Abstract
Objective
To examine African-American prostate cancer (PCa) survivors’ involvement in treatment decision-making (TDM), and examine the association between TDM and quality of life (QOL), using secondary data.
Methods
African-American PCa survivors (181) were recruited from the North Carolina Central Cancer Registry. Participants completed a cross-sectional survey that asked about their chosen cancer treatment, TDM factors, and PCa-specific QOL (using the Expanded Prostate Cancer Index – EPIC). Multivariate analysis of covariance was conducted to determine the association between TDM and QOL, controlling for confounders.
Results
Most men reported being active (44.2%) or collaborative (38.1%) in TDM, while 14.4% preferred a passive role. Adjusting for marital status, education and treatment, passive patients reported somewhat better QOL compared to active patients in the following QOL domains: urinary summary (p=.04), urinary function (p=.01), and urinary incontinence (p=.03).
Conclusion
Most African-American PCa survivors preferred to be, and were, actively or collaboratively involved in TDM. However, those who preferred a passive role reported better PCa-specific QOL for the urinary domain compared to others.
Practice Implications
It is important to assess patients’ TDM preference. Patients’ QOL may differ by their TDM role, such that active patients may be more bothered by treatment side effects than other patients.
Keywords: prostate cancer, quality of life, treatment decision making
1. Introduction
Prostate cancer is the most common non-skin cancer diagnosed among men and the second leading cause of cancer deaths among men in the United States [1]. Unfortunately, there are no optimal treatment guidelines for prostate cancer to improve survival without the risk of diminishing quality of life (QOL) [2]. Although several treatment options are available, they all have multiple adverse side effects – including urinary and bowel problems, hormonal symptoms, and sexual problems – that may result in psychological distress and decreased QOL [3,4]. The burden of disease is greatest among African American men, who have the highest incidence and are at least twice as likely to die from prostate cancer compared to Caucasians [1]. African American prostate cancer survivors also report poorer QOL compared to Caucasians [5–7].
Due to the complex considerations of prostate cancer treatment, patients have been encouraged to participate in informed treatment decision-making (TDM) [8]. The informed decision-making model focuses on the patient’s perspective, in which patients receive all pertinent information, understand the risks and benefits of treatment options, and make decisions based on personal preference [9]. Research has shown that patients vary in their preference for involvement in medical decision-making [10,11], ranging from desiring active involvement [12,13] to preferring passive involvement where the physician is the primary decision maker [14–16]. Numerous studies have assessed medical decision-making preferences among a variety of cancer survivors [12,14,17–28]. In studies of TDM among prostate cancer survivors, most patients prefer a collaborative or active role in TDM [12,18,23–28]. Only one study reported that most prostate cancer survivors preferred a passive role [14]. These study samples predominantly consisted of Caucasian men.
Research primarily among breast cancer survivors has found that taking an active role in TDM is associated with less depression and anxiety [29], greater satisfaction with care and treatment choice [17], and better QOL [30,31]. There are limited data on TDM and QOL among prostate cancer patients. Two studies have assessed TDM preference or involvement and subsequent QOL among Caucasian prostate cancer survivors. In an Australian cohort, 11% of whom were prostate cancer survivors, those who reported a collaborative role in TDM reported better satisfaction with the consultation, information about treatment, and emotional support received, compared to those who reported a passive or active role in TDM [17]. However, a US study of predominately Caucasian prostate cancer survivors found no association between TDM and subsequent QOL [18]. To our knowledge, this relationship has not been studied among African American prostate cancer survivors.
A few studies have assessed general medical decision-making among African American men [19,32–34]. One study of healthy men and women found African Americans were more likely to prefer passive medical decision-making compared to Caucasians [34]. However, other studies conducted with only healthy African American men reported that most preferred a collaborative or active role [32,33]. For example, Williams and colleagues (2008) conducted a study among healthy African American men considering prostate cancer screening; most preferred collaborative (57%) and active (36%) decision-making [33]. Keating and colleagues (2010) reported that African American colorectal and lung cancer survivors preferred to be active or collaborative in medical decision-making [19]. These discrepant findings may be due to gender and racial differences in the study population, and also respondents’ health status – cancer survivors versus a healthy population.
Little is known regarding the relationship between TDM and QOL among African American prostate cancer survivors. The current study was designed to address these gaps in knowledge by assessing self-reported TDM among a sample of African American prostate cancer patients and the association between TDM and QOL. The study addresses the following research questions:
To what extent do African American prostate cancer survivors report their preferences and involvement in treatment decision-making?
Is self-reported preference and involvement in treatment decision-making associated with prostate cancer-specific quality of life among African American prostate cancer survivors?
2. Methods
This is a secondary analysis of data from a cross-sectional, case-control study investigating genetic risks of prostate cancer in African Americans [35]. Only cases were included in the current analyses. All study activities were approved by the Institutional Review Board of Wake Forest School of Medicine.
2.1. Study participants
Prostate cancer cases were identified using the rapid case ascertainment of the North Carolina Central Cancer Registry (NCCCR). The NCCCR registry includes all cancer cases diagnosed among North Carolina residents, as reported by hospitals in the state and health care providers who are required by law to report cases. Eligible cases were men with prostate cancer diagnosed within the last six months, as documented by the NCCCR, African American race, age 40–75 years, and a resident in one of the 15 counties surrounding Winston-Salem, NC. This region allowed for reasonable travel for interviewers and identification of cases available to meet recruitment goal.
2.2. Study procedures
Study participants were enrolled between November 2006 and July 2009. In accordance with state laws, passive permission from the reporting physician was obtained before contacting participants. Passive permission was achieved by the following protocol: before being contacted about the study, each potential participant received a NCCCR brochure describing a) legal requirements for physicians to report newly diagnosed cancer cases to the registry, b) confidentiality of the data reported, and c) possibility of contact by researchers working to improve the health and lives of North Carolina residents.
When physicians either authorized or did not object to researchers contacting their patients, patients received an introductory postcard providing an overview of the study. After one week, a follow-up letter describing the study in greater detail was mailed. Both the postcard and letter asked the patient to call a toll-free number if he was interested in participating in the study. After an additional week, non-respondents were contacted by telephone. Patients were ineligible or not contacted further if they (1) declined interest or participation, (2) denied a prostate cancer diagnosis, (3) had problems with hearing, speaking, or understanding English, or (4) were unreachable after multiple attempts to contact. An overall response rate of 65% for the cases was obtained, consistent with rates reported in other population-based studies of African American men [35].
Men who agreed to participate were scheduled for in-person interviews and blood draws (for genetic testing) at their convenience. Two trained study interviewers administered a questionnaire at the patient’s home or in the clinic. Interviews lasted between 60 and 90 minutes and patients received a $50 incentive for their time and participation in the study. A total of 185 men were consented and enrolled, however four men were excluded from these analyses due to lack of answering questions pertinent to the main analysis.
2.3. Measures
Data relevant to the current analyses included socio-demographic and clinical characteristics, treatment decision-making, and prostate cancer-specific QOL. Genetic test results and questions on medication use, family medical history, and diet that were part of the original study were not included in this secondary analysis.
2.3.1. Socio-demographic and Clinical Characteristics
Socio-demographic questions included year of birth, race/ethnicity, marital status (married, not married), and education. Education was classified into three categories (less than high school, high school graduate, college graduate or higher). Prostate cancer-specific information included Gleason score and treatment received. We used the Gleason scoring system to assess cancer severity [36]. Gleason scores were calculated from the prostatectomy pathology report or the biopsy pathology report when the prostatectomy pathology report was not available. Pathology reports were obtained through the cancer registry from the reporting physician. We categorized the Gleason scores into low (2–6), intermediate (7) and high (8–10) risk [1,2]. Patients were also asked what treatment they received for prostate cancer (i.e., prostatectomy, hormonal therapy, brachytherapy, chemotherapy, external beam radiation, internal radiation therapy, watchful waiting, and other). Prostatectomy treatment was confirmed with prostatectomy pathology report. No other treatment options were confirmed; however a previous study of prostate cancer patients reported 90% agreement between self-reported treatment and medical records for all treatments [37]. Treatment was classified into four categories (surgery, radiation, watchful waiting and other) for the main analyses. Self-reported health status was measured with a single item using a 5-point scale (1=Excellent; 5=Poor).
2.3.2. Treatment Decision-Making
Factors relevant to treatment decision-making (TDM) were assessed using five questions derived from a study of decision-making strategies for primarily Caucasian prostate cancer patients [38]. These questions included: (1) whether another physician was consulted for a second opinion about prostate cancer treatment and if so, the type of physician; (2) sources of information used to learn more about prostate cancer; (3) perceived severity of prostate cancer; (4) reasons for choosing a treatment; and (5) distress while making their treatment decision. Each reason for choosing a treatment (e.g., least painful, less invasive, few side effects, convenient, offered the best chance for cure) and distress were assessed using a 5-point scale (1=not at all; 5=very much); perceived severity was measured with a single item using a 5-point scale (1=not at serious; 5=very serious). These questions have also been used in another study of TDM in prostate cancer [26].
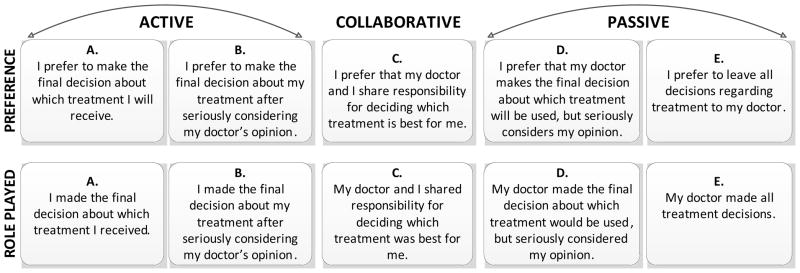
Patients’ TDM preference and self-reported role played in TDM (see Figure 1) were measured using two questions, modified from the Control Preference Scale [39]. The first question was, which of the following statements best describes your preferred approach to making medical decisions? A second, similarly worded question was asked about patients’ perceived role played in choosing their treatment for prostate cancer. For both questions, patients who chose a preference for making the final decision (A or B) were categorized as active, those who chose sharing responsibility (C) were categorized as collaborative, and those who preferred the doctor making the final decision (D or E) were categorized as passive, as done in previous studies [23,27,28,39,40].
Figure 1.
Response options to questions regarding medical decision-making preference and role played during treatment decision-making. Adapted from Degner and Sloan 1992.
2.3.3. Quality of Life
Prostate cancer-specific QOL was measured by the Expanded Prostate Cancer Index Composite (EPIC) [41]. The EPIC is a validated, comprehensive, prostate cancer-specific measure of 50 items that include subscales of patient function and bother after prostate cancer treatment. EPIC comprises four domains: urinary, bowel, sexual, and hormonal. The urinary domain has two additional subscales beyond function and bother, including urinary incontinence and urinary irritation/obstruction. Response options form a Likert scale; and domain and subscale scores were standardized to a 0 to 100 point scale, with higher scores indicating better function and less bother in prostate cancer-specific QOL [41]. The domains showed good internal consistency in this study sample. Cronbach’s alpha was greater than or equal to 0.76 for each of the domain summary scores, and the domain subscales ranged from 0.48 to 0.93, similar to that reported earlier [41].
2.4. Statistical Analysis
All data were analyzed using the Statistical Package for Social Sciences (SPSS 19, Chicago, IL). Statistical significance was established if two-sided p-values were less than 0.05. We used descriptive statistics (means and frequencies) to characterize participants’ socio-demographic and clinical characteristics, TDM factors, and QOL. We examined the agreement between men’s identified TDM preference and self-reported role-played, and agreement was measured using total percent agreement. We conducted chi-square (X2) analyses or Fisher’s Exact Test to examine the differences in TDM preference/role-played (active, collaborative, passive) according to marital status, education, information source used, second opinion, perceived severity, distress and treatment. Analysis of variance (ANOVA) was used to examine differences in age, reasons for choosing treatment, and QOL scores by TDM preference/role played. We also employed the Tukey HSD multiple comparisons procedure for post hoc comparisons. Preliminary results showed that marital status and education were associated with TDM preference/role-played, and treatment was associated with QOL. No other variables were associated with TDM or QOL. Therefore, these variables were controlled for in the multivariate analyses [42]. Multivariate analyses of covariance (MANCOVA) was used to evaluate the association between TDM preference/role-played and QOL, adjusting for marital status, education and treatment.
Because treatment was associated with QOL, we conducted a sensitivity analysis to further control for the effect of treatment on QOL. We conducted a subgroup analysis of patients who had surgery only, as confirmed by prostatectomy pathology reports. We calculated the total percent agreement for accuracy in reporting surgery as treatment, using the agreement between men’s self-reported treatment received and surgical pathology reports confirming treatment. Similar to the main analysis, we used MANCOVA and adjusted for marital status and education, to examine the association between TDM role and QOL.
3. Results
3.1. Sample Characteristics
A total of 181 African American men with prostate cancer were included in these analyses. Table 1 shows demographic and clinical characteristics of the sample. Participants ranged in age from 43 to 75 years (M±SD = 61.3±7.0), 59% were married; and a high percentage (50%) had completed college. Forty-seven percent had an intermediate risk Gleason score of 7. The majority of patients had only one treatment (87%), prostatectomy being the most common (63%), followed by radiation (17%). Most patients rated their health between “good” and “excellent” (77%).
Table 1.
Socio-demographic and Clinical Characteristics of African American Prostate Cancer Survivors (N=181)
| No. | % | |
|---|---|---|
| Age in years a | ||
| 40–49 | 13 | 7.2 |
| 50–54 | 16 | 8.8 |
| 55–59 | 39 | 21.5 |
| 60–64 | 51 | 28.2 |
| 65–75 | 62 | 34.3 |
| Marital status b, c | ||
| Married | 106 | 58.6 |
| Not married | 72 | 39.8 |
| Highest level of education d | ||
| Less than high school | 30 | 16.6 |
| High school | 60 | 33.1 |
| College or higher | 90 | 49.7 |
| Gleason Score c | ||
| Low (2–6) | 66 | 36.5 |
| Intermediate (7) | 85 | 47.0 |
| High (8–10) | 27 | 14.9 |
| Primary Treatment c | ||
| Surgery | 114 | 63.0 |
| Radiation | 31 | 17.1 |
| Other | 13 | 7.2 |
| Watchful Waiting | 23 | 12.7 |
| Self-Reported Health Status d | ||
| Excellent | 18 | 9.9 |
| Very Good | 38 | 21.0 |
| Good | 83 | 45.9 |
| Fair | 38 | 21.0 |
| Poor | 3 | 1.7 |
Mean ± Standard Deviation = 61.3 ± 7.0
Not married includes single, divorced/separated and widowed
Missing 3
Missing 1
3.2. Treatment Decision-Making
A high number of men (87%) rated prostate cancer as being “quite” or “very serious” (Table 2). Despite the complexities of TDM, almost 40% of men said they were not at all distressed while making their prostate cancer treatment decisions. However, 29% were a little or somewhat distressed and almost 30% were quite a bit or very much distressed. Only a fourth of the sample (27%) sought a second opinion on prostate cancer treatment, with urologists being the most common consultant.
Table 2.
Treatment Decision-Making Factors
| No. | (%) | |
|---|---|---|
| Rating Seriousness of Prostate Cancera | ||
| Not at all serious | 2 | (1.1) |
| A little / somewhat serious | 21 | (11.6) |
| Quite / Very serious | 157 | (86.7) |
| Level of Decision-Making Distress b | ||
| Not at all | 72 | (39.8) |
| A little bit | 32 | (17.7) |
| Somewhat | 20 | (11.0) |
| Quite a bit | 18 | (9.9) |
| Very much | 36 | (19.9) |
| Sought 2nd Opiniona, from | 49 | (27.1) |
| Urologist | 25 | (51.0) |
| Surgeon | 11 | (22.4) |
| Other Physician | 10 | (20.4) |
| Radiation Oncologist | 6 | (12.2) |
Missing 1, Mean ± Standard Deviation = 4.7 ± 0.8
Missing 3, M±SD = 2.5 ± 1.6
Table 3 presents patients’ self-reported preferred role in TDM and their self-reported role played in TDM. Four patients were excluded from this analysis because they did not answer both questions. Most men preferred making the final decision about medical decisions after seriously considering their doctor’s opinion (option B, 42%), or preferred to share the responsibility with their doctor (option C, 39%). There was a 95% agreement between their TDM preference and reported role played. As suggested by others [27,28,39], we combined the five options shown in Table 3 into three categories: active (options A and B), collaborative (option C) and passive (options D and E). Within these combined categories, we examined concordance between TDM preference and role played. Almost 97% (n=175) of the men were concordant. Of the concordant men, 44% were in the active category, 38% were in the collaborative category, and 14% were in the passive category.
Table 3.
Patients’ Preferred Approach to Decision-Making Versus Patients’ Role Played in Prostate Cancer Treatment Decision-Making
| Patient’s Preferred Approach to Making Medical Decisions | Patients’ Role Played in Treatment Decision | Total Patients | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| (A) Patient Alone | (B) Patient w/Physician’s Opinion | (C) Shared Decision | (D) Physician w/Physician Patient’s Opinion | (E) Alone | No. | % | |
| (A) Patient Alone | 5 | 0 | 0 | 0 | 0 | 5 | 2.8 |
|
|
|||||||
| (B) Patient w/ Physician’s Opinion | 0 | 75 | 0 | 0 | 1 | 76 | 42.0 |
|
|
|||||||
| (C) Shared Decision | 1 | 1 | 69 | 0 | 0 | 71 | 39.2 |
|
|
|||||||
| (D) Physician w/ Patient’s Opinion | 0 | 2 | 1 | 13 | 2 | 18 | 9.9 |
|
|
|||||||
| (E) Physician Alone | 0 | 0 | 0 | 1 | 10 | 11 | 6.1 |
|
|
|||||||
| Total Patients | |||||||
| No. | 6 | 78 | 70 | 14 | 13 | 181 | |
| % | 3.3 | 43.1 | 38.7 | 7.7 | 7.2 | ||
| Combined Categories | Active Collaborative | Passive | ||
|---|---|---|---|---|
|
| ||||
| (A + B) | (C) | (D + E) | Discordant | |
|
|
||||
| No. | 80 | 69 | 26 | 6 |
| % | 44.2 | 38.1 | 14.4 | 3.3 |
Although most patients sought information about prostate cancer from physicians, active patients were significantly more likely to seek information from the Internet (p<0.001) (Table 4). Additionally, active and collaborative patients utilized more sources of information compared to passive patients (M±SE=3.1±0.2 and M±SE=2.9±0.2 versus M±SE=1.9±0.2, p=0.004 and p=0.019, respectively). No other TDM factors were associated with TDM role.
Table 4.
Information Sources Used, displayed by Treatment Decision-Making Role
| Active | Collaborative | Passive a | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Information Source | No. | % | (n=80) | (n=69) | (n=25) | FE | |||
| n | (%) | n | (%) | n | (%) | p-value | |||
| Physicians | 168 | 92.8 | 75 | (93.8) | 63 | (91.3) | 25 | (100) | .367 |
| Books/magazines | 100 | 55.2 | 50 | (62.5) | 40 | (58.0) | 9 | (36.0) | .062 |
| Family and Friends | 79 | 43.6 | 34 | (42.5) | 32 | (46.4) | 8 | (32.0) | .482 |
| Internet | 77 | 42.5 | 43 | (53.8) | 28 | (40.6) | 2 | (8.0) | <.001 |
| Nurses | 41 | 22.7 | 20 | (25.0) | 17 | (24.6) | 2 | (8.0) | .164 |
| NCI Cancer Info. 1–800 no. | 22 | 12.2 | 12 | (15.0) | 10 | (14.5) | 0 | (0.0) | .097 |
| Other cancer organization | 21 | 11.6 | 11 | (13.8) | 9 | (13.0) | 1 | (4.0) | .483 |
NCI: National Cancer Institute. FE, Fisher’s Exact Test.
Missing 1.
Across all TDM roles, having the best chance for cure was rated as the most important reason for choosing treatment, followed by doctor’s recommendation (Table 5). Patients significantly differed by TDM role in their reasons for choosing treatment with active patients having higher scores on all reasons except doctor’s recommendation. Compared to passive patients, active and collaborative patients were more likely to choose treatment because it had the fewest side effects (p=0.001 and p=0.025, respectively). Active patients were also more likely to choose treatment because it is less invasive compared to collaborative (p=0.009) and passive (p<0.001) patients. Active patients were more likely to score treatment being convenient as a reason for choosing treatment (p=0.016) higher than collaborative patients. Compared to collaborative and passive patients, active patients also scored know people satisfied with treatment (p=0.021) and treatment being least painful (p=0.004) higher. When we dichotomized the scale (1–3 = low versus 4–5 = high), the majority of active patients (53.2%) scored treatment being less invasive as high, compared to collaborative (31.3%) or passive (8.3%) patients (p<0.001). More active patients (51.3%) rated treatment side effects as high, compared to collaborative (40.9%) and passive (12.5%) patients (p=0.003). Active patients also rated convenience and least painful high compared to collaborative or passive patients (p=0.033 and p=0.009, respectively).
Table 5.
Reasons for Choosing Treatment by Treatment Decision-Making Role
| Reasons for Choosing Treatment | Total | Active | Collaborative | Passive | ANOVA |
|---|---|---|---|---|---|
| I chose treatment because … | M (SD) | M (SD) | M (SD) | M (SD) | p-value |
| Best chance for cure | 4.5 (1.1) | 4.5 (1.0) | 4.6 (1.1) | 4.1 (1.5) | .154 |
| Doctors’ recommendation | 3.4 (1.7) | 3.2 (1.7) | 3.6 (1.6) | 3.7 (1.7) | .356 |
| Fewest side effects a | 2.7 (1.8) | 3.1 (1.8) | 2.7 (1.7) | 1.6 (1.3) | .002 |
| Less invasive b | 2.5 (1.8) | 3.1 (1.8) | 2.3 (1.7) | 1.4 (1.0) | <.001 |
| Convenient c | 2.3 (1.6) | 2.6 (1.7) | 1.9 (1.5) | 2.0 (1.6) | .014 |
| Know people satisfied with it d | 2.3 (1.7) | 2.5 (1.8) | 2.2 (1.6) | 1.5 (1.1) | .028 |
| Least painful e | 1.8 (1.4) | 2.2 (1.6) | 1.4 (1.1) | 1.5 (1.1) | .003 |
| Most affordable | 1.6 (1.2) | 1.7 (1.4) | 1.3 (1.0) | 1.6 (1.3) | .134 |
5-point scale range: 1 – 5 (not at all – very much).
Active vs. Passive, mean difference = 1.46 (SE=0.4), p=.001; Collaborative vs. Passive, mean difference = 1.07 (SE=0.4), p=.025
Active vs. Collaborative, mean difference = 0.83 (SE=0.3), p=.009; Active vs. Passive, mean difference = 1.73 (SE=0.4), p<.001
Active vs. Collaborative, mean difference = 0.74 (SE=0.3), p=.016
Active vs. Passive, mean difference = 1.03 (SE=0.4), p=.021
Active vs. Collaborative, mean difference = 0.75 (SE=0.2), p=.004
TDM role was associated with marital status (X2=7.8, df=2, p=0.020) and education (X2=16.6, df=4, p=0.002). No other patient characteristics were associated with TDM role.
3.3. Quality of Life
Treatment was associated with various subscales of QOL: urinary summary (p<0.001), urinary function (p<0.001), urinary bother (p=0.014), urinary incontinence (p<0.001), sexual summary (p<0.001), sexual function (p<0.001), sexual bother (p=0.031), hormonal summary (p=0.007), hormonal function (p=0.004), and hormonal bother (p=0.047). Therefore, we controlled for treatment in the main analysis [42]. We then evaluated the association between self-reported TDM role and QOL, controlling for marital status, education and treatment (Table 6). Overall, men had relatively high bowel (mean=94.9, standard error=1.0) and hormonal (M=90.1, SE=1.2) summary scores for QOL. The scores were lower in the urinary domain (M=82.7, SE=1.6), particularly urinary incontinence (M=73.5, SE=2.5); and the sexual domain (M=42.7, SE=2.3) was most problematic, with lowest scores in sexual function (M=33.4, SE=2.4).
Table 6.
EPIC Mean Scores by Treatment Decision-Making Role
| Domains & Subscales | Total | Active | Collaborative | Passive | MANCOVA P |
|---|---|---|---|---|---|
|
| |||||
| M (SE) | M (SE) | M (SE) | M (SE) | ||
| Urinary Summary | 82.7 (1.6) | 77.3 (2.1) | 81.9 (2.1) | 89.0 (4.1) | 0.036 |
| Urinary Function | 80.9 (1.8) | 74.0 (2.3) | 79.4 (2.3) | 89.1 (4.4) | 0.011 |
| Urinary Bother | 84.1 (1.8) | 79.7 (2.3) | 83.6 (2.4) | 88.9 (4.5) | 0.173 |
| Urinary Incontinence | 73.5 (2.5) | 65.2 (3.2) | 72.2 (3.2) | 83.1 (6.1) | 0.032 |
| Urinary Irritation/Obstruction | 89.7 (1.5) | 86.1 (2.0) | 89.5 (2.0) | 93.6 (3.8) | 0.192 |
| Bowel Summary | 94.9 (1.0) | 93.6 (1.2) | 93.4 (1.3) | 97.7 (2.4) | 0.263 |
| Bowel Function | 93.9 (1.0) | 92.8 (1.3) | 91.9 (1.4) | 97.0 (2.6) | 0.230 |
| Bowel Bother | 95.9 (1.0) | 94.4 (1.3) | 94.8 (1.3) | 98.5 (2.5) | 0.356 |
| Sexual Summary | 42.7 (2.3) | 38.1 (2.9) | 45.1 (3.0) | 44.9 (5.7) | 0.224 |
| Sexual Function | 33.4 (2.4) | 29.2 (3.1) | 35.3 (3.2) | 35.7 (6.0) | 0.338 |
| Sexual Bother | 63.7 (3.5) | 58.2 (4.5) | 67.2 (4.6) | 65.7 (8.7) | 0.362 |
| Hormonal Summary | 90.1 (1.2) | 88.0 (1.6) | 90.2 (1.6) | 92.0 (3.1) | 0.434 |
| Hormonal Function | 85.7 (1.6) | 84.5 (2.0) | 85.5 (2.1) | 87.1 (4.0) | 0.834 |
| Hormonal Bother | 93.7 (1.2) | 90.9 (1.5) | 94.1 (1.5) | 96.1 (2.9) | 0.172 |
MANCOVA, multivariate analysis of covariance. Controlled for marital status, education and treatment. Mean scores are shown with standard errors in parentheses. EPIC scores range from 0 to 100 with higher scores representing better function or less bother. 6 patients were excluded due to TDM discordance.
Active participants reported significantly lower overall urinary summary scores (p=0.036) compared to passive patients, with significant differences also noted in urinary function (p=0.011) and urinary incontinence (p=0.032). Although differences were not statistically significant for the other domains and subscales, passive patients tended to have higher scores in all EPIC domains and subscales, except for the sexual domain.
As another way to control for the impact of treatment on prostate cancer-specific QOL, we conducted a subgroup analysis among patients who received surgery only (n=87), confirmed by prostatectomy pathology report. Significant differences were found in the urinary summary (p=0.038) and the urinary function subscale (p=0.022), where active patients reported lower scores compared to passive patients. These results suggest type of treatment is not the main determinant of the associations found between TDM role and QOL.
4. Discussion and Conclusion
4.1. Discussion
Previous studies have shown that type of treatment for prostate cancer impacts QOL [43–46]. However, TDM is also important regarding QOL, as studies have also shown that participation in TDM is associated with QOL [30,31]. The present study assessed TDM and post-treatment QOL in a sample of African American men who were recently diagnosed with and treated for prostate cancer. We sought to characterize patients’ TDM preference and self-reported role played in TDM, and to determine the association between TDM and QOL, taking into consideration the strength of the association between treatment received and post-treatment QOL.
The majority of African American prostate cancer survivors reported preferring and having an active or collaborative role in TDM. Only a small percentage preferred and played a passive role. These results are consistent with other studies that included African American cancer survivors [19], healthy African American men [32,33], and studies of predominantly Caucasian prostate cancer survivors [23–25]. However, our results differ from one previous study of healthy men and women, in which minorities were more likely to prefer passive medical decision-making [34], and one study in which Caucasian prostate cancer patients preferred a passive role in decision-making [14]. These differences may be due to variations in education, health status, how decision-making preference was measured, or differences in health care systems.
In general, African American prostate cancer survivors in this study reported fairly high QOL scores on the bowel and hormonal domains, but lower scores on the urinary and sexual domains. These scores vary somewhat from previous studies. For example, a study of predominately Caucasian prostate cancer survivors reported lower bowel and sexual summary scores [45,47,48], while hormonal summary scores were higher [45,47], compared to the present study’s sample. Interestingly, a pilot study of African American prostate cancer survivors consistently reported lower QOL scores in all domains and subscales of EPIC, compared to the present study’s sample [49]. These differences could be due to age variation, education level, and treatment received.
A key finding was that passive patients reported better prostate cancer-specific QOL compared to active patients. However, previous studies suggest that active participation in TDM is associated with better QOL in other cancer populations [30,31]. In a study of Caucasian prostate cancer patients [18], researchers also found that passive patients reported better QOL in unadjusted analyses, but the association became non-significant when adjusted for treatment modality and pre-treatment QOL. Our cross-sectional design, did not measure pre-treatment QOL, which may be a better predictor of post-treatment QOL [18]. The present study did not find significant differences in TDM role by treatment, but we did find significant differences in QOL by treatment. Further controlling for treatment, subgroup analysis resulted in similar significant associations, indicating treatment does not appear to be the driving force of the association between TDM role and QOL.
Although treatment type can affect QOL, it is unlikely that TDM role has a direct effect on physical symptoms. The differences in QOL may be explained by patients’ reasons for choosing treatment. Our study found that active patients consistently rated various reasons for choosing treatment higher than passive patients. While passive patients seemed most concerned with having the best chance for cure and following the doctors’ recommendation, active patients were also very concerned with having fewest side effects and the treatment being less invasive. Active patients may have been more concerned about side effects and thereby reported more functional issues and bother.
This study has several limitations. First, the cross-sectional secondary data analysis study design precludes causal inferences. Correspondingly, questions about TDM preference and TDM role played were asked sequentially and retrospectively, which means one could have biased the other. Future studies should examine TDM prospectively, beginning at prostate cancer screening through the cancer continuum. Second, selection bias may have occurred because proactive patients may be more inclined to participate in research studies. This could result in an overestimate of the percentage of men preferring active TDM. However, this is a potential bias of any decision-making study. Third, single item measures were used to assess TDM factors (i.e., disease severity and distress). However, these questions were derived from work conducted by Diefenbach et al. [38] and used by Gwede et al. [26]. Our results were also similar to these studies [26,38]. More detailed measures of TDM factors may reveal different results that could enhance our understanding of TDM. Fourth, although, treatment received was self-reported we do not believe this had an impact on our results. Among the men who had a prostatectomy pathology report indicating surgery, 97% self-reported surgery. Previous studies have reported over 90% agreement between self-reported and medical record confirmed cancer treatment received [37,50]. Finally, our sample of African American prostate cancer survivors were from one state and highly educated and may not be representative of all African American prostate cancer survivors elsewhere. Nevertheless, we believe this is the first study to assess TDM and explore the associations between TDM and QOL in a population of African American men. This is an understudied population that merits further investigation, since they have the highest incidence and mortality rates of all prostate cancer patients.
4.2. Conclusion
In summary, the majority African American prostate cancer patients reported preferring an active or collaborative role in medical decision-making, and felt they played these roles when choosing their treatment. Intriguingly, passive patients reported better prostate cancer-specific QOL compared to active patients; this may reflect active patients being more bothered by treatment side effects. These results suggest the need for prospective studies to assess patients’ preferences, pre-treatment symptoms, information needs and support. Furthermore, such studies should also include the mental and social domains of QOL, to move the current research beyond the physical functioning and bothersome symptoms.
4.3. Practice Implications
Acknowledging the limitations of this study, we believe our results have important practice implications. Patients should be well-informed about prostate cancer, its treatment options and the potential side effects associated with each. Understanding patients’ preference for TDM role and their reasons for choosing treatment are important in assisting patients with information and prostate cancer decision-making. Providers should assess patients’ decision-making preferences and consider patients’ individual preference in all patient-physician communication. In particular, active patients may be more concerned and bothered by treatment side effects, may seek additional information sources, and may have more reasons for choosing treatment, compared to passive patients.
Acknowledgments
The authors thank the patients who participated in this study and Karen Klein for her editing expertise. The original study was funded by the Department of Defense Congressionally Directed Medical Research Program, Award: W81XWH-06-1-0245. Dr. Palmer is supported by NCI/NIH Grant #5R25CA122061.
References
- 1.American Cancer Society. Cancer Facts and Figures for African Americans 2011–2012. 2011. [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Prostate cancer. 2011. [DOI] [PubMed] [Google Scholar]
- 3.Litwin MS, Flanders SC, Pasta DJ, Stoddard ML, Lubeck DP, Henning JM. Sexual function and bother after radical prostatectomy or radiation for prostate cancer: multivariate quality-of-life analysis from CaPSURE. Cancer of the Prostate Strategic Urologic Research Endeavor Urology. 1999;54:503–8. doi: 10.1016/s0090-4295(99)00172-7. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt ME, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, D.C: The National Academies Press; 2006. [Google Scholar]
- 5.Powe BD, Hamilton J, Hancock N, Johanson N, Finnie R, Ko J, Brooks P, Boggan M. Quality of life of African American cancer survivors. Cancer. 2007;109:435–45. doi: 10.1002/cncr.22358. [DOI] [PubMed] [Google Scholar]
- 6.Penedo FJ, Dahn JR, Shen B-J, Schneiderman N, Antoni MH. Ethnicity and determinants of quality of life after prostate cancer treatment. Urology. 2006;67:1022–7. doi: 10.1016/j.urology.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Lubeck DP, Kim H, Grossfeld G, Ray P, Penson DF, Flanders SC, Carroll PR. Health related quality of life differences between black and white men with prostate cancer: data from the cancer of the prostate strategic urologic research endeavor. J Urol. 2001;166:2281–5. [PubMed] [Google Scholar]
- 8.Sheridan SL, Harris RP, Woolf SH. Shared decision making about screening and chemoprevention. a suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26:56–66. doi: 10.1016/j.amepre.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Rimer BK, Briss PA, Zeller PK, Chan ECY, Woolf SH. Informed decision making: what is its role in cancer screening? Cancer. 2004;101:1214–28. doi: 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- 10.Guadagnoli E, Ward P. Patient participation in decision-making. Soc Sci Med. 1998;47:329–39. doi: 10.1016/s0277-9536(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 11.Frosch DL, Kaplan RM. Shared decision making in clinical medicine: past research and future directions. Am J Prev Med. 1999;17:285–94. doi: 10.1016/s0749-3797(99)00097-5. [DOI] [PubMed] [Google Scholar]
- 12.Davison BJ, Parker PA, Goldenberg SL. Patients’ preferences for communicating a prostate cancer diagnosis and participating in medical decision-making. BJU Int. 2004;93:47–51. doi: 10.1111/j.1464-410x.2004.04553.x. [DOI] [PubMed] [Google Scholar]
- 13.Degner LF, Kristjanson LJ, Bowman D, Sloan JA, Carriere KC, O’Neil J, Bilodeau B, Watson P, Mueller B. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277:1485–92. [PubMed] [Google Scholar]
- 14.Davison BJ, Degner LF, Morgan TR. Information and decision-making preferences of men with prostate cancer. Oncol Nurs Forum. 1995;22:1401–8. [PubMed] [Google Scholar]
- 15.Beaver K, Bogg J, Luker KA. Decision-making role preferences and information needs: a comparison of colorectal and breast cancer. Health Expect. 1999;2:266–76. doi: 10.1046/j.1369-6513.1999.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaver K, Luker KA, Owens RG, Leinster SJ, Degner LF, Sloan JA. Treatment decision making in women newly diagnosed with breast cancer. Cancer Nurs. 1996;19:8–19. doi: 10.1097/00002820-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Gattellari M, Butow PN, Tattersall MH. Sharing decisions in cancer care. Soc Sci Med. 2001;52:1865–78. doi: 10.1016/s0277-9536(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 18.Giedzinska-Simons, Antoinette S. Health-related quality of life correlates of the treatment decision making process of newly diagnosed prostate cancer patients. University of Southern California; 2007. [Google Scholar]
- 19.Keating NL, Beth Landrum M, Arora NK, Malin JL, Ganz PA, van Ryn M, Weeks JC. Cancer patients’ roles in treatment decisions: do characteristics of the decision influence roles? J Clin Oncol. 2010;28:4364–70. doi: 10.1200/JCO.2009.26.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh JA, Sloan JA, Atherton PJ, Smith T, Hack TF, Huschka MM, Rummans TA, Clark MM, Diekmann B, Denger LF. Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the Control Preferences Scale. Am J Manag Care. 2010;16:688–96. [PMC free article] [PubMed] [Google Scholar]
- 21.Bruera E, Sweeney C, Calder K, Palmer L, Benisch-Tolley S. Patient preferences versus physician perceptions of treatment decisions in cancer care. J Clin Oncol. 2001;19:2883–5. doi: 10.1200/JCO.2001.19.11.2883. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard CG, Labrecque MS, Ruckdeschel JC, Blanchard EB. Information and decision-making preferences of hospitalized adult cancer patients. Soc Sci Med. 1988;27:1139–45. doi: 10.1016/0277-9536(88)90343-7. [DOI] [PubMed] [Google Scholar]
- 23.Davison BJ, Breckon EN. Impact of health information-seeking behavior and personal factors on preferred role in treatment decision making in men with newly diagnosed prostate cancer. Cancer Nurs. 2011 doi: 10.1097/NCC.0b013e318236565a. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22067700. [DOI] [PubMed]
- 24.Sidana A, Hernandez DJ, Feng Z, Partin AW, Trock BJ, Saha S, Epstein JI. Treatment decision-making for localized prostate cancer: what younger men choose and why. Prostate. 2012;72:58–64. doi: 10.1002/pros.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer M, Visser A, Voerman B, Garssen B, van Andel G, Bensing J. Treatment decision making in prostate cancer: patients’ participation in complex decisions. Patient Educ Couns. 2006;63:308–13. doi: 10.1016/j.pec.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Gwede CK, Pow-Sang J, Seigne J, Heysek R, Helal M, Shade K, Cantor A, Jacobson PB. Treatment decision-making strategies and influences in patients with localized prostate carcinoma. Cancer. 2005;104:1381–90. doi: 10.1002/cncr.21330. [DOI] [PubMed] [Google Scholar]
- 27.Davison BJ, Gleave ME, Goldenberg SL, Degner LF, Hoffart D, Berkowitz J. Assessing information and decision preferences of men with prostate cancer and their partners. Cancer Nurs. 2002;25:42–9. doi: 10.1097/00002820-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Wong F, Stewart DE, Dancey J, Meana M, McAndrews MP, Bunston T, Cheung AM. Men with prostate cancer: influence of psychological factors on informational needs and decision making. J Psychosom Res. 2000;49:13–9. doi: 10.1016/s0022-3999(99)00109-9. [DOI] [PubMed] [Google Scholar]
- 29.Fallowfield LJ, Hall A, Maguire P, Baum M, A’Hern RP. Psychological effects of being offered choice of surgery for breast cancer. BMJ. 1994;309:448. doi: 10.1136/bmj.309.6952.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psychooncology. 2006;15:9–19. doi: 10.1002/pon.907. [DOI] [PubMed] [Google Scholar]
- 31.Andersen MR, Urban N. Involvement in decision-making and breast cancer survivor quality of life. Ann Behav Med. 1999;21:201–9. doi: 10.1007/BF02884834. [DOI] [PubMed] [Google Scholar]
- 32.Hart A, Jr, Smith WR, Tademy RH, McClish DK, McCreary M. Health decision-making preferences among African American men recruited from urban barbershops. J Natl Med Assoc. 2009;101:684–9. doi: 10.1016/s0027-9684(15)30977-9. [DOI] [PubMed] [Google Scholar]
- 33.Williams RM, Zincke NL, Turner RO, Davis JL, Davis KM, Schwartz MD, Johnson L, Kerner JF, Taylor KL. Prostate cancer screening and shared decision-making preferences among African-American members of the Prince Hall Masons. Psychooncology. 2008;17:1006–13. doi: 10.1002/pon.1318. [DOI] [PubMed] [Google Scholar]
- 34.Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med. 2005;20:531–5. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Kibel AS, Hu JJ, Turner AR, Pruett K, Zheng SL, Sun J, Isaacs SD, Wiley KE, Kim S, Hsu F, Wu W, Torti FM, Walsh PC, Chang B, Isaacs WB. Prostate cancer risk associated loci in African Americans. Cancer Epidemiol Biomarkers Prev. 2009;18:2145–9. doi: 10.1158/1055-9965.EPI-09-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 37.Clegg LX, Potosky AL, Harlan LC, Hankey BF, Hoffman RM, Stanford JL, Hamilton AS. Comparison of self-reported initial treatment with medical records: results from the prostate cancer outcomes study. Am J Epidemiol. 2001;154:582–7. doi: 10.1093/aje/154.6.582. [DOI] [PubMed] [Google Scholar]
- 38.Diefenbach MA, Dorsey J, Uzzo RG, Hanks GE, Greenberg RE, Horwitz E, Newton F, Engstrom PF. Decision-making strategies for patients with localized prostate cancer. Semin Urol Oncol. 2002;20:55–62. doi: 10.1053/suro.2002.30399. [DOI] [PubMed] [Google Scholar]
- 39.Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45:941–50. doi: 10.1016/0895-4356(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 40.Davison BJ, Breckon E. Factors influencing treatment decision making and information preferences of prostate cancer patients on active surveillance. Patient Education and Counseling. 2012;87:369–74. doi: 10.1016/j.pec.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 42.Day NE, Byar DP, Green SB. Overadjustment in case-control studies. Am J Epidemiol. 1980;112:696–706. doi: 10.1093/oxfordjournals.aje.a113042. [DOI] [PubMed] [Google Scholar]
- 43.Eton DT, Lepore SJ. Prostate cancer and health-related quality of life: a review of the literature. Psychooncology. 2002;11:307–26. doi: 10.1002/pon.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eton DT, Lepore SJ, Helgeson VS. Early quality of life in patients with localized prostate carcinoma: an examination of treatment-related, demographic, and psychosocial factors. Cancer. 2001;92:1451–9. doi: 10.1002/1097-0142(20010915)92:6<1451::aid-cncr1469>3.0.co;2-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller DC, Sanda MG, Dunn RL, Montie JE, Pimentel H, Sandler HM, McLaughlin WP, Wei JT. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772–80. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 46.Litwin MS, Gore JL, Kwan L, Brandeis JM, LE SP, Withers HR, Reiter RE. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109:2239–47. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- 47.Wei JT, Dunn RL, Sandler HM, McLaughlin W, Montie JE, Litwin MS, Nyquist L, Sanda MG. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–66. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 48.Northouse LL, Mood DW, Montie JE, Sandler HM, Forman JD, Hussain M, Pienta KJ, Smith DC, Sanda MG, Kershaw T. Living with prostate cancer: patients’ and spouses’ psychosocial status and quality of life. J Clin Oncol. 2007;25:4171–7. doi: 10.1200/JCO.2006.09.6503. [DOI] [PubMed] [Google Scholar]
- 49.Campbell LC, Keefe FJ, Scipio C, McKee DC, Edwards CL, Herman SH, Johnson LE, Colvin OM, McBride CM, Donatucci C. Facilitating research participation and improving quality of life for African American prostate cancer survivors and their intimate partners. A pilot study of telephone-based coping skills training. Cancer. 2007;109:414–24. doi: 10.1002/cncr.22355. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Diamant AL, Thind A, Maly RC. Validity of self-reports of breast cancer treatment in low-income, medically underserved women with breast cancer. Breast Cancer Res Treat. 2010;119:745–51. doi: 10.1007/s10549-009-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]