Monocular deprivation (MD) has been widely used to measure plasticity in the early visual system [1]. It is widely known that during an early critical period of an observer’s life, the ocular dominance in the primary visual cortex is severely disrupted when measured immediately after the offset of MD. In contrast, hardly any change was observed when MD is conducted after the critical period [2]. Here we report that long-term plasticity occurred significantly more rapidly with the non-deprived eye than with the deprived eye of human adults when induced by training on a visual task conducted after three-day MD. Thus, the present results challenge the long-standing view that MD has no long-term influence on the visual function of normal adults.
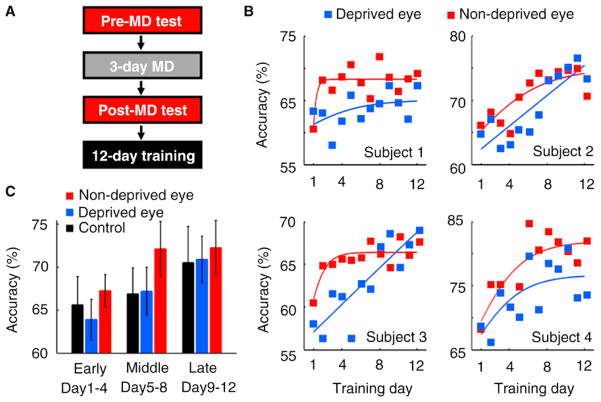
The experiment consisted of pre-MD test, MD, post-MD test, and training stages (Figure 1A; see the Supplemental Information available on-line with this issue for details). In the pre-MD and post-MD test stages, subjects’ (N = 4) performance was measured on a contrast detection task (Supplemental Figure S1A) separately for each eye. In the MD stage, one of the subjects’ eyes was occluded with an eye-patch for three days during which the subjects performed no task. We found no significant effect of test (pre-MD versus post-MD tests), eye (deprived versus non-deprived), or their interaction (Supplemental Figure S1B), indicating no effect of MD or eyes on task performance before training.
Figure 1.
Experimental design and training results after MD.
(A) Experimental procedure. (B) Training results for each subject (logistically fitted). (C) Mean (±SEM) performance in each period for the deprived eye, non-deprived eye, and control subjects during the training stage (see Figure S1C for performance for each eye).
Following the post-MD test stage, subjects underwent 12-day training on the same detection task in a separate trial for each eye. Despite some degree of variability, the same tendency was observed across the subjects (Figure 1B). While performance for the non-deprived eye was generally better than for the deprived eye in the early and middle periods of the training stage for all subjects, performance levels were similar between the eyes in the late period (Figure 1B). Significant differences were observed in the early (paired t-test, P = 0.015) and middle (P = 0.001) periods, but no significant difference in the late period (P = 0.380) (Figure 1C). These results suggest that MD selectively modulates the speed of performance improvement.
Did MD boost performance for the non-deprived eye, or impair performance for the deprived eye? To address this question, four new subjects participated in a control experiment, in which no MD was conducted during a three-day interval between the pre- and post-test stages. Mean performance for the non-deprived eye among the experimental subjects was significantly better than that of control subjects in the middle period (unpaired t-test, P = 0.039), but not in the early (P = 0.124) or late (P = 0.480) periods (Figure 1C). No significant performance differences were observed between the deprived eyes of experimental subjects and control subjects for any period (P > 0.315). That is, MD selectively boosted visual plasticity for the non-deprived eye without significantly influencing an initial level of visual performance before the onset of training. MD in adults can modulate subsequent plasticity.
Recent studies of adult vision have reported no or only slight visual function changes after MD [1,2]. Such changes, if any, lasted for a very short time (~3 h) [3,4]. In contrast, the boosting effect found here lasted for several days, which was longer than previously reported. While some studies suggest a plastic change after MD [5,6], it is unclear whether the change was caused by MD itself or by the training imposed during the MD stage. In contrast, our results clearly demonstrate that MD itself induced a greater degree of plasticity, as there was no training during the MD stage and that MD’s subsequent boosting effect on perceptual learning lasted for several days. These findings are important, because MD has been used under the assumption that it does not change the long-term plasticity of adult vision.
What is the underlying mechanism for the boosting effect? In a normal adult, cross-inhibition occurs from each eye [7]. The boosting effect suggests that no cross-inhibition from the deprived to non-deprived eyes during MD reduces effectiveness of plasticity brake, which is usually effective after the offset of a critical period [1]. If so, the facilitation of visual plasticity after binocular deprivation [8] may be due to the removal of mutual inhibitions between the two eyes. Moreover, the improvement in performance with an amblyopic eye as a result of occluding the fellow eye [5] might be at least partially accounted for by this boosting effect, although the combination of training with an amblyopic eye and deprivation of the fellow eye may be more effective [5,7].
Supplementary Material
Acknowledgments
This research was supported by NIH R01 EY019466, R01 MH091801, NSF 0964776, and Japanese MEXT SRPBS. We thank Jonathan Dobres for comments on a draft and Yuka Furukawa for technical assistance.
Footnotes
Supplemental Information
Supplemental Information includes one figure and supplemental experimental procedures and can be found with this article online at doi: 10.1016/j.cub.2012.03.010.
References
- 1.Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. Eur J Neurosci. 2003;17:167–173. doi: 10.1046/j.1460-9568.2003.02420.x. [DOI] [PubMed] [Google Scholar]
- 3.Lou AR, Madsen KH, Paulson OB, Julian HO, Prause JU, Siebner HR, Kjaer TW. Monocular visual deprivation suppresses excitability in adult human visual cortex. Cereb Cortex. 2011;21:2876–2882. doi: 10.1093/cercor/bhr082. [DOI] [PubMed] [Google Scholar]
- 4.Lunghi C, Burr DC, Morrone C. Brief periods of monocular deprivation disrupt ocular balance in human adult visual cortex. Curr Biol. 2011;21:R538–R539. doi: 10.1016/j.cub.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc Natl Acad Sci USA. 2004;101:6692–6697. doi: 10.1073/pnas.0401200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prusky GT, Alam NM, Douglas RM. Enhancement of vision by monocular deprivation in adult mice. J Neurosci. 2006;26:11554–11561. doi: 10.1523/JNEUROSCI.3396-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu JP, He ZJ, Ooi TL. Effectively reducing sensory eye dominance with a push-pull perceptual learning protocol. Curr Biol. 2010;20:1864–1868. doi: 10.1016/j.cub.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He HY, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat Neurosci. 2007;10:1134–1136. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.