Abstract
BACKGROUND
Cytogenetics and multicolor flow cytometry (MFC) are useful tools for monitoring outcome of treatment in acute myeloid leukemia (AML). However, no data are available regarding the meaning of results when the two tests do not agree.
METHODS
We analyzed 1464 pairs of concurrent cytogenetics and flow results from 424 patients, both pre- and post- hematopoietic cell transplantation (HCT), and compared the prognostic impact of discordant and concordant results.
RESULTS
Informative discordant results were found in 22% of patients. Compared with patients with double negative testing results, either positive result had a significant impact on overall survival (OS) and relapse-free survival (RFS). The hazard ratios (HR) with either cytogenetics or MFC positive pre-transplant were 3.1 (P = 0.009) and 2.5 (P = 0.0008), respectively, for reduced OS, and 2.7 (P = 0.01) and 4.1 (P < 0.0001), respectively, for decreased RFS. Similar findings were obtained post-transplant. Molecular cytogenetics, i.e. fluorescence in situ hybridization (FISH), further added value to the evaluation of discordant cases.
CONCLUSION
Detection of residual disease of AML by either cytogenetics or flow cytometry in HCT patients predicts early relapse and shortened survival.
Keywords: Acute myeloid leukemia, Cytogenetics, Multicolor flow cytometry, Fluorescence in situ hybridization
INTRODUCTION
Cytogenetics and immunophenotyping by flow cytometry are part of the standard evaluation of newly diagnosed acute myeloid leukemia (AML), and both techniques are also used to monitor results of therapy 1–4. Most of the available information about the utility of these techniques to monitor therapeutic response comes from their application following conventional chemotherapy. In that setting, abnormal cytogenetics in patients in morphologic complete remission predicts for significantly shorter disease-free and overall survival 5. However, due to limited sensitivity, the false-negative rate for conventional cytogenetics as an indicator of minimal residual disease (MRD) is estimated to be approximately 50%, and, in addition, almost 50% of all AML cases are cytogenetically normal at diagnosis 3, 6. Molecular cytogenetics with interphase fluorescence in situ hydribization (FISH) has greater sensitivity for the detection of specific chromosome abnormalities, especially for patients with poor chromosome morphology, or low or no yield of metaphase cells 7. Exactly how much FISH adds to the predictive power of cytogenetics in monitoring AML patients following therapy is uncertain.
Flow cytometric methods for MRD detection rely on the degree of immunophenotypic deviation of abnormal populations from normal hematopoietic maturation and hence have a variable sensitivity that for myeloid neoplasms typically ranges from 0.1% to 0.001% of leukocytes8 depending on the specific reagent panel employed. The advent of high-level multicolor flow cytometry analysis (MFC) allows for the routine detection of up to 10 simultaneous fluorochromes and offers theoretical improvements in both specificity and sensitivity9. Several studies have shown that the level of MRD is a valuable tool for monitoring continued disease response, detection of early relapse, and for predicting patient outcome primarily in the non-transplant setting10–12. It should be noted that the lack of standardization for these assays prevents generalizations about assay sensitivity and in clinical practice one must inquire about the performance characteristics of the specific assay to be used for MRD assessment.
Because conventional cytogenetics, FISH and newer, very sensitive MFC techniques are useful for monitoring response to conventional chemotherapy, they are increasingly performed in patients with AML undergoing hematopoietic cell transplantation (HCT). In this setting, we have observed that the results of the three tests do not always agree. Currently, no data are available to help the physician interpret such discordant results. While intuitively any positive finding should predict for a poor outcome, the sensitivities of current MFC techniques are approaching those of polymerase chain reactions (PCR), and at least in the setting of PCR testing for t(8;21) and for BCR/ABL1 in the early post-transplant setting, positive results have not necessarily been predictive of subsequent relapse13–15. Because a direct comparison of cytogenetic and MFC results for their prognostic impact in the transplant setting is not available, especially when the analyses yield discordant results, we conducted a comparison of 1464 paired results from 424 patients with AML receiving allogeneic HCT. In this study, a positive result from either cytogenetic or MFC testing was associated with poor prognosis of similar degree. FISH testing provided further prognostic information in both concordant and discordant cases. These data demonstrated that MRD either pre- or post-transplant, regardless of how is measured, predicts outcome.
MATERIALS AND METHODS
Human Subject
Among consecutive AML patients treated with allogeneic HCT at the Fred Hutchinson Cancer Research Center (FHCRC)/Seattle Cancer Care Alliance (SCCA) between 2006 and 2009, we evaluated all who had both cytogenetic and flow cytometric results available on the same sample, regardless of specific regimen and treatment protocol. Each patient may have multiple samples tested both pre- and post-transplant. Results were analyzed for concordance and discordance between the two diagnostic methods based on the presence or absence of abnormal cells. A total of 404 patients contributed to the outcome analysis. The study was approved by the institutional review office at the FHCRC/SCCA.
Conventional Cytogenetic Studies
At diagnosis or pre- and post-transplant evaluations, samples from bone marrow aspirates were tested for cytogenetic abnormalities using standard culturing and G-banding analysis at SCCA. Karyotype designation was based on the International System for Human Cytogenetic Nomenclature (ISCN)16, 17. Only clonal abnormalities were considered as positive results. The karyotype analysis was based on 20 metaphases for each sample as a routine procedure. The presence of one or more clonal abnormalities was considered a positive cytogenetic result; the absence of any clonal aberrations was considered a negative result.
FISH Studies
FISH was performed at SCCA according to standard procedures at the time of testing in a subset of 215 patients. FISH probes were purchased from Abbott-Vysis (Abbott Park, IL) and Cytocell-Rainbow Scientific (Windsor, CT). A false-positive cutoff was established for each probe based on the number of abnormal signal patterns seen in 500 cells per control specimen for a total of 20 controls (mean+3SD). An enumeration above the false-positive cutoff was considered a positive result; an enumeration below the cutoff was considered a negative result.
Flow Cytometry Studies
Ten-color MFC was performed as previously described18, 19. The panel consisted of three tubes as follows: (1) HLA-DR-Pacific Blue (PB), CD15-FITC, CD33-Phycoerythrin (PE), CD19-PE-Texas Red (PE-TR), CD117-PE-Cy5, CD13-PE-Cy7, CD38-Alexa 594 (A594), CD34-allophycocyanin (APC), CD71-APC-A700 and CD45-APC-H7. (2) HLA-DR-PB, CD64-FITC, CD123-PE, CD4-PE-TR, CD14-PE-Cy5.5, CD13-PE-Cy7, CD38-A594, CD34-APC, CD16-APC-A700 and CD45-APC-H7. (3) CD56-Alexa 488, CD7-PE, CD5-PE-Cy5, CD33-PE-Cy7, CD38-A594, CD34-APC and CD45-APC-H7. All antibodies were obtained from Beckman-Coulter (Fullerton, CA) or Becton-Dickinson (San Jose, CA). Up to 1 million events per tube were acquired on a custom-built LSRII and data compensation and analysis performed using software developed in our institution. Disease was identified as a population of 10 or more events showing deviation from the normal patterns of antigen expression seen on specific cell lineages at specific stages of maturation as compared with either normal or regenerating marrow. When identified, the abnormal population was quantified as a percentage of the total CD45+ white cell events. Any level of detectable disease was considered positive.
Statistical Analysis
Overall survival (OS) and relapse-free survival (RFS) were estimated by the Kaplan-Meier method. For pre-transplant test results, survival was calculated relative to the day of transplant. For post-transplant test results, survival was calculated relative to the day of the test. In cases where a patient had multiple test results within a given time window, analysis was based on either the first or the last test result as specified in Results. Hazard ratio analysis of survival between groups was performed using Cox regression. For the analysis of RFS, patients who relapsed prior to testing were excluded. Relapse was defined per standard criteria, such as 5% or more blasts in the bone marrow by morphological assessment, reappearance of leukemic blasts in the peripheral blood, or reappearance or development of cytologically proven extramedullary disease.
RESULTS
Incidence of discordant cytogenetic and flow cytometry results
We first asked how frequently discordant results were observed. Of the 1464 test entries from 424 patients (see Table 1 for patient characteristics), 267 samples (18%) showed apparently discordant results, two thirds of which were cytogenetically negative and MFC positive (C−F+) and one third were cytogenetically positive and MFC negative (C+F−). Once patients with normal cytogenetics at diagnosis, Y loss only, or known constitutional abnormalities were excluded as non-informative, the incidence of discordant results was 186 samples (12.7%), which accounted for 95 total patients (22%). Among these, 127 samples from 65 were C−F+ while 59 samples from 38 were C+F−.
Table 1.
Patient characteristics (n=424)
| Age, median (range) | 50 (0–74) |
| 0–17 | 33 (8%) |
| 18–60 | 299 (71%) |
| 61–80 | 90 (21%) |
| Sex | |
| Male | 224 (53%) |
| Female | 200 (47%) |
| Conditioning | |
| myeloablative | 288 (68%) |
| nonmyeloablative | 136 (32%) |
| Stem cell source | |
| Bone marrow | 64 (15%) |
| Peripheral blood | 311 (73%) |
| Cord blood | 49 (12%) |
| Cytogenetic risk pre-tx | |
| Good | 29 (7%) |
| Intermediate | 250 (59%) |
| Poor | 85 (34%) |
| Status at tx | |
| Remission | 349 (82%) |
| Relapse | 75 (18%) |
Because MFC analysis can detect abnormalities in as few as 0.001% abnormal cells whereas cytogenetic analysis has a sensitivity of 5–10% for G-banding analysis, the discordant rate was expected to inversely correlate with the frequency of abnormal cells by MFC. Indeed, negative cytogenetics were found in 127 MFC+ samples from 65 patients and showed a higher discordant rate when the percent abnormal cells detected by MFC was low (Table 2).
Table 2.
Discordant rate among cases with positive flow and negative cytogenetic results (C−F+) based on percent abnormal cells identified by flow cytometric analysis.
| Abnormal rate by flow cytometric analysis |
Number of samples |
Normal cytogenetics (known abnormal at diagnosis) |
|
|---|---|---|---|
| Number | Proportion | ||
| 20% and above | 76 | 0 | 0% |
| 5 – <20% | 92 | 4 | 4.3% |
| 1 – < 5% | 88 | 18 | 20% |
| 0.1 – < 1% | 93 | 43 | 46% |
| 0.01 – <0.1% | 68 | 46 | 68% |
| 0.001 – <0.01% | 19 | 16 | 84% |
Negative MFC results (C+F−) in cytogenetically abnormal samples were found in 34 patients with aberrations of host origin and 4 patients with donor-derived abnormalities (mostly deletion 20q). The majority were low-level positive by cytogenetics; i.e., less than five abnormal metaphase cells out of twenty total analyzed.
Prognostic impact of discordant results among transplant AML patients
As a primary goal of this study, we determined the impact of discordant results on the overall survival of patients. For the initial analysis, C+ cases included those abnormal by G-banding analysis and/or FISH. In cases where a patient had multiple test results within a given time window, analysis was based on the last test result for the pre-transplant window and the first test result post-transplant.
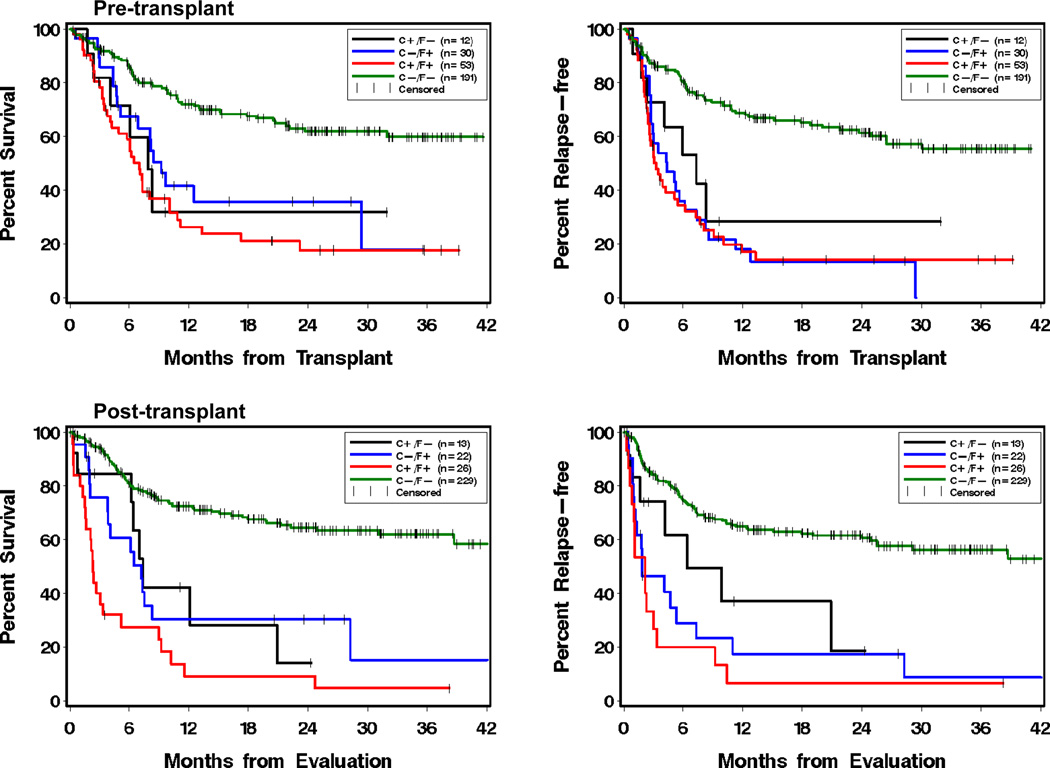
As expected, for both pre-transplant and post-transplant testing, positive results for both cytogenetics and flow (C+F+) predicted a significantly worse outcome than negative results (C−F−) (Figure 1 and Table 3a). Patients with discordant results (C+F− or C−F+) demonstrated significantly worse outcome than patients with negative results (C−F−). Overall, the prognosis was almost as poor for the discordant patients as for the C+F+ group.
Figure 1.
Kaplan-Meier plots of overall survival (left) and relapse-free survival (right) by test results obtained pre-transplant (upper) and post-transplant (lower). Analysis was based on the last test result for the pre-transplant window (day −60 to −1) and the first test result for the post-transplant window (day 1 to 100). C+/F− represents the group of patients who had abnormal cytogenetic (including FISH) results but normal flow cytometry results. C−/F+ represents the group of patients who had normal cytogenetic results but abnormal flow cytometry results. C+/F+ represents those who had both abnormal cytogenetic and flow cytometry results. C−/F− represents those who had normal results for both cytogenetics and flow.
Table 3.
Statistical analysis of overall survival and relapse-free survival as a function of cytogenetic and flow cytometric testing.
| a. | Overall Mortality | Relapse1 + NRM2 | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Pre-Tx (last value day −60 to day −1) | ||||
| C+F− (n=12) | 3.1 (1.3–7.2) | 0.009 | 2.7 (1.3–6.0) | 0.01 |
| C−F+ (n=30) | 2.5 (1.5–4.4) | 0.0008 | 4.1 (2.6–6.5) | <0.0001 |
| C+F+ (n=53) | 3.7 (2.5–5.6) | <0.0001 | 4.2 (2.8–6.2) | <0.0001 |
| C−F− (n=191) | 1 | 1 | ||
| Post-Tx (first value day 1 to day 100) | ||||
| C+F− (n=13) | 3.1 (1.5–6.5) | 0.002 | 2.8 (1.3–5.8) | 0.006 |
| C−F+ (n=22) | 3.2 (1.8–5.5) | <0.0001 | 4.2 (2.5–7.0) | <0.0001 |
| C+F+ (n=26) | 7.2 (4.5–12) | <0.0001 | 8.1 (5.0–13) | <0.0001 |
| C−F− (n=229) | 1 | 1 | ||
| b. | ||||
|---|---|---|---|---|
| Day 28 (first result day 14 to day 42) | ||||
| C+F− (n=12) | 3.5 (1.6–7.3) | 0.001 | 3.2 (1.5–6.6) | 0.002 |
| C−F+ (n=19) | 3.7 (2.0–6.6) | <0.0001 | 5.0 (2.9–8.7) | <0.0001 |
| C+F+ (n=17) | 5.7 (3.2–10) | <0.0001 | 5.8 (3.3–10) | <0.0001 |
| C−F− (n=176) | 1 | 1 | ||
| Day 80 (first result day 70 to day 100) | ||||
| C+F− (n=7) | 4.6 (1.8–12) | 0.002 | 6.4 (2.7–15) | <0.0001 |
| C−F+ (n=21) | 3.6 (1.9–6.9) | 0.0001 | 3.7 (2.0–6.9) | <0.0001 |
| C+F+ (n=25) | 11.2 (6.5–19) | <0.0001 | 25.6 (13–49) | <0.0001 |
| C−F− (n=177) | 1 | 1 | ||
| Post Day 100 (first result day 101 to day 365) | ||||
| C+F− (n=6) | 13.0 (3.3–50) | 0.0002 | 16.8 (3.9–71) | 0.0001 |
| C−F+ (n=11) | 9.4 (3.5–25) | <0.0001 | 10.5 (2.6–41) | 0.0008 |
| C+F+ (n=18) | 22.2 (8.9–55) | <0.0001 | 41.4 (11–150) | <0.0001 |
| C−F− (n=81) | 1 | 1 | ||
| Post 1 Year (first result 1 year to 5 years) | ||||
| C+F−/C−F− (n=6) | 2.3 (0.5–10) | 0.27 | 1.5 (0.2–12) | 0.69 |
| C+F+ (n=8) | 5.7 (1.8–18) | 0.003 | 78.6 (17–364) | <0.0001 |
| C−F− (n=107) | 1 | 1 | ||
| c. | ||||
|---|---|---|---|---|
| Myeloablative Pre-Tx (last value day −60 to day −1) | ||||
| C+F− (n=9) | 5.2 (2.0–14) | 0.0009 | 3.8 (1.5–9.9) | 0.006 |
| C−F+ (n=20) | 2.7 (1.3–5.8) | 0.01 | 5.4 (2.9–9.9) | <0.0001 |
| C+F+ (n=44) | 5.6 (3.3–9.4) | <0.0001 | 6.1 (3.7–10) | <0.0001 |
| C−F− (n=124) | 1 | 1 | ||
| Myeloablative Post-Tx (last value day 1 to day 100) | ||||
| C+F− (n=5) | 3.8 (0.9–16) | 0.07 | 5.8 (1.8–19) | 0.004 |
| C−F+ (n=14) | 2.6 (1.1–6.3) | 0.03 | 3.0 (1.4–6.5) | 0.004 |
| C+F+ (n=32) | 9.2 (5.4–16) | <0.0001 | 16.6 (8.9–31) | <0.0001 |
| C−F− (n=147) | 1 | 1 | ||
| Nonmyeloablative Pre-Tx (last value day −60 to day −1) | ||||
| C+F− (n=3) | 1.0 (0.1–7.1) | 0.97 | 1.7 (0.4–7.2) | 0.46 |
| C−F+ (n=10) | 2.5 (1.1–5.4) | 0.02 | 2.9 (1.4–6.0) | 0.005 |
| C+F+ (n=9) | 2.2 (0.9–5.2) | 0.09 | 2.4 (1.0–5.8) | 0.05 |
| C−F− (n=67) | 1 | 1 | ||
| Nonmyeloablative Post-Tx (last value day 1 to day 100) | ||||
| C+F− (n=6) | 2.5 (0.9–7.3) | 0.1 | 3.5 (1.3–9.4) | 0.01 |
| C−F+ (n=9) | 2.8 (1.2–6.6) | 0.02 | 4.0 (1.7–9.4) | 0.002 |
| C+F+ (n=14) | 8.1 (3.9–17) | <0.0001 | 16.1 (7.2–36) | <0.0001 |
| C−F− (n=63) | 1 | 1 | ||
for post-transplant analysis, only relapses subsequent to test result are counted
NRM: non-relapse mortality
The next question we addressed was whether any specific post-transplant time point carried more weight than the others for prognosis when abnormal testing result(s) were obtained. We divided the post-transplant testing results into four time frames. Typically, patients were scheduled to be evaluated at 28 days, 80 days and one year post transplant at our institution. We took the first result from each of the following 4 time frames for analysis: day 28 (first result day 14 to day 42), day 80 (first result day 70 to day 100), post day 100 (first result day 101 to day 365), and post 1-year (first result 1 year to 5 years). In general, each time frame showed a similar prognostic impact to the aggregated post-transplant results. Detailed statistical results are summarized in Table 3b.
Since the benefit of allogeneic HCT is attributable to both the conditioning regimen and a potent GVL effect20, one question was whether the presence of MRD would have the same prognostic significance in non-myeloablative (NMA) patients as in myeloablative (MA) patients. We compared the two groups in the pre- and post-transplant settings. The results did not show significant differences between the NMA and the MA patients (Table 3c).
The entire analysis was also performed on the subset of patients in remission; i.e., with <5% blasts. The results remained the same although P-values were slightly different due to smaller sample size (data not shown).
FISH Results add value to prognostication of discordant and concordant negative cases
FISH studies enhance the detection sensitivity of specific chromosome abnormalities and are frequently used in pre- and post-transplant patient assessment as an adjunct test to cytogenetic testing when a “FISHable” marker is available. Of the 1464 total samples, 564 (38.5%) had concurrent FISH studies performed on 215 total patients. We used this subset to address the question of whether FISH results add prognostic value to the samples with either concordant or discordant cytogenetic and MFC results.
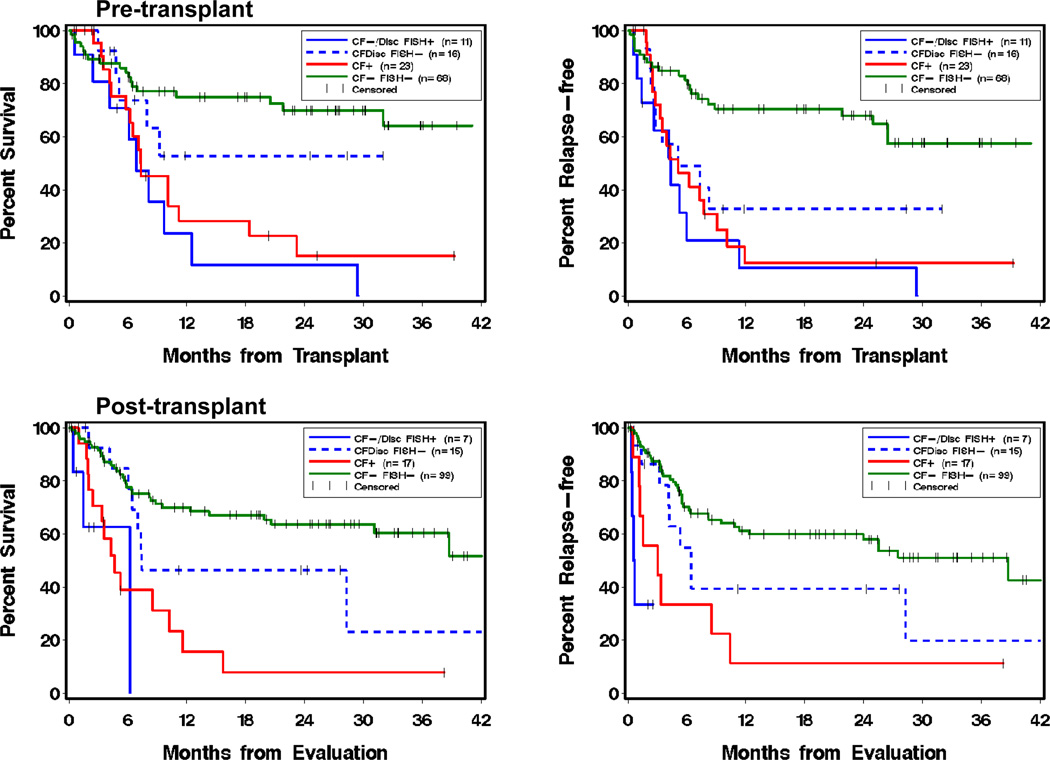
Five categories were compared in both the pre- and post- transplant settings: (1) discordant samples (either C+F− or C−F+) with negative FISH results, (2) discordant samples with positive FISH results, (3) concordant negative samples (CF−) with positive FISH results, (4) concordant positive samples (CF+ regardless of FISH results, as positive control), and (5) concordant negative samples (CF− and FISH-, as negative control). Groups (2) and (3) were similar and thus combined. Demonstrated by Figure 2 and Table 4, in both pre- and post-transplant settings, patients with positive FISH results had a significantly worse outcome than those with concordant negative results (P <=0.001); during post-transplant, FISH positivity increased the risk among patients with either discordant or negative CF results (HR 4.0 for OS and 4.8 for RFS).
Figure 2.
Impact of fluorescence in situ hybridization (FISH) testing results on overall survival (left) and relapse-free survival (right) when obtained pre-transplant (upper) and post-transplant (lower). Analysis was based on the last test result for the pre-transplant window (day −60 to −1) and the first test result for the post-transplant window (day 1 to 100). CF− represents patients with concordant negative results for both conventional G-banding cytogenetics and flow cytometry. CF+ represents patients with concordant positive results for both conventional cytogenetics and flow. CFDisc represents all patients with discordant results between conventional cytogenetics and flow, including either normal cytogenetics but abnormal flow or abnormal cytogenetics but normal flow. Additional FISH results are shown as abnormal (FISH+) or normal (FISH−).
Table 4.
Statistical analysis results for overall survival and relapse-free survival as a function of FISH result in addition to cytogenetic and flow testing results.
| Overall Mortality | Relapse1 + NRM | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Pre-Tx (last value day −60 to day −1) | ||||
| CFDisc FISH+ (n=11) | 5.1 (2.3–12) | <0.0001 | 5.2 (2.5–11) | <0.0001 |
| CFDisc FISH− (n=16) | 1.8 (0.6–4.8) | 0.27 | 2.7 (1.2–5.8) | 0.01 |
| CF+ (n=23) | 3.6 (1.8–7.2) | 0.0002 | 3.6 (1.9–6.9) | <0.0001 |
| CF− FISH− (n=68) | 1 | 1 | ||
| Disc FISH+ vs FISH− | 2.9 (1.0–8.7) | 0.06 | 2.0 (0.8–4.8) | 0.14 |
| Post-Tx (first value day 1 to day 100) | ||||
| CF−/Disc FISH+ (n=7) | 7.3 (2.2–25) | 0.001 | 8.4 (2.8–25) | 0.0001 |
| CFDisc FISH− (n=15) | 1.8 (0.8–4.0) | 0.13 | 1.8 (0.9–3.6) | 0.12 |
| CF+ (n=17) | 4.4 (2.3–8.4) | <0.0001 | 6.0 (3.2–11) | <0.0001 |
| CF− FISH− (n=99) | 1 | 1 | ||
| Disc FISH+ vs FISH− | 4.0 (1.0–16) | 0.04 | 4.8 (1.4–16) | 0.01 |
for post-transplant analysis, only relapses subsequent to test result are counted
Prognostic impact of minimal residual disease detected by flow cytometry analysis
With increasing sensitivity of MFC, an unanswered question was how prognostically significant are low levels of abnormal results in AML by MFC, especially when cytogenetic/FISH results were normal. We compared two groups of post-transplant patients with negative cytogenetic/FISH results using 0.1% flow abnormal rate as a division point because many clinical MFC laboratories do not report positive results lower than 0.1%. The testing time frame was out to 1 year post-transplant, using the first test result if there were multiple. Although the group with abnormal cells < 0.1% (n=20) appeared to have a slightly better outcome than the > 0.1% group (n=23), the difference was not statistically significant (P > 0.05). There was no obvious difference in the proportion of high-risk cytogenetic patients between groups.
DISCUSSION
Although conventional cytogenetics is unarguably an important prognostic marker for AML, it is limited by the requirement for the presence of metaphase cells and hence the low detection sensitivity. This limitation is clearly demonstrated by data presented in Table 2. When disease burden is high, both cytogenetics and MFC can detect the disease and therefore are usually concordant in testing results. As disease burden decreases, the false-negative rate increases with conventional cytogenetic analysis.
Interphase FISH improves the detection sensitivity by approximately ten fold compared with conventional cytogenetics. FISH has been shown as more reliable than morphology for defining CR21. It has therefore been proposed as a valid MRD parameter in AML patients. However, FISH cannot be performed in all patients; it is only possible and informative if there is a known cytogenetic abnormality that is detectable by a specific FISH probe. Frequently, no FISH probe is available for a given cytogenetic aberration.
MFC for MRD also has been shown to correlate strongly with the clinical course21. Moreover, MFC can be performed in most patients without specific cytogenetic marker(s) and is the most sensitive of the three methods described so far. Although PCR allows detection of one abnormal cell from a million normal cells, it requires an informative marker and thus cannot be currently performed in a large subset of patients. The sensitivity of up to 0.001% achieved by the new MFC techniques makes it attractive for MRD detection, which is increasingly important in the risk-adapted management of AML patients. However, it should be noted that the flow cytometric technique requires an informative immunophenotype, which although present in the large majority of AML, does not yet allow for a uniformly high level of sensitivity in all cases.
We asked how often might there be discordant results between cytogenetics, FISH and MFC. Our data showed an incidence of 22%, although a more accurate rate of discordance requires results on all patients at initial diagnosis, which is generally not feasible for most tertiary care environment where a large percentage of patients are referred for therapy following diagnosis outside the institution and pretreatment data may be difficult to obtain or of inconsistent quality. In this study, we provide data to demonstrate the prognostic impact of cytogenetic, FISH and MFC testing results in AML patients undergoing allogeneic HCT, particularly focusing on those cases where test results do not agree. Detection of disease both pre- and post-transplant predicts poor outcome (Figure 1). When both cytogenetic and flow results were positive (C+F+), survival was significantly inferior to patients with negative results. Patients with discordant results both pre- and post-transplant, i.e., either cytogenetically abnormal (C+F−) or MFC abnormal (C−F+), showed an outcome almost as poor as the C+F+ group (Table 3a). In fact, the overlapping 95% CI of the HR between discordant results and C+F+ results indicated the significant prognostic value of any detectable disease.
Whether and when to intervene are typical questions for an oncologist during post-transplant follow-up when an abnormal test result is obtained. For CML patients, PCR detection of BCR/ABL1 transcript at 3 months post allogeneic HCT is of no predictive value, and only at later time points is the test useful15. In this study of AML transplant, however, the timing of detecting an abnormal result did not appear to be a significant factor for outcome (Table 3b). Rather, anytime an abnormality is detected by either cytogenetics or MFC, there is an increased risk for subsequent relapse and mortality. Furthermore, some believe it might take longer for a patient who undergoes non-myeloablative (NMA) transplant to fully benefit from the GVL effect; hence abnormalities detected early post-transplant may not be as informative as those detected later. We attempted to separate the MA and NMA patients into two analysis groups for comparison using the last values during the pre- and post-transplant windows (Table 3c). Some of the subgroup comparisons do not have enough statistical power to be truly informative, especially for the NMA groups whose sample size is small. It will be of interest to perform follow-up analyses once sufficient numbers of NMA patients are available for study.
FISH has been proven to be a reliable MRD parameter in AML in the non-transplant setting21. We further confirmed that FISH was useful in the transplant setting as well (Figure 2 and Table 4). A positive FISH result in a post-transplant patient, whether cytogenetics and MFC are both negative or discordant, increases the risk for relapse and mortality by 7.3 and 8.4 fold, respectively, compared with patients with a negative FISH result along with normal cytogenetics and MFC. Therefore, FISH testing is valuable when there is an informative marker. It is worth noting that FISH probes have various detection sensitivities due to the nature of the probes and the way each clinical laboratory validates its probes, including the number of cells scored for each sample.
The advantages of MFC - high sensitivity and near universal applicability - make it a desirable tool for MRD monitoring. Its prognostic value has been reported in the pre-transplant setting19. Our data further demonstrated its utility in predicting outcome both pre- and post-transplant, in the context of comparison with cytogenetics. Although many flow cytometry laboratories do not report abnormal populations below 0.1% in AML, we found that very low levels of involvement (0.001–0.1%) are associated with a prognosis comparable to those with higher frequencies of abnormal cells (>0.1%). While this may reflect the biology of the disease, it also may be in part due to the difficulty in providing consistent estimates of population frequency in bone marrow samples due to a variable degree of hemodilution by peripheral blood that is inevitably present. It is also possible that the abnormal population identified only represents the portion of the neoplasm that exhibits recognizable deviation from normal patterns of antigen expression and hence underestimates the total level of involvement when the MRD frequency is low. A corollary is that the ultimate sensitivity of the technique for MRD detection currently varies between patients dependent on their degree of immunophenotypic aberrancy and the composition of the background populations in which detection is attempted, believed to be in the range of 0.1–0.01% for most patients. Reduced assay sensitivity in a subset of patients is the likely explanation for the small number of discordant cases where cytogenetics/FISH are positive and flow cytometry is negative, and further methodologic improvements are desirable. Despite these limitations, the positive identification of MRD supports potential medical intervention even when a very low level abnormality is detected by MFC.
In summary, our study is the first to examine the prognostic significance of concordant and discordant cytogenetic and flow cytometry test results among the AML transplant population. The evidence is clear that both abnormal cytogenetic and/or flow cytometric test results prior to and following transplant are associated with an increased risk for relapse and mortality. How to clinically apply these findings is an obvious question. The finding pre-transplant of minimal residual disease by cytogenetics, MFC, or FISH predicts for a poor outcome and raises the question of whether survival might be improved by administering additional therapy pre-transplant, by intensifying the preparative regimen, or by the addition of pre-emptive post-transplant therapy. Similarly, the detection of minimal residual disease post transplant predicts for subsequent clinical relapse and provides a setting in which to test various post transplant therapies. While our studies do not answer the question of how to intervene, they do provide tools to better select patients in which to test such interventions and to monitor the results of such treatments.
Acknowledgments
The authors thank Gary Schoch, a clinical research database manager from Fred Hutchinson Cancer Research Center, and Stacie Thomas (secretary) and Lisa Zhang (clinical technologist) from the Cytogenetics Laboratory of Seattle Cancer Care Alliance (SCCA), for their help with data collection.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Authorship Contributions: MF and FA conceptualized the project. MF and BW collected and reviewed all the data. BG and BMS collected NMA data. MF and BS analyzed the data. All authors contributed to writing and editing the manuscript.
REFERENCES
- 1.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–4083. [PubMed] [Google Scholar]
- 2.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92(7):2322–2333. [PubMed] [Google Scholar]
- 3.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100(13):4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 5.Marcucci G, Mrozek K, Ruppert AS, Archer KJ, Pettenati MJ, Heerema NA, et al. Abnormal cytogenetics at date of morphologic complete remission predicts short overall and disease-free survival, and higher relapse rate in adult acute myeloid leukemia: results from cancer and leukemia group B study 8461. J Clin Oncol. 2004;22(12):2410–2418. doi: 10.1200/JCO.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Freireich EJ, Cork A, Stass SA, McCredie KB, Keating MJ, Estey EH, et al. Cytogenetics for detection of minimal residual disease in acute myeloblastic leukemia. Leukemia. 1992;6(6):500–506. [PubMed] [Google Scholar]
- 7.Frohling S, Skelin S, Liebisch C, Scholl C, Schlenk RF, Dohner H, et al. Comparison of cytogenetic and molecular cytogenetic detection of chromosome abnormalities in 240 consecutive adult patients with acute myeloid leukemia. J Clin Oncol. 2002;20(10):2480–2485. doi: 10.1200/JCO.2002.08.155. [DOI] [PubMed] [Google Scholar]
- 8.Wood BL. Myeloid malignancies: myelodysplastic syndromes, myeloproliferative disorders, and acute myeloid leukemia. Clin Lab Med. 2007;27(3):551–575. doi: 10.1016/j.cll.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Wood B. 9-color and 10-color flow cytometry in the clinical laboratory. Arch Pathol Lab Med. 2006;130(5):680–690. doi: 10.5858/2006-130-680-CACFCI. [DOI] [PubMed] [Google Scholar]
- 10.San Miguel JF, Vidriales MB, Lopez-Berges C, Diaz-Mediavilla J, Gutierrez N, Canizo C, et al. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood. 2001;98(6):1746–1751. doi: 10.1182/blood.v98.6.1746. [DOI] [PubMed] [Google Scholar]
- 11.Kern W, Voskova D, Schoch C, Schnittger S, Hiddemann W, Haferlach T. Prognostic impact of early response to induction therapy as assessed by multiparameter flow cytometry in acute myeloid leukemia. Haematologica. 2004;89(5):528–540. [PubMed] [Google Scholar]
- 12.Maurillo L, Buccisano F, Del Principe MI, Del Poeta G, Spagnoli A, Panetta P, et al. Toward optimization of postremission therapy for residual disease-positive patients with acute myeloid leukemia. J Clin Oncol. 2008;26(30):4944–4951. doi: 10.1200/JCO.2007.15.9814. [DOI] [PubMed] [Google Scholar]
- 13.Appelbaum FR. Molecular diagnosis and clinical decisions in adult acute leukemia. Semin Hematol. 1999;36(4):401–410. [PubMed] [Google Scholar]
- 14.Miyamoto T, Nagafuji K, Akashi K, Harada M, Kyo T, Akashi T, et al. Persistence of multipotent progenitors expressing AML1/ETO transcripts in long-term remission patients with t(8;21) acute myelogenous leukemia. Blood. 1996;87(11):4789–4796. [PubMed] [Google Scholar]
- 15.Radich JP, Gehly G, Gooley T, Bryant E, Clift RA, Collins S, et al. Polymerase chain reaction detection of the BCR-ABL fusion transcript after allogeneic marrow transplantation for chronic myeloid leukemia: results and implications in 346 patients. Blood. 1995;85(9):2632–2638. [PubMed] [Google Scholar]
- 16.Shaffer LG, Slovak ML, Campbell LJ. An International System for Human Cytogenetic Nomeclature. S. Karger Publishers, Inc.; 2009. 2009. [Google Scholar]
- 17.Shaffer LG, Tommerup N. An International System for Human Cytogenetic Nomeclature. S. Karger Publishers, Inc.; 2005. 2005. [Google Scholar]
- 18.Wood BL. Ten-color immunophenotyping of hematopoietic cells Curr. Protoc. Cytom. 2005 doi: 10.1002/0471142956.cy0621s33. Chapter 6: Unit 6.21. [DOI] [PubMed] [Google Scholar]
- 19.Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 29(9):1190–1197. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. [PubMed] [Google Scholar]
- 21.Bacher U, Kern W, Schoch C, Schnittger S, Hiddemann W, Haferlach T. Evaluation of complete disease remission in acute myeloid leukemia: a prospective study based on cytomorphology, interphase fluorescence in situ hybridization, and immunophenotyping during follow-up in patients with acute myeloid leukemia. Cancer. 2006;106(4):839–847. doi: 10.1002/cncr.21665. [DOI] [PubMed] [Google Scholar]