmenopausal women that suffered from subtrochanteric atypical low-energy fractures after prolonged bisphosphonate therapy that failed to heal after intramedullary nailing that healed after few months of strontium ranelate oral therapy.
Case 1
A 79 year old white woman was diagnosed osteoporosis in 1998. She had had the menopause at age 49. Se was treated with calcium salts, vitamin D and alendronate 70 mg/week for 8 years and afterward with ibandronate 150 mg/month for another four more years. In January 2010, she began with acute pain at the mid left thigh. Few weeks later she suffered a spontaneous fracture of the same femur. Intramedullary nailing of the femur was performed and bisphosphonate therapy was stopped. After several months the fracture had not healed. She was sent for consultation at our institution. A diagnosis of atypical subtrochanteric femoral fracture after prolonged bisphosphonate therapy was made and ibandronate was stopped. Laboratory data showed: total calcium: 9.9 mg/dl, serum phosphorus: 3.8 mg/dl, iPTHi: 26 pg/ml, serum ALP: 231 IU, serum crosslaps: 183 pg/ml. She was given strontium ranelate 2 gr/day plus calcium and vitamin D. Closure of the fracture line was seen after five months treatment with strontium ranelate (Figures 1 and 2) with complete resolution of thigh pain.
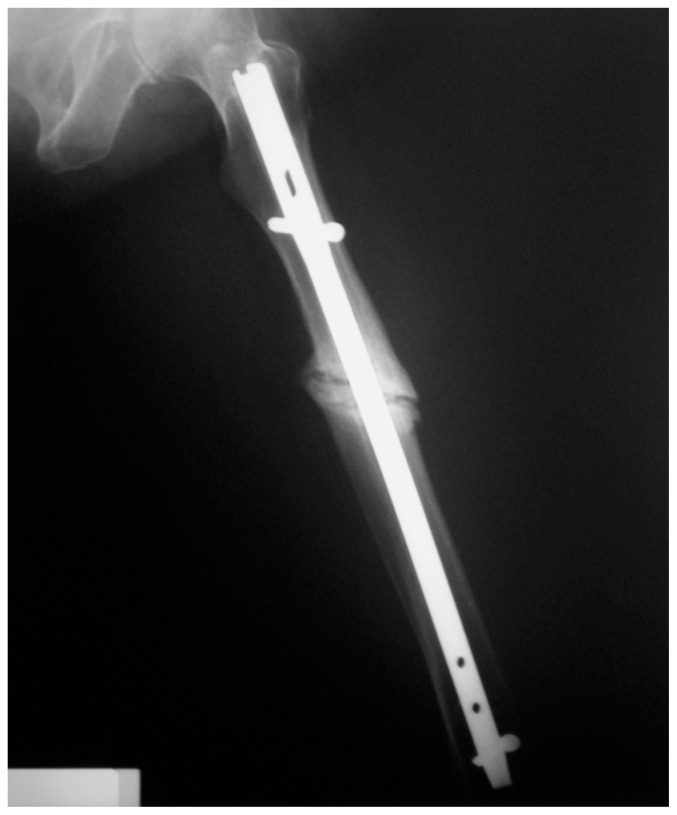
Figure 1.
Case 1: Postoperative non-comminuted fracture of the femur diaphysis, before strontium ranelate treatment.
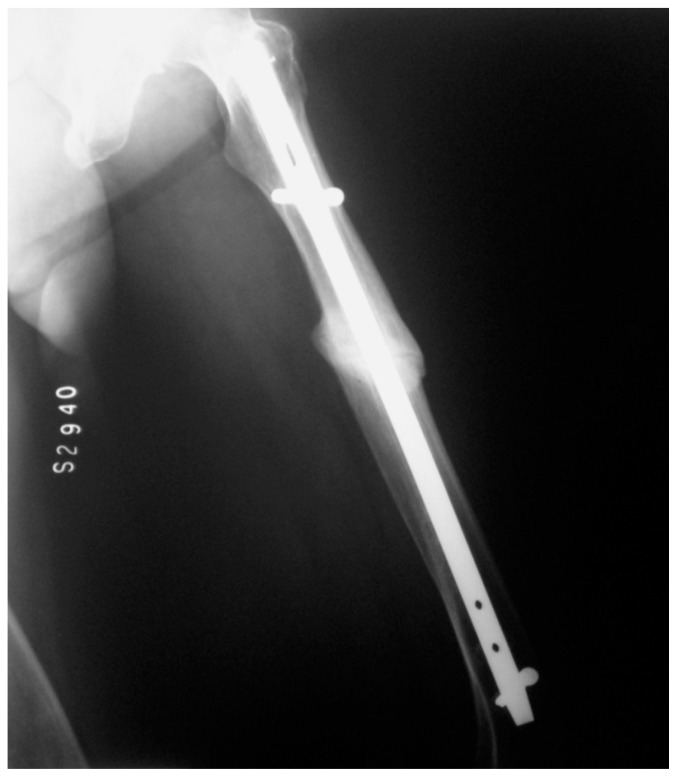
Figure 2.
Case 1: After five months of strontium ranelate treatment.
Case 2
A 68 year old white woman was diagnosed osteoporosis in 1993. She had had surgical menopause (hysterectomy) at age 48. She was treated initially with pamidronate 200 mg/day and calcium salts for 1 year after which her gynecologist changed her treatment to transdermal estrogen. She continued with that treatment until May 1995 when she was changed to alendronate 10 mg/day plus calcium salts returning to transdermal estrogen in October 1996. In July 2003, her medication was changed back to alendronate 70 mg once a week and then to risedronate which she took until July 2005. During 2006 she stopped taking medications for osteoporosis. In May 2007 she began taking ibandronate 150 mg once a month. In September 2009, she began with acute pain at the mid right thigh. While walking in the beach, she had a spontaneous fracture of the right femur. She had x-rays of both femurs that revealed cortical thickening in the midshafts of both femurs with an oblique complete loss of continuity of the right femoral cortex with a unicortical beak at the fracture site. She was operated and a Kuncher nail was introduced as fixation in her right femur. Ibandronate was stopped. In September 2010, despite the intramedullar nail, she continued with pain and failure of the fracture to heal. Laboratory data at that moment showed: total calcium: 9.8 mg/dl, serum phosphorus: 4.2 mg/dl, serum crosslaps 191, 25 OH D: 41.6 ng/ml, serum osteocalcin 13.8, serum ALP: 175 IU. She was given Strontium ranelate 2 g/day. By March 2010 her pain had completely resolved and the fracture showed complete healing (Figures 3 and 4).
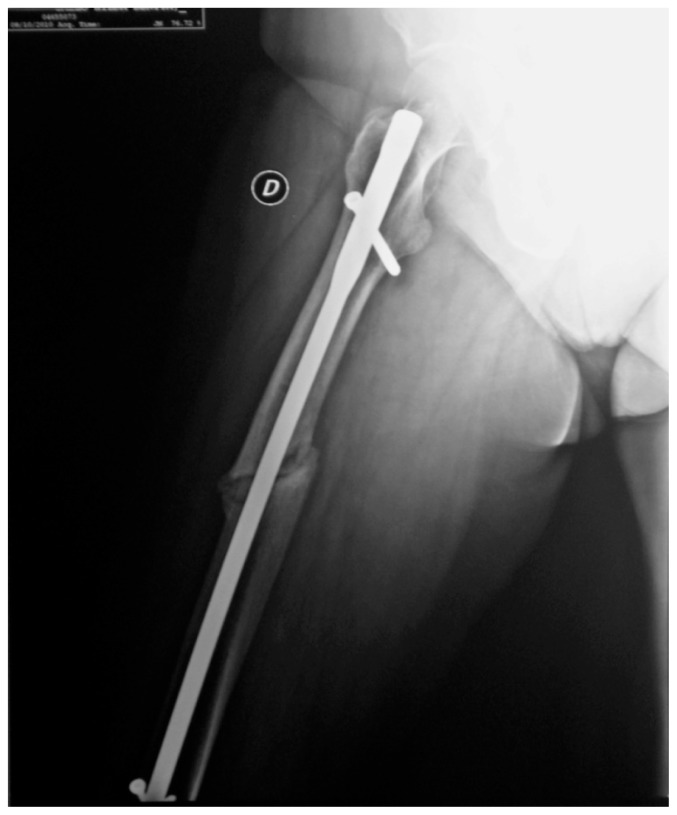
Figure 3.
Case 2: Postoperative oblique fracture of the femur diaphysis before strontium ranelate treatment.
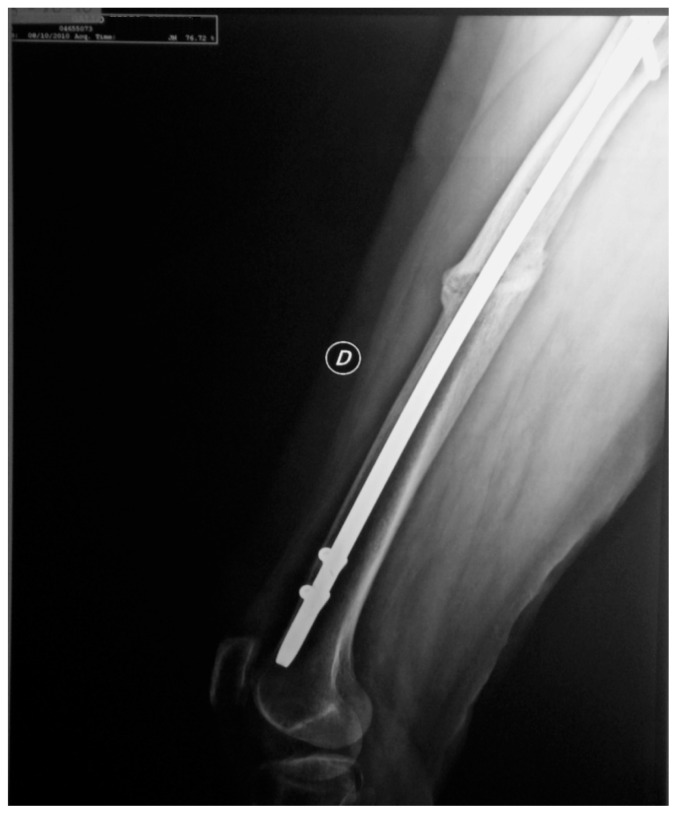
Figure 4.
Case 2: After five months of strontium ranelate treatment.
Discussion
In this report we present two patients who had atypical subtrochanteric femoral fractures after long-term bisphosphonate therapy. Despite intramedullary rodding in both cases, healing of the fractures and pain disappearance did not occur until the introduction of several months of strontium ranelate treatment. Spontaneous healing related to time cannot be assumed in these cases, as several months had elapsed after fracture with adequate juxtaposition of fracture ends with the nailing. Thus we suggest that anabolic treatment with stromtium ranelate has been the cure for these atypical fractures.
Recent evidence indicates suppression of the bone turnover and increased risk of bone fragility after long term bisphosphonate therapy (11). The delayed recovery from fracture and evidences of severe suppression of bone metabolism were first reported by Odvina (12). Between 2006 and 2007 several case reports about atypical insufficiency fractures after long term exposure to bisphosphonates were published (4, 13–15). These case reports and conflicting findings from small observational studies (7, 16, 17) have left clinicians and patients uncertain about whether bisphosphonates increase the risk of subtrochanteric or femoral shaft fractures. A recent population-based study have confirmed the association between increased risk of subtrochanteric or femoral shaft fractures and more than 5 years bisphosphonate usage in older women, however the absolute risk seems to be low (18).
There has been little recommendation on the way to treat these atypical fractures. Some clinicians have recommended cessation of bisphosphonate therapy and protected weight bearing (19). Others recommend prophylactic intramedullary rodding (3, 20). More recently, based on its anabolic effects, teriparatide has been used to promote the healing of this type of fracture (9, 10).
Strontium ranelate is a new orally administered agent for the treatment of osteoporosis licensed for use in Europe, and several other countries, but not yet in the United States. It is composed of an organic moiety (ranelic acid) and two atoms of stable strontium. Although its molecular mechanism of action has not been completely elucidated, strontium ranelate has been shown, in preclinical models, to reduce concomitantly bone resorption and increase bone formation (21). Strontium ranelate was recently demonstrated to reduce significantly risk of vertebral (22), nonvertebral (23), and, in a high-risk population, hip fracture risk in women with post-menopausal osteoporosis. Moreover, significant increases in lumbar spine, femoral neck, and total hip BMD have been consistently reported in all populations exposed to strontium ranelate (24). In vitro studies suggest that strontium ranelate potentially increases the bone formation activity of osteoblasts and decreases the bone resorption activity of osteoclasts, thus leading to the prevention of bone loss and an increase in bone mass and strength. No defect of mineralization due to strontium ranelate was observed in studies on animals (25) and humans (26).
Recently strontium ranelate has been shown to increase bone repair and favor callus formation. In a model of screw fixation in ovariectomized rats, treatment with strontium enhanced the process after 4 and 8 weeks of treatment (27). Two recent studies also revealed that 4 and 8 weeks of treatment with strontium ranelate promoted fracture healing in OVX rats. This effect resulted from enhancement of bone and tissue volume within the callus and from formation of a more mature and tightly arranged bone after 8 weeks (28).
Our results suggest potential benefits of strontium ranelate on bone formation in vivo, with the cure of atypical fractures, albeit more clinical studies are required to confirm this effect.
References
- 1.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 2.Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11(1):83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 3.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster D, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Koval K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Sen HT, van der Meulen MC, Weinstein RS, Whyte M. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 4.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 5.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358:1304–1306. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 6.Armamento-Villareal R, Napoli N, Diemer K, Watkins M, Civitelli R, Teitelbaum S, Novack D. Bone turnover in bone biopsies of patients with low-energy cortical fractures receiving bisphosphonates: a case series. Calcif Tissue Int. 2009;85:37–44. doi: 10.1007/s00223-009-9263-5. [DOI] [PubMed] [Google Scholar]
- 7.Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, van der Meulen MC, Lorich DG, Lane JM. Association of low energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;20:1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362:1761–1771. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- 9.Gomberg SJ, Wustrack RL, Napoli N, Arnaud CD, Black DM. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. Clin Endocrinol Metab. 2011;96(6):1627–32. doi: 10.1210/jc.2010-2520. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho NN, Voss LA, Almeida MO, Salgado CL, Bandeira F. Atypical Femoral Fractures during Prolonged Use of Bisphosphonates: Short-Term Responses to Strontium Ranelate and Teriparatide. J Clin Endocrinol Metab. 2011 Jul; doi: 10.1210/jc.2011-0593. [DOI] [PubMed] [Google Scholar]
- 11.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–1565. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 12.Odvina CV, Zerwekh JE, Rao DE, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 13.Charopoulos I, Orme S, Giannoudis PV. Fracture risk associated with chronic use of bisphosphonates: evidence today. Expert Opin Drug Saf. 2011;10(1):67–76. doi: 10.1517/14740338.2010.517192. [DOI] [PubMed] [Google Scholar]
- 14.Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 15.Kwek EB, Goh SK, Koh JS, PNG MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long term complication of alendronate therapy? Injury. 2008;39:224–231. doi: 10.1016/j.injury.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Rizzoli R, Akesson K, Bouxsein M, et al. Subtrochanteric fractures after long-term treatment with bisphosphonates: a European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int. 2011;22(2):373–390. doi: 10.1007/s00198-010-1453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrahamsen B, Eiken P, Eastell R. Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res. 2009;24(6):1095–1102. doi: 10.1359/jbmr.081247. [DOI] [PubMed] [Google Scholar]
- 18.Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunrai N, Austin PC, Whelan DB, Weiler PJ, Laupacis A. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA. 2011;305(8):783–789. doi: 10.1001/jama.2011.190. [DOI] [PubMed] [Google Scholar]
- 19.Capeci CM, Tejwani NC. Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J Bone Joint Surg Am. 2009;91:2556–2561. doi: 10.2106/JBJS.H.01774. [DOI] [PubMed] [Google Scholar]
- 20.Das De S, Setiobudi T, Shen L. A rational approach to management of alendronate-related subtrochanteric fractures. J Bone Joint Surg Br. 2010;92:679–686. doi: 10.1302/0301-620X.92B5.22941. [DOI] [PubMed] [Google Scholar]
- 21.Marie PJ, Ammann P, Boivin G, Rey C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif Tissue Int. 2001;69:121–129. doi: 10.1007/s002230010055. [DOI] [PubMed] [Google Scholar]
- 22.Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, Cannata J, Balogh A, Lemmel EM, Pors-Nielsen S, Rizzoli R, Genant HK, Reginster JY. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350:459–468. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 23.Reginster JY, Seeman E, De Vernejoul MC, Adami S, Compston J, Phenekos C, Devogelaer JP, Curiel MD, Sawicki A, Goemaere S, Sorensen OH, Felsenberg D, Meunier PJ. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90:2816–2822. doi: 10.1210/jc.2004-1774. [DOI] [PubMed] [Google Scholar]
- 24.Meunier PJ, Slosman DO, Delmas PD, Sebert JL, Brandi ML, Albanese C, Lorenc R, Pors-Nielsen S, De Vernejoul MC, Roces A, Reginster JY. Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis–a 2-year randomized placebo controlled trial. J Clin Endocrinol Metab. 2002;87:2060–2066. doi: 10.1210/jcem.87.5.8507. [DOI] [PubMed] [Google Scholar]
- 25.Boivin G, Deloffre P, Perrat B, Panczer G, Boudeulle M, Mauras Y, Allain P, Tsouderos Y, Meunier PJ. Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S 12911) administration. J Bone Miner Res. 1996;11(9):1302–11. doi: 10.1002/jbmr.5650110915. [DOI] [PubMed] [Google Scholar]
- 26.Arlot ME, Jiang Y, Genant HK, Zhao J, Burt-Pichat B, Roux JP, Delmas PD, Meunier PJ. Histomorphometric and microCT analysis of bone biopsies from postmenopausal osteoporotic women treated with strontium ranelate. J Bone Miner Res. 2008;23(2):215–22. doi: 10.1359/jbmr.071012. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Feng G, Gao Y, Luo E, Liu X, Hu J. Strontium ranelate treatment enhances hydroxyapatite-coated titanium screws fixation in osteoporotic rats. J Orthop Res. 2009;28:578–582. doi: 10.1002/jor.21050. [DOI] [PubMed] [Google Scholar]
- 28.Li YF, Luo E, Feng G, Zhu SS, Li JH, Hu J. Systemic treatment with strontium ranelate promotes tibial fracture healing in ovariectomized rats. Osteoporos Int. 2010;21(11):1889–97. doi: 10.1007/s00198-009-1140-6. [DOI] [PubMed] [Google Scholar]