Abstract
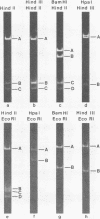
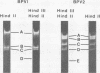
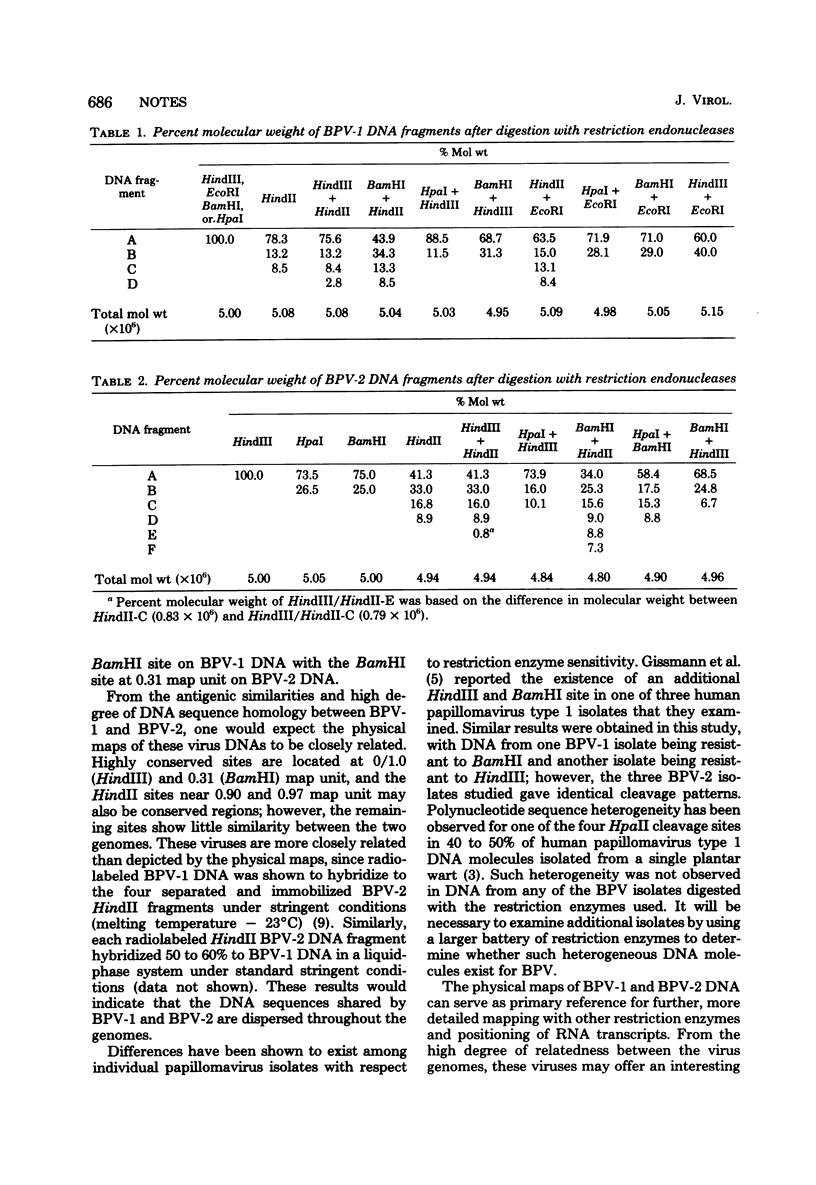
Physical maps of bovine papillomavirus type 1 and type 2 (BPV-1 and BPV-2) DNA were constructed from analysis of the electrophoretic mobilities of restriction endonuclease cleavage fragments from dual digests. BPV-1 DNA was sensitive to Hind III, HindIII, EcoRI, HpaI, AND BamHI, with all but HindII yielding single scissions. BPV-2 DNA was resistant to EcoRI, and HindIII had one cleavage site whereas HpaI, BamHI, and HindII yielded multiple fragments. Of four BPV-1 isolates examined, DNA from one isolate was resistant to HindIII, and another DNA isolate was resistant to BamHI. The three BPV-2 isolates examined were uniformly sensitive to the restriction endonucleases employed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthold S. W., Koller L. D., Olson C., Studer E., Holtan A. Atypical warts in cattle. J Am Vet Med Assoc. 1974 Aug 1;165(3):276–280. [PubMed] [Google Scholar]
- Favre M., Orth G., Croissant O., Yaniv M. Human papillomavirus DNA: physical map. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4810–4814. doi: 10.1073/pnas.72.12.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M., Orth G., Croissant O., Yaniv M. Human papillomavirus DNA: physical mapping of the cleavage sites of Bacillus amyloliquefaciens (BamI) and Haemophilus parainfluenzae (HpaII) endonucleases and evidence for partial heterogeneity. J Virol. 1977 Mar;21(3):1210–1214. doi: 10.1128/jvi.21.3.1210-1214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfin D. E., Goodman H. M. Nucleotide sequences at the cleavage sites of two restriction endonucleases from Hemophilus parainfluenzae. Biochem Biophys Res Commun. 1974 Jul 10;59(1):108–116. doi: 10.1016/s0006-291x(74)80181-6. [DOI] [PubMed] [Google Scholar]
- Gissmann L., Pfister H., Zur Hausen H. Human papilloma viruses (HPV): characterization of four different isolates. Virology. 1977 Feb;76(2):569–580. doi: 10.1016/0042-6822(77)90239-2. [DOI] [PubMed] [Google Scholar]
- Jarrett W. F., McNeil P. E., Grimshaw W. T., Selman I. E., McIntyre W. I. High incidence area of cattle cancer with a possible interaction between an environmental carcinogen and a papilloma virus. Nature. 1978 Jul 20;274(5668):215–217. doi: 10.1038/274215a0. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C. Demonstration of two distinct classes of bovine papilloma virus. Virology. 1978 Sep;89(2):372–379. doi: 10.1016/0042-6822(78)90179-4. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Theilen G. H., Olson C. Hybridization of bovine papilloma virus type 1 and type 2 DNA to DNA from virus-induced hamster tumors and naturally occurring equine tumors. Intervirology. 1979;11(4):227–233. doi: 10.1159/000149038. [DOI] [PubMed] [Google Scholar]
- Law M. F., Lancaster W. D., Howley P. M. Conserved polynucleotide sequences among the genomes of papillomaviruses. J Virol. 1979 Oct;32(1):199–207. doi: 10.1128/jvi.32.1.199-207.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]