Abstract
Altered peptide ligands (APLs) with enhanced binding to MHC class I (MHC-I) can increase the CD8+ T cell response to native antigens, including tumor antigens. Here we investigate the influence of peptide-MHC (pMHC) stability on recruitment of tumor antigen-specific CD8+ T cells through cross-priming. Among the four known H-2b-restricted CD8+ T cell determinants within SV40 large tumor antigen (TAg), the site V determinant (489QGINNLDNL497) forms relatively low-stability pMHC and is characteristically immunorecessive. Absence of detectable site V-specific CD8+ T cells following immunization with wild type TAg is due in part to inefficient cross-priming. We mutated non-anchor residues within the TAg site V determinant that increased pMHC-stability but preserved recognition by both T cell receptor transgenic and polyclonal endogenous T cells. Using a novel approach to quantify the fraction of naïve T cells triggered through cross-priming in vivo, we show that immunization with TAg variants expressing higher-stability determinants increased the fraction of site V-specific T cells cross-primed and effectively overcame the immunorecessive phenotype. In addition, using MHC-I tetramer-based enrichment we demonstrate for the first time that endogenous site V-specific T cells are primed following wild type TAg immunization despite their low initial frequency, but that the magnitude of T cell accumulation is enhanced following immunization with a site V variant TAg. Our results demonstrate that site V APLs cross-prime a higher fraction of available T cells, providing a potential mechanism for high-stability APLs to enhance immunogenicity and accumulation of T cells specific for the native determinant.
Introduction
The T cell response to viral and tumor antigens is typically skewed toward a subset of immunodominant determinants. Such determinants have been the main focus of research for vaccine development and immunotherapy. However, T cells targeting subdominant determinants, which induce fewer responder T cells, can also play a role in the control of tumors (1, 2) and virus infections (3–5). Limited immunological tolerance toward subdominant determinants found in self tumor associated antigens in mice (6–8) and in humans (9) makes them attractive targets for CD8+ T cell-based immunotherapy approaches. Understanding the mechanisms contributing to the subdominant phenotype may facilitate inclusion of T cells targeting a broader repertoire of determinants for vaccination and immunotherapy.
Expression of the TAg oncoprotein from SV40 in primary mouse fibroblasts leads to cell immortalization and transformation in vitro and also serves as a tumor rejection antigen when these cells are injected into immunocompetent mice (10). The CD8+ T cell response to SV40 TAg in C57BL/6 mice is characterized by a reproducible immunodominance hierarchy directed toward four determinants, designated sites I, II/III, IV, and V (11). T cells specific for sites I, II/III and IV are detected following immunization with wild type TAg (wt-TAg) whereas the endogenous site V-specific T cell response is detected only following immunization with TAg variants lacking sites I, II/III and IV or following immunization with a site V minigene-based vaccine (12, 13). We previously found that injection of site V-specific TCR transgenic T cells (TCR-V) into C57BL/6 mice was not sufficient to overcome the weak response to site V in the context of wt-TAg immunization, indicating that precursor frequency is not the sole limiting factor (14). Rather, we found that site V is weakly cross-presented in vivo compared to the more dominant determinants from TAg. Based on previous data showing a kinetic difference in the stability of site V/H-2Db complexes relative to pMHC formed with the other known TAg determinants (13), we proposed that unstable pMHC interactions may contribute to limited cross-presentation and the immunorecessive phenotype of site V.
Amino acid substitutions within weakly immunogenic T cell determinants can lead to improved immunogenicity. Such APLs have been shown to induce higher magnitude CD8+ T cell responses, particularly in the setting of self tolerance and can increase CD8+ T cell-mediated anti-tumor immunity (15–18). APLs with engineered substitutions at TCR contact residues can directly enhance T cell activation by increasing TCR signal transduction (17). A second class of APLs effects residues that improve peptide affinity for MHC-I and/or increase stability of pMHC. The basis for improved immunogenicity of this latter class of APLs may result from structural changes in the pMHC complex that improves TCR/pMHC affinity (19). Alternatively, changes in peptide affinity for MHC class I may promote higher levels of antigen presentation that could lead to more durable T cell/APC interactions and more efficient T cell activation (18, 20). While evidence suggests that a minimal affinity for MHC class I is necessary for a peptide to be immunogenic (21, 22), multiple studies additionally show a strong correlation between pMHC stability (half-life) and immunogenicity (23–25). In fact, a recent extensive study by Harndahl and colleagues provides strong support that pMHC stability can accurately predict immunogenicity (26). Thus APLs containing amino acid substitutions that increase pMHC stability are likely to be more immunogenic, although no consensus has been reached regarding the basis for this effect.
Cell-associated antigens, such as tumor antigens, can induce CD8+ T cell responses by accessing the cross-presentation pathway that is active in professional APCs (27, 28). Thus, pMHC stability could potentially impact the efficiency of cross-priming by influencing the level and/or duration of pMHC cross-presentation. We evaluated the extent to which site V APLs promote increased immunogenicity following expression of variant TAgs in C57BL/6 fibroblasts. Using newly defined APLs and a unique approach to monitor the initial recruitment of naïve TCR transgenic T cells, we find that APLs with enhanced pMHC-stability increase the fraction of naïve TCR transgenic T cells recruited by cross-priming and overcome the immunorecessive nature of site V by the endogenous T cells. In addition, using MHC tetramer-enrichment, we show that wt-TAg immunization induces limited accumulation of endogenous site V-specific T cells and that this response is dramatically enhanced by immunization with APL-expressing TAg.
Materials and Methods
Mice
C57BL/6 (B6), B6.129S2-Tap1tm1Arp (tap1−/−), and B6.PL-Thy1a/CyJ (Thy1.1) mice were purchased from The Jackson Laboratory and bred on-site. TCR transgenic mice specific for the TAg site V (TCR-V) (14) and site I (TCR-I) (29) were described previously. TCR-V mice were bred onto the B6.SJL-PtprcαPepcb/BoyJ (SJL; Taconic Farms) background. All mice were maintained under SPF conditions in the Penn State Hershey animal facility. All studies were performed in accordance with an approved Penn State Hershey Institutional Animal Care and Use Committee protocol.
Synthetic peptides
Peptides were synthesized at the Penn State Hershey Macromolecular Core Facility using Fmoc chemistry. Peptides were dissolved in DMSO then diluted to the appropriate concentration in RPMI 1640 medium with GlutaMAX™ (Invitrogen) and stored at −80° C. Peptides correspond to the SV40 TAg site V (QGINNLDNL; Pep-V), site I (SAINNYAQKL; Pep-I), and a control H-2Db-binding peptide corresponding to influenza virus nucleoprotein 366–374 (ASNENMETM; Pep-Flu). The following site V variant peptides were used: Q489A (AGINNLDNL), G490A (QAINNLDNL), I491F (QGFNNLDNL), N492A (QGIANLDNL), L494A (QGINNADNL), D495A (QGINNLANL) and N496A (QGINNLDAL).
Site-directed mutagenesis
TAg constructs expressing the Q489A mutation were produced via Quikchange II XL (Stratagene) site directed mutagenesis using oligos 5′-TTGCCT TCA GGT GCT GGA ATT AAT AAC CTG GAC-3′ (sense) and 5′-GTC CAG GTT ATT AAT TCC AGC ACC TGA AGG CAA-3′ (anti-sense). G490A substitutions were introduced into SV40 TAg by the Altered Sites II mutagenesis procedure (11) (Promega) alone or in combination with MHC anchor residue alanine substitutions inactivating sites I, II/III and IV. The mutagenic oligonucleotide encoding G490A was 5′-TTG CCT TCA GGT CAG GCT ATT AAT AAC CTG GAC-3′. Determinants I, II/III and IV were inactivated by alanine substitutions using the mutagenic oligonucleotides N210A; 5′-GTG TCT GCT ATT AAT GCT TAT GCT CAA AAA TTG-3′, N227A; 5′-ATT TGT AAA GGG GTT GCT AAG GAA TAT TTG ATG-3′, and F408A; 5′-TCA GTG GTG TAT GAC GCT TTA AAA TGC ATG GTG-3′.
Cells
Cell lines are summarized in Table I and are referred to in the text using the ‘common name.’ Q489A, TAP Q489A, and TAP G490A cell lines were produced by immortalization of primary mouse kidney cells from B6 or TAP1−/− mice following transfection with TAg plasmids by the calcium phosphate precipitation method (30). G490A and G490A V-only cells were derived using the Fugene 6 method of transfection (Roche). TAg transformed cell lines were maintained in DMEM supplemented with 5% or 10% FCS and 100 U penicillin/ml, 100 μg streptomycin/ml, 100 μg kanamycin/ml, 2 mM L-glutamine, 10 mM HEPES buffer, 0.075% (w/v) NaHCO3. Primary site V-specific T cell cultures were initiated by incubation of 1×107 RBC-depleted spleen cells from previously immunized B6 mice with 5×105 gamma-irradiated (100 Gy) K-3,1,4 cells per well of 12-well plates. Thereafter, T cell cultures were passed every 7 days into fresh wells containing stimulator cells with 5 U/ml of rh-IL-2 (Amgen). TCR-V and TCR-I T cell lines were initiated in-vitro with RBC-depleted spleen cells from naïve TCR-V and TCR-I mice. At the time of primary culture, 100 nM synthetic site V or site I peptide was used for stimulation. TCR-V and TCR-I cultures were maintained as described above by restimulation with irradiated WT-TAg cells. The site I specific clone K-11 (33), site II/III specific CTL clone K-19 (33) and site IV-specific CTL line SV2168T (34) were maintained as described previously. T cells were used for assays on days 4–5 of the growth cycle. Lymphocytes and TAP2-deficient RMA/s cells (35) were maintained in complete RPMI 1640 medium with GlutaMAX™ supplemented with 10% FBS, 100 U/ml penicillin, 100 ug/ml streptomycin, 25ng/ml sodium pyruvate, 50 μM β-Mercaptoethanol, and 10 mM HEPES.
Table I.
Cell lines used in this study
| Common name | Background | Transforming agent | Determinants expressed | Formal name | Reference |
|---|---|---|---|---|---|
| WT-TAg | B6 | SV40 | I, II/III, IV, V | B6/WT-19 | (30) |
| V-Only | B6 | pSLM-361-11 | V | B6/T 116A1 Cl-C | (12) |
| Q489A | B6 | pAMW 4-8 | I, II/III, IV, Q489A | Q489A WT Bulk 4-8 | This Study |
| G490A | B6 | pMS02-7 | I, II/III, IV, G490A | G490A WT C1.1 | This Study |
| G490A V-Only | B6 | p413S06 C3-13 | G490A | G490A V-only D2.2 | This Study |
| Null | B6 | pLMTS364-1 | None | B6/122B1 | (12) |
| TAP WT | TAP1−/− | pPVU0 | I, II/III, IV, V | TAP WT-DNA | (14, 31) |
| TAPV-Only | TAP1−/− | pSLM-361-11 | V | TAP(361-11)c | (14) |
| TAP Q489A | TAP1−/− | pAMW 4-4 | I, II/III, IV, Q489A | Tap−/− Q489A WT 4-4 B1 | This Study |
| TAP G490A | TAP1−/− | pMS02-7 | I, II/III, IV, G490A | Tap−/− G490A WT A1 | This Study |
| K-3,1,4 | B6 | V | B6/K-3,1,4 | (32) | |
| K-1,4,5 | B6 | None | B6/K-1,4,5 | (32) |
Measurement of relative affinity and H-2Db complex stability
Assays to estimate relative affinity have been previously described (36, 37). RMA/s cells (38) were grown overnight at 26° C, 5% CO2 and then 5×105 cells were plated per well of a 96-well round-bottom plate and the indicated concentrations of peptide added. Cultures were incubated at 37° C, 5% CO2 for 4 hours and then stained with 28-14-8s anti-Db mAb for 30 minutes at 4° C. Bound primary antibody was detected by addition of goat anti-mouse IgG-FITC (MP Biomedicals) for 30 minutes at 4° C. Samples were collected on a FACSCanto instrument and the Geometric Mean of FITC staining determined using Flowjo Software (Tree Star, Inc.). To calculate the % maximum mean fluorescence intensity (MFI), the following formula was used: (MFIpeptide −MFIno peptide)/(MFIHi−MFIno peptide) × 100, where MFIHi = the peptide concentration where the highest MFI was achieved. Data were plotted using Prism software and the relative affinity (Kd) of peptide binding to H-2Db was estimated using nonlinear regression analysis fitted to one-site saturation binding kinetics. The relative decay rate of synthetic peptide-stabilized H-2Db complexes on RMA/s cells was determined as previously described (13).
Cytotoxicity assay
Cytotoxicity assays were performed as previously described (39). Briefly, T-25 flasks of target fibroblasts were labeled overnight with 200 μCi of sodium 51chromate. Target cells were treated with trypsin in versene and 104 cells were seeded into 96-well V-bottom plates. Effector cells were plated with targets in triplicate at the indicated effector to target ratio (E:T) in a total volume of 200 μl. The plates were incubated for 4 hours at 37°C and 5% CO2 and then centrifuged to pellet cells and debris. 100 μl of supernatant was removed from each well. Percent specific lysis was determined as described previously (39). Effector cell lines specific for sites I, II/III, IV, and V were as follows: TCR-I cells, clone K-19, line SV2168T, and TCR-V cells.
Immunizations
Cell lines for immunization were grown under uniform conditions in a single batch for each experiment and were frozen at a concentration of 2 × 107 cells/ml at −80°C in DMEM supplemented with 10% FBS and 5% DMSO. On the day of immunization, cells were quickly thawed at 37°C, washed twice with PBS, and resuspended at a concentration of 108 cells/ml in PBS. Mice were immunized i.p. with 5 × 107 cells (0.5 ml). Peptide immunizations were performed by subcutaneous injection at the base of the tail with 100 μg of Pep-V, Pep-Q489A, or Pep-I and 160 μg of the HBV core helper peptide 128–140 in 100 μl of IFA as previously described (40). Immunization with recombinant vaccinia viruses rVV-ES-V and rVV-ES-I (13) was carried out by i.v. injection of 107 PFU in 0.2 ml of PBS into the tail vein.
Fine specificity measurements and intracellular cytokine staining
Fine specificity of responding T cells was determined following primary immunization with 5×107 of the indicated cells followed by a booster immunization 21 days later with either B6/V-only cells or B6/G490A V-only cells as indicated. After eight days, splenocytes were restimulated ex vivo with 1 μM site V peptide or each of the site V single amino acid substitution analogues and the percent of CD8+ cells producing IFNγ determined by intracellular cytokine staining and flow cytometry. The percentage of wt site V response was calculated according to the formula (% response to analogue / % response to site V) × 100.
To detect the intracellular production of IFN-γ, 1–2×106 spleen cells or cultured T cells were stimulated for 5–6 hours with synthetic peptides in a solution of 1 μg/ml brefeldin A as previously described (12). For assays using TAg transformed cells as stimulators, T cells lines were mixed 1:1 with stimulator cells (3×105 cells each) for 1 hour followed by addition of 1 μg/ml brefeldin A and incubation at 37° C, 5% CO2 for a further 5 hours. Intracellular IFN-γ was detected using the Cytofix/Cytoperm kit (BD Pharmingen) according to the manufacturer’s instructions.
In-vivo proliferation assays
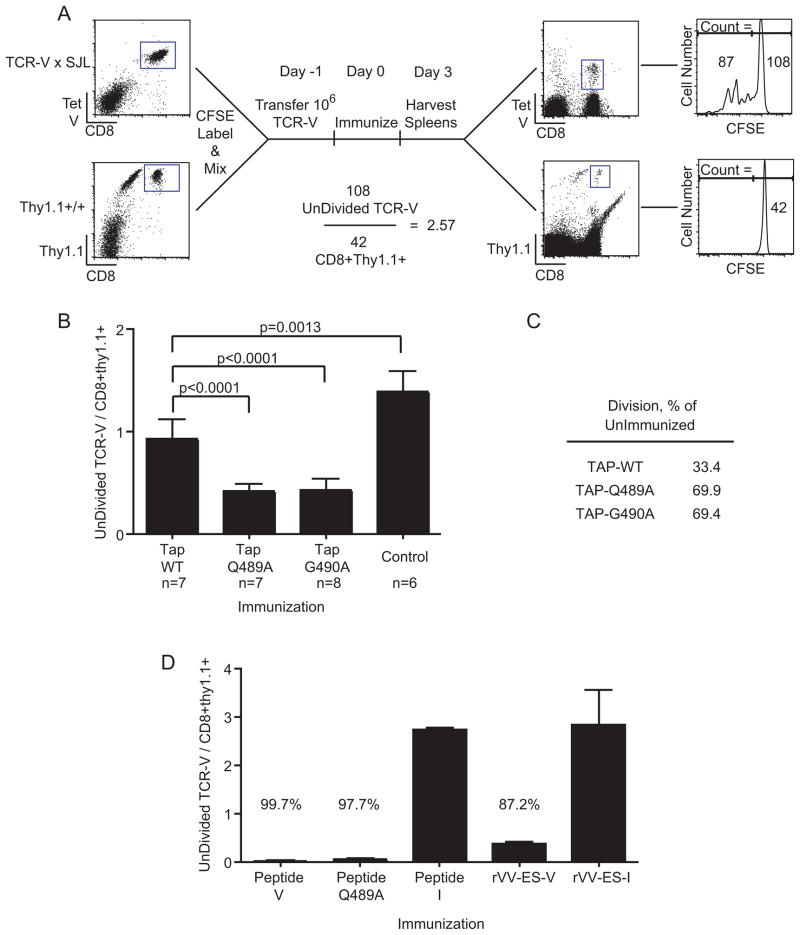
Single cell suspensions of lymphocytes from TCR-V/SJL-transgenic mice and/or Thy1.1+ mice were labeled with 5μM CFSE (Molecular Probes) as previously described (14). Mice received 1×106 (unless otherwise noted) CD8+/Tet-V+ cells by i.v. injection and the following day were immunized. 3–7 days following immunization, spleens were harvested and TCR-V cells were visualized by FACS. For “Thy1.1-spike” experiments, total lymphocytes from TCR-V/SJL and Thy1.1+ mice were mixed prior to transfer. The number of undivided (UD) TCR-V cells (CFSE-Hi TCR-V) and UD-Thy1.1 cells (CFSE-Hi CD8+thy1.1+) was determined and a ratio was calculated according to the equation UD-TCR-V / UD-Thy1.1 to define the fraction of TCR-V cells that had not undergone initial cell division.
Flow cytometry and antibodies
Ex-vivo staining of lymphocytes was performed on single cell suspensions from spleens created by pushing the particulate tissues through a stainless-steel 60 mesh screen (Bellco Glass Inc.) as previously described (12). All staining was performed in FACS buffer (PBS supplemented with 2% FBS and 0.1% Sodium Azide) and completed at room temperature. Cells were incubated in a 1:100 dilution of rat anti-mouse CD16/CD32 (FC block) for 15 minutes at room temperature then washed 1× with FACS buffer. Fluorophore conjugated antibodies (1:150 dilution) and MHC-tetramer (1:200 dilution) were added to the cells and incubated for 15 minutes at room temperature. Cells were washed 3× with FACS buffer, fixed in 2% paraformaldehyde in PBS and analyzed using a FACScan II, FACSCalibur, FACSCanto or LSR II flow cytometer (BD Biosciences). Unless otherwise noted, 100,000–300,000 live events were recorded based on FSC-A/SSC-A gating, and data were analyzed using FlowJo software. MHC class I tetramers specific for H-2Db/TAg site V (Tet-V) or H-2Db/TAg site I (Tet-I) were produced and characterized as described previously (12). The following antibodies were purchased from BD Biosciences or eBiosciences: CD8α (clone 53-6-7), CD45.1 (clone A20), Thy1.1 (clone H1S51), Thy1.2 (53-2.1), IFN-γ (clone XMG1.2), DUMP [CD19 (MB19-1), MHCII (M5/114.15.2), F4/80 (BM8), CD11b (M1/70), and CD4 (GK1.5)].
Tetramer-based enrichment of site I- and site V-specific CD8+ T cells from mice
At least 10 days prior to the experiment, positive control mice were immunized with WT-TAg or Q489A cells, and at least 1 day prior to the experiment mice received 105 naïve TCR-V cells i.v. Immunization plus adoptive transfer of TCR-V cells in positive control mice yielded detectable populations of both site I- and site V-specific T cells to facilitate identification of tetramer positive cells during gating. A variation of previously published protocols (41–43) was utilized for tetramer enrichment. Briefly, on the day of enrichment, the spleen and the superficial cervical, brachial, inguinal, lumbar, and mesenteric lymph nodes were harvested. Organs were mechanically disrupted, and the resulting suspension was incubated in RPMI-1640 supplemented with 150 U/ml collagenase (Gibco, 17018-029) and 100 U/ml recombinant DNAse I (Roche, 04 536 282 001) at 37°C for 30 minutes with rocking. Tissues were passed through a 60-mesh screen, RBCs were lysed using tris-ammonium-chloride and the cells were washed once with FACS buffer. Cells were resuspended in 0.75 ml of FACS buffer, and co-stained with Tet-V-PE and Tet-I-APC for 30 minutes at room temperature in FACS buffer containing Fc block.
MHC tetramer stained cells were washed twice with 5 ml of MACS buffer (PBS supplemented with 0.5% BSA and 2 mM EDTA), and stained with 0.1 ml each anti-PE and anti-APC MicroBeads (Miltenyi Biotech) in a total volume of 1 ml for 15 minutes at 4°C. Cells were washed with 10 ml MACS buffer and passed through a cell strainer (70-mesh, Becton Dickinson or Fischer Brand) before centrifugation. Cells were resuspended in 3 ml MACS buffer and passed over an LS column (Miltenyi Biotech) at 4°C. Columns were washed 3× with 3 ml MACS buffer, and cells bound to the column were eluted twice with 5 ml of MACS buffer. Eluted cells were washed with MACS buffer.
Eluted cells were stained in a 96-well plate with the following antibodies: unlabeled αCD16/CD32, FITC DUMP – (antibodies to CD19, MHCII, F4/80, CD11b, and CD4), anti-CD8-PerCP Cy5.5, anti-Thy1.2-PE-C7, Tet-V-PE, and Tet-I-APC for 15 min at RT. Cells were washed 3× with FACS buffer and resuspended in 2% PFA. All samples were run on a BD FACSCantoII or LSRII. The entire eluted cell fraction was acquired. First, live cells were determined by FSC-A/SSC-A gating followed by gating on FITC-negative/CD8+ cells and then thy1.2+ cells. Dot plots were displayed as Tet-I+ vs. Tet-V+ cells. Tetramer positive populations from positive control mice were used as a guide to set up the tetramer+ gates.
Statistics
All data are displayed as mean ± s.d. P values were evaluated using an unpaired Student’s t-test unless otherwise noted.
Results
Identification of site V analogues with increased pMHC stability
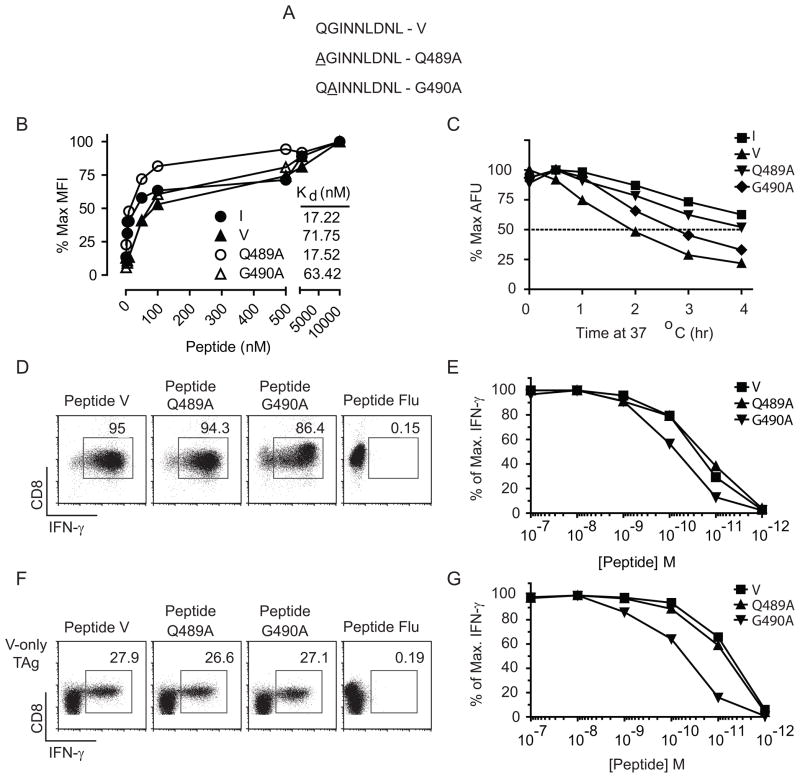
Immunogenicity requires that a peptide determinant have a minimum affinity for the presenting MHC molecule (21, 22). In addition, several studies suggest that the stability of pMHC complexes is predictive of the immunogenicity of CD8+ T cell determinants (23–26) and has been directly linked to the establishment of immunodominance hierarchies for CD4+ T cells (44). We sought to identify site V APLs that could form pMHC with increased stability. Despite the formation of relatively unstable pMHC complexes, the wt site V determinant, encompassing TAg residues 489–497 (QGINNLDNL), harbors the canonical Db anchor residues at position (P) P5-N and P9-L (Fig. 1A). Thus to stabilize pMHC interactions, we considered the role of non-anchor residues to enhance pMHC binding without disrupting TCR recognition (36, 45, 46). P3-I and P8-N were previously shown to be critical for recognition by site V-specific CTL (47) and as demonstrated in other systems, P3, P4, P6, P7, and P8 can protrude from the MHC binding cleft and/or serve as TCR contact residues (48, 49). Thus, we identified positions P1 and P2 for substitution with alanine residues. The resulting analogues are designated Q489A and G490A, respectively. We evaluated both the relative binding affinity of the Q489A and G490A peptides for H-2Db (Fig. 1B) as well as the stability of H-2Db molecules formed with each peptide (Fig. 1C). To determine the relative affinity of peptide binding to H-2Db, TAP2 mutant RMA/s cells were incubated overnight at 26° C and then aliquots of cells mixed with increasing concentrations of each peptide. After 4 hours at 37° C, cells were stained for H-2Db surface expression and the relative affinity determined (Fig. 1B). The results indicate that peptide Q489A has an increased relative affinity for Db (4-fold) while that of G490A is similar to wild type site V. Thus, only the Q489A analogue showed an increased relative affinity, which was similar to the dominant site I peptide.
Figure 1.
Site V substitution mutants, Q489A and G490A, enhance pMHC-stability and conserve recognition by site V-specific T cells. (A) Sequence of synthetic peptides representing the Q489A and G490A mutants. (B) Relative affinity of synthetic peptides bound to H-2Db was determined by incubation of RMA/s cells overnight at 26° C followed by addition of the indicated concentrations of each peptide to aliquots of RMA/s cells and incubation at 37° C for 4 hours. Cells were stained for H-2Db expression and the data expressed as the % maximum mean fluorescence intensity of Db expression for each peptide. Relative affinity (Kd) was determined as described in the Materials and Methods using nonlinear regression analysis. (C) RMA/s cells were incubated with the indicated peptides over night at 29°C, excess peptide was removed by washing and the cells were incubated at 37°C for the indicated times. Cells were held on ice prior to staining for H-2Db. Data are presented as percent of maximum arbitrary fluorescence units (AFU) for each peptide. Cultures of TCR-V T cells (D–E) or polyclonal endogenous site V-specific T cells (F–G) were stimulated with 10−7M (D, F) or 10−7– 10−12M (E, G) peptide in the presence of BFA, and intracellular IFN-γ was assessed by flow cytometry. Values represent the percent of CD8+ T cells producing IFN-γ (D, F) or the percent of maximum CD8+IFN-γ+ cells for each peptide (E, G). All results are representative of at least two independent experiments in which similar results were obtained.
The stability of peptide loaded Db complexes was estimated by measuring the decay of pMHC from RMA/s cells following removal of excess peptide. Complexes formed with the Q489A, G490A and site V peptides had half-lives of approximately 4, 2.75, and 2 hours, respectively (Fig. 1C). The site I peptide was the most stable with a half-life greater than 4 hours. These results indicate that both Q489A and G490A formed more stable pMHC than the wild type site V peptide, although only Q489A peptide showed a measurable change in relative affinity for H-2Db.
We next evaluated cross-recognition of Q489A and G490A by site V-specific T cells. T cell lines derived from TCR-V (Fig. 1D & E) or polyclonal site V-specific T cells isolated from immunized mice (Fig. 1F & G) were stimulated with decreasing concentrations of site V, Q489A, G490A or control Flu (NP366–374) peptide. At the highest concentration, site V, Q489A and G490A peptides stimulated a similar percentage of cells to produce IFN-γ for both TCR transgenic and polyclonal T cells (Fig. 1D and F). Wild type site V and Q489A peptides demonstrated similar functional avidity for clonal TCR-V (Fig. 1E) and polyclonal (Fig. 1G) T cell lines at all peptide dilutions, indicating no discernable difference in T cell recognition. Functional avidity for G490A was reduced between five- and ten-fold for TCR-V and polyclonal T cells, respectively. Overall these results demonstrate that compared with wild type site V, Q489A binds to Db with a higher relative affinity, forms more stable pMHC and is recognized by site V-specific T cells with a similar efficiency. The G490A analogue forms pMHC with moderately increased stability compared to wt site V, but has a similar relative affinity for Db and provides a less efficient target for site V-specific T cells in vitro, indicating that the functional avidity of site V-specific T cells for the G490A variant is reduced. Thus, two site V APLs were identified with increased pMHC stability, but which varied in the other parameters measured.
Q489A and G490A TAg variants are processed and presented for T cell recognition
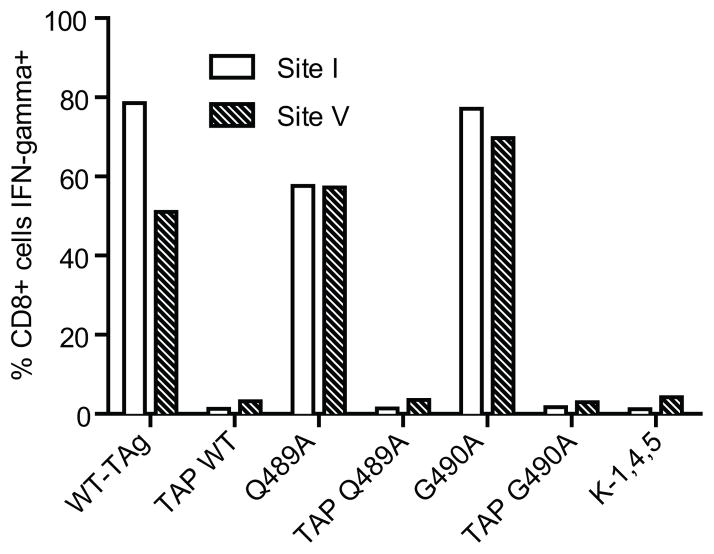
We produced full-length TAg variants containing the Q489A or G490A substitutions in the context of both wild type and site V-only TAg. Primary mouse B6 and tap1 knockout primary kidney cells were transfected with plasmid DNA and immortalized cell lines were derived for each of the constructs except Q489A V-only TAg (see Table I), which was unable to immortalize primary kidney cells. Relative expression of TAg in each cell line was verified by IP/western blot and did not vary by more than 39% (unpublished observations). Incorporation of the particular site V variant in each cell line was verified by sequencing of PCR amplified genomic DNA (unpublished observations). We confirmed that the TAg determinants were processed and presented from the Q489A and G490A TAg variants using in vitro recognition by site I and site V-specific CTL lines. T cells produced IFN-γ in response to B6-derived, but not tap1 knockout-derived cell lines (Fig. 2), demonstrating direct presentation of the expected determinants by the B6-derived cell lines. Furthermore, CTL killing of Q489A or G490A B6-derived cell lines was similar to that of wild type TAg-expressing cells and the TAg variants did not interfere with target cell killing by site I-, II/III-, and IV-specific T cells (Supplemental Fig. 1). Thus, Q489A and G490A are processed and presented in a TAP-dependent manner and the B6-derived cells present the expected array of TAg determinants.
Figure 2.
Direct presentation of Q489A and G490A determinants by B6-derived cells. B6-derived and tap1 knockout-derived cell lines expressing wt TAg or TAg variants expressing the Q489A or G490A substitutions were tested for their ability to stimulate IFN-γ production by either site I-specific CTL clone K-11 or bulk polyclonal site V-specific CTL. K-1,4,5 cells lack expression of the four known H-2b-restricted determinants. Cells were stained for intracellular IFN-γ production and analyzed by flow cytometry.
Immunization with Q489A or G490A induces endogenous site V-specific T cell responses
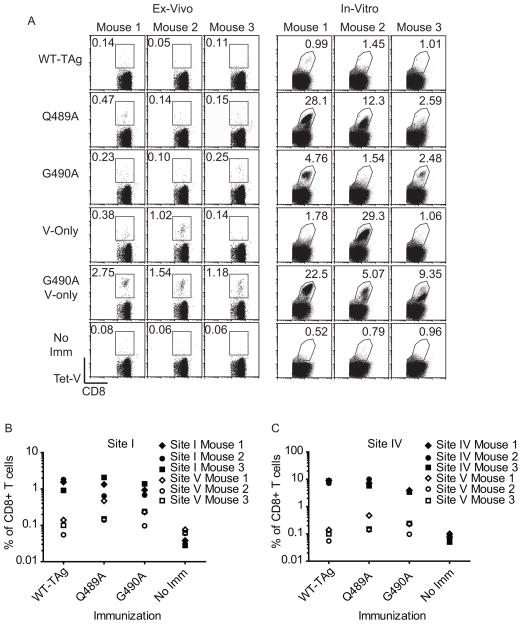
Endogenous site V-specific T cell responses have not previously been detected following immunization with TAg-expressing cells that encode both site V and the dominant TAg determinants. To test the immunogenicity of the Q489A and G490ATAg’s, mice were immunized with WT-TAg, V-only, Q489A, G490A or G490A V-only B6-derived cell lines (Fig. 3A, left panels). Discernable tetramer V+ CD8+ T cells were observed for one of 3 mice and two of three mice immunized with Q489A and G490A, respectively. Two of three mice had detectable site V-specific CD8+ T cell responses following immunization with V-only cells. While previous studies had used pooled lymphocytes from V-only immunized mice (12), we consistently found using individual mice that an average of one in three mice could mount a detectable ex vivo response to site V (data not shown). In contrast, immunization with G490A V-only cells produced strong ex-vivo responses in all mice ranging from 1.18% to 2.75% of CD8+ T cells (Fig. 3A, left panels), indicating that the combination of the G490A analogue and the absence of the dominant determinants was optimal for the induction of site V cross-reactive CD8+ T cells. No significant accumulation of site V-specific T cells was detected in WT-TAg-immunized or unimmunized mice. We observed no discernable relationship between the magnitude of the site V-specific T cell response and the site I- or IV-specific T cell response in individual mice (Fig. 3B and Fig. 3C, respectively), indicating that the site V variants did not compromise the response to the dominant determinants and likewise the detection of site V-specific T cells was not facilitated by reduced responses to the dominant determinants.
Figure 3.
Q489A and G490A prime endogenous site V-specific T cell responses in the presence of the dominant TAg determinants. (A–C) B6 mice were immunized with the indicated B6-derived cell lines. 10 days following immunization, spleens were harvested and cells were stained with anti-CD8 and site V MHC-tetramer. The percentage of endogenous site V-specific CD8+ T cells was assessed ex-vivo or following two rounds of in-vitro restimulation with K-3,1,4 cells. (A) Values indicate the percent of CD8+ T cells that are site V MHC-tetramer positive. For comparison purposes, the data for site I (B) and site IV (C) are plotted along with the CD8+Tet-V+ T cell response to site V. Each data-point represents an individual mouse, and the open and closed paired symbols represent data from the same animal. Results are representative of at least two independent experiments with three mice per group.
To discern whether low numbers of site V-specific T cells were primed in some immunized mice, splenocytes were restimulated in vitro with K-3,1,4 cells, which express a TAg variant lacking sites I, II/III and IV. In general we found that mice with detectable ex-vivo Tet-V+ cells demonstrated marked expansion of T cells in culture (Fig. 3A, right panels), with the exception of mouse 1 immunized with V-only TAg. Additionally, mouse 2 from the Q489A-immunized group showed accumulation of a prominent population of Tet-V+ cells despite no detection ex-vivo. Consistent with previous studies, site V-specific T cells did not expand from WT-TAg-immunized mice or from unimmunized mice following in-vitro culture, indicating that the expansion of site V-specific T cells was not due to in-vitro priming. These data demonstrate that Q489A and G490A are immunogenic when co-expressed with the dominant TAg determinants, overcoming the immunorecessive phenotype of site V. Even so, the site V-specific response remained quantitatively subdominant to both site I and site IV.
Site V-reactive T cells primed by Q489A and G490A have a similar phenotype to those primed by wild type site V
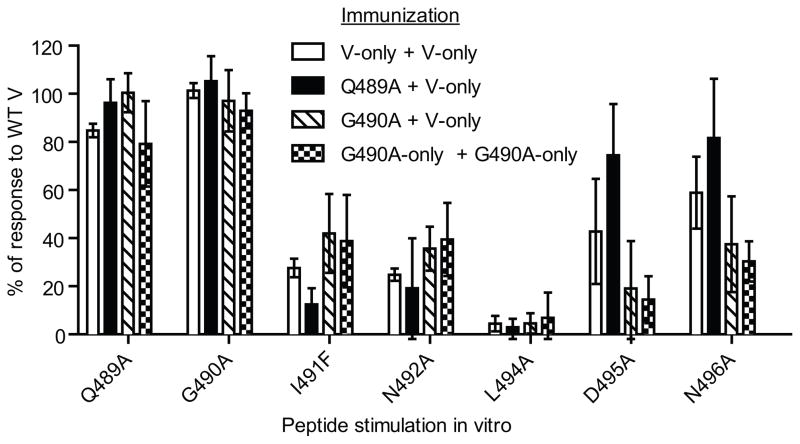
The increased immunogenicity of Q489A and G490A could be explained by recruitment of additional T cell clones. To investigate whether an overall change in the site V-reactive population occurs following immunization with Q489A and G490A, we evaluated both the fine specificity of the responding site V-reactive T cells and the TCR beta chain usage by these T cells. Since only low levels of T cells can be detected ex vivo after primary immunization with Q489A and G490A cells (Fig. 3), we used a prime/boost approach in which mice were first immunized with Q489A or G490A cells followed by booster immunizations with V-only cells to expand any site V cross-reactive T cells. Some mice received prime/boost with either V-only cells or G490AV-only cells for comparison. Ex vivo analysis of responding cells was performed at day 8 post-boost, prior to the time when CD8+ T cell responses to primary site V-only immunization can be detected. CD8+ T cell responses were tested against a panel of site V variant peptides containing single amino acid substitutions at all positions except for N493 and L497, which serve as the primary anchor residues and for which substitutions interrupt peptide binding. CD8+ T cells from each of the four groups of mice showed similar fine specificities (Fig. 4), with substitutions at Q489 and G490 having the least impact, residue L494 having the greatest impact and the remaining 4 residues having intermediate impacts on T cell recognition. For the G490A and G490A V-only immunized groups there was a trend toward greater impact for substitutions at D495 and N496, but these changes were not statistically different from the V-only immunized group. These results indicate that the fine specificity of site V-reactive T cells is maintained following priming with the Q489A and G490A variants.
Figure 4.
Fine specificity of CD8+ T cells primed with Q489A and G490A is similar to those primed against wild type site V. Groups of three C57BL/6 mice were immunized i.p. with the indicated B6-derived cell lines and rested for three weeks. Mice received booster vaccinations with either V-only cells or G490A V-only cells. On day 8 splenocyte responses to site V wt and variant peptides containing single amino acid substitutions were tested ex vivo using intracellular staining for IFN-γ production by CD8+ cells. Data are presented as the percentage of response to the wt site V peptide. All mice were tested individually and the error bars represent standard deviation. No statistically significant differences were observed between the immunization groups. The data are representative of two independent experiments in which similar results were obtained.
We also broadly determined the TCRβ chain usage of site V-reactive T cells derived from mice primed with wild type site V or the site V variants. We first compared the profiles of site V-reactive CD8+ T cells that accumulate following a prime and boost with either V-only cells or G490A V-only cells, since these immunizations induce higher levels of T cells facilitating direct ex vivo analysis. All mice showed dominant accumulation of Vβ7+ T cells, with some mice in both groups also utilizing Vβ2 (supplemental Fig. 2A). One mouse in the G490A V-only group had a minor population of Vβ17+ cells, which was not observed in the V-only-immunized mice. We also evaluated TCRβ expression by site V-reactive CD8+ T cells expanded in vitro from mice initially primed with V-only or Q489A cells (supplemental Fig. 2B). In vitro expansion was necessary due to the limited accumulation of cells following immunization with Q489A. This analysis also revealed predominant accumulation of Vβ7+ T cells in the V-only and Q489A groups. Taken together, the fine specificity and TCRβ chain analyses suggest that the responsive repertoire of T cells is similar following priming with wild type site V compared to the site V variants. Although we cannot exclude CDR3 differences among the responding clones, the results indicate that site V-reactive TCRs use a restricted set of TCRβ chain families.
Q489A and G490A variants enhance cross-priming of TCR-V T cells
Previous evaluation of mechanisms regulating the immunogenicity of site V indicated that the wild type determinant is inefficiently cross-presented, resulting in proliferation of only a fraction of adoptively transferred naïve TCR-V T cells (14). To determine whether the site V variants would promote more efficient triggering of TCR-V T cells through cross-priming, we developed an assay to quantify the fraction of cells remaining undivided following immunization. In this approach, naïve CFSE-labeled TCR-V cells are co-transferred with CFSE-labeled spleen cells from naïve Thy1.1+ mice prior to immunization (Fig. 5A). Since all mice received the same ratio of TCR-V to CD8+Thy1.1+ cells, and the Thy1.1+ cells did not divide following immunization (Fig. 5A, bottom right panel), the CFSE-hi CD8+Thy1.1+ population provides an internal reference to evaluate changes in the undivided TCR-V population. In this way the frequency of cells that initially proliferate can be quantified independent of the expansion and survival of the divided cells. Data are represented as # undivided CFSE-hi TCR-V cells / # CFSE-hi Thy1.1+CD8+ cells such that a reduced ratio indicates more naïve cells are triggered.
Figure 5.
A greater fraction of naïve TCR-V cells undergoes proliferation in response to Q489A or G490A. (A–D) Total CFSE labeled splenocytes from TCR-V mice and Thy1.1 congenic mice were mixed together (Thy1.1-spike) and aliquots containing 1×106 TCR-V T cells were transferred into B6 mice. The following day, mice were immunized with the indicated cell lines (A–C) or with the indicated peptide or recombinant vaccinia virus expressing ER-targeted minigene products (D). Three days following immunization, splenocytes were stained with Tet-V, anti-CD8 and anti-Thy1.1. The ratio of CFSE-Hi “UnDivided” TCR-V / CFSE-Hi CD8+Thy1.1+ cells was calculated as in the example shown in (A). (B) Results show the fraction of undivided TCR-V cells in each immunization group. The control group consists of unimmunized mice or mice immunized with Null cells. (C) The percent of naive T cells that divided was calculated by the formula 100*(1-(I/U)) where I = the mean ratio from a group of immunized mice and U = the mean ratio from the group of unimmunized mice. (B–C) Results from two independent experiments were normalized and combined. P values were determined by unpaired t-tests. (D) Data are presented as in B and the percentages shown as in C. For mice immunized with peptide, U = the mean ratio from peptide-I immunized mice. For mice immunized with rVV-ES-V, U = the mean ratio from rVV-ES-I immunized mice. The data are representative of two independent experiments with groups of three mice.
To restrict the T cell response to cross-presented antigen, we immunized mice with TAP1−/− cell lines TAP-WT, TAP-Q489A and TAP-G490A. As expected (14), immunization with TAP-WT cells triggered a small fraction of TCR-V cells to divide (Fig. 5B). Immunization with TAP-Q489A or TAP-G490A cells resulted in significantly more TCR-V cells dividing compared to TAP-WT immunization. In summary, 33.4% of TCR-V cells divided following immunization with TAP-WT, whereas 69.9% and 69.4% of TCR-V cells divided following immunization with TAP-Q489A and TAP-G490A, respectively (Fig. 5C). These results demonstrate that Q489A and G490A cross-prime a higher fraction of TCR-V cells than the wild type TAg.
In contrast to these results, we previously found that almost all TCR-transgenic T cells specific for site I (TCR-I) divided in response to cross-presented antigen following immunization with WT-TAg cells (14) raising the possibility that a subset of TCR-V cells may be incapable of responding to the antigen in vivo. To examine this question, we asked whether bypassing cross-presentation would initiate priming of a higher fraction of the TCR-V donor cells. Mice were immunized with synthetic peptides (40) or recombinant vaccinia virus expressing an ER-targeted minigene (eg. rVV-ES-V) (13). These approaches bypass the need for antigen processing and cross-presentation of antigen from donor cells. The results revealed that almost all of the TCR-V cells underwent division following immunization with peptide V and peptide Q489A or rVV-ES-V but not with control site I immunizations (Fig. 5D). These results rule out the possibility of an unresponsive subpopulation of TCR-V T cells and support the conclusion that cross-priming constitutes a major limitation in the response to TAg site V. Taken together these data reveal that enhanced cross-presentation of site V is associated with increased pMHC stability, but that methods of immunization that bypass cross-presentation are most efficient in activation of TCR-V cells in vivo.
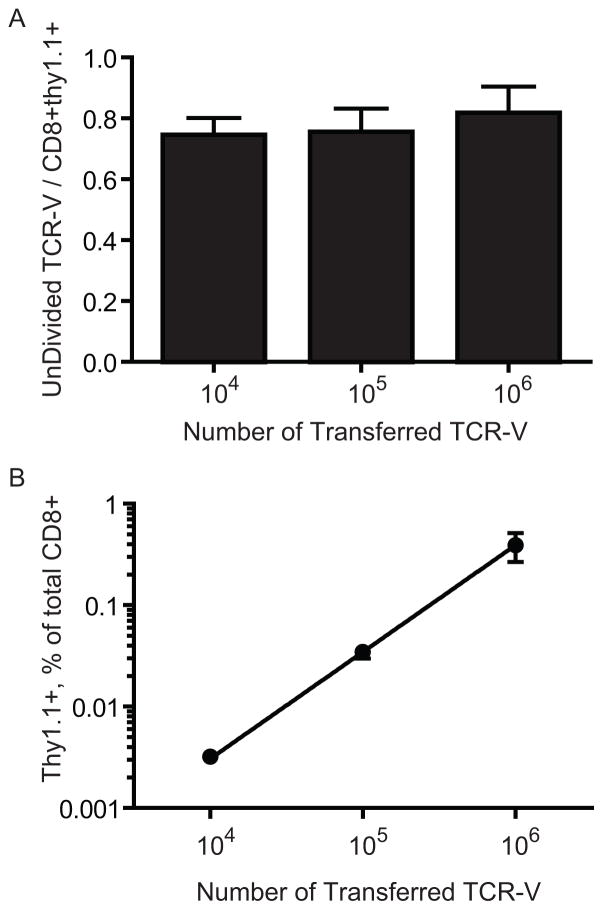
TCR-V precursor number does not influence the efficiency of cross-priming
Incomplete cross-priming of TCR-V cells following immunization with TAg transformed cells could result from transfer of large numbers of TCR transgenic T cells since T cells of the same specificity can compete for available pMHC during priming (50). Thus, we transferred decreasing numbers (106–104) of TCR-V cells plus Thy1.1+ cells into mice prior to immunization with TAP-WT cells. Three days following immunization, CD8+ T cells were enriched from splenocytes and the fraction of undivided TCR-V T cells was measured. The results indicate that a similar fraction of naïve TCR-V cells divided regardless of the number of transferred TCR-V cells (Fig. 6A). This result is not due to altered T cell seeding efficiencies because the percentage of CD8+Thy1.1+ cells detected at each dilution was reduced proportionately (Fig. 6B). These data indicate that the fraction of naive TCR-V cells that is cross-primed remains the same when the number of precursors is between 106–104 per mouse, and suggest that competition among responding TCR-V T cells does not contribute toward the incomplete response. Extrapolating these results suggests that as naïve T cells approach more physiologic numbers [103 and below (41–43)], incomplete cross-priming of available site V-specific T cells remains a limiting factor.
Figure 6.
The number of precursor TCR-V cells does not alter the fraction of naïve T cells that are cross-primed. (A–B) B6 mice received the indicated number of CFSE-labeled TCR-V cells plus Thy1.1-spike as in Figure 5 so that the initial ratio of TCR-V cells to CD8+Thy1.1+ cells remained the same in all groups. Three days following immunization with TAP-WT cells, spleens were harvested and cells enriched using CD8 magnetic beads. The ratio of UnDivided TCR-V / CD8+Thy1.1+ cells (A) or Thy1.1+ cells as a percentage of total CD8+ cells (B) is plotted. The line in (B) is the linear regression of the data (r2=0.8844).
Endogenous site V-specific T cells are primed following immunization with WT-TAg
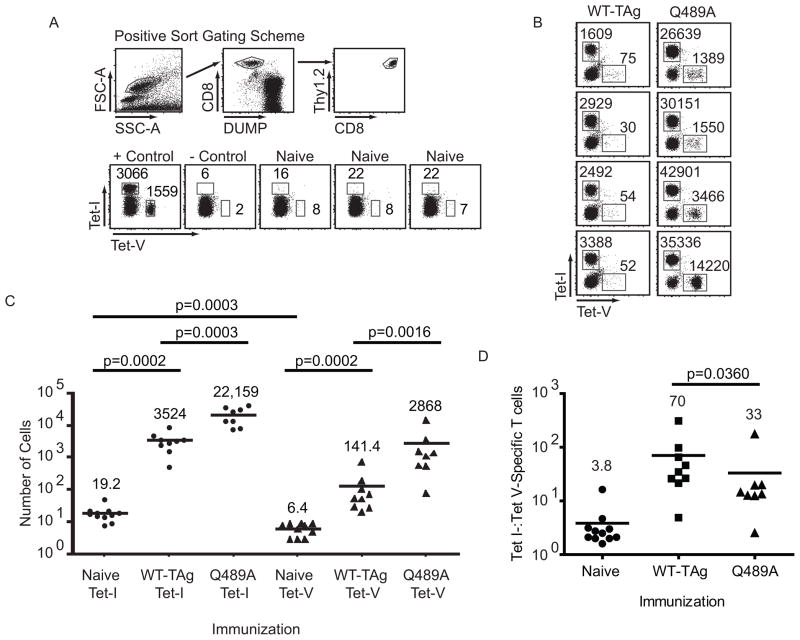
Although an endogenous site V-specific T cell response has not been detected following wt-TAg immunization, our results using transferred TCR-V transgenic cells suggest that limited priming might occur. Lack of detection could be explained by a combination of inefficient cross-priming and low precursor number. We determined the number of site V-specific precursor T cells available in naïve mice using MHC-tetramer-based magnetic enrichment (41–43). Site I-specific T cells were co-enriched from each mouse so that relative levels of site I- and site V-specific T cells could be compared in individual mice. Cells isolated from spleens and lymph nodes of individual mice were stained and gated as described in the Materials and Methods and depicted in figure 7A. The values reported represent the number of cells directly detected per mouse following analysis of the entire cell population. On average, 6.4 site V-specific precursor T cells and 19.2 site I-specific T cells were detected per naïve mouse (Fig. 7C). Thus, naïve mice contained approximately 3:1 Tet-I:Tet-V-specific precursor T cells, indicating that naive site V-specific T cells are initially present at lower numbers than site I-specific T cells.
Figure 7.
Endogenous site V-specific T cells proliferate following immunization with WT-TAg cells. (A–C) Total splenocytes and lymphocytes were stained with Tet-I-APC and Tet-V-PE and isolated by magnetic enrichment. (A) The flow-cytometric gating scheme was based on forward-scatter (FSC-A) versus side-scatter (SSC-A) followed by cells positive for CD8 and negative for the DUMP stain and finally CD8+ Thy1.2+ cells (A, top panels). Representative dot-plots from positive and negative control mice and naïve mice are displayed (A, bottom panels). Positive control mice received immunization with WT-TAg cells and transfer of TCR-V cells prior to the day of the experiment. The negative controls were naïve mice that received no tetramer staining prior to magnetic enrichment. (B) Representative plots of TAg-specific T cells obtained from mice seven days following immunization with WT-TAg or Q489A cells. (C) Data compiled from all experiments. Values indicate the number of cells in each gate (A–B) or the mean value of all Tet-V+ or Tet-I+ cells in all experiments (C). The data are representative of two (B) or three (A) experiments comprising at least three mice per group. (D) The ratio of Tet I+ cells to Tet V+ cells from panel C is plotted for individual mice. Bars indicate mean values. P values were calculated using the Mann-Whitney U-Test.
The ability to detect low numbers of endogenous site V-specific T cells provided an opportunity to readdress whether endogenous site V-specific T cells are primed following WT-TAg immunization and to evaluate the early kinetics of response to the site V analogues. Mice were immunized with WT-TAg cells or Q489A cells and isolated at day seven post immunization. For the first time, we found that endogenous site V-specific T cells accumulated in mice following WT-TAg immunization (Fig. 7B). Our cumulative results demonstrate a 22-fold expansion (141.4 cells per mouse; Fig. 7C) of endogenous site V-specific T cells compared to naïve mice. By comparison, site I-specific T cells expanded 184-fold (3524 cells per mouse) following immunization with WT-TAg, suggesting that site I-specific T cells accumulate at a higher rate over the 7-day period. Immunization with Q489A cells resulted in endogenous site V-specific T cells expanding to 2868 cells per mouse, a 450-fold expansion of the precursor population, representing a ~20 fold (2868/141.4) greater expansion of site V-specific T cells relative to WT-TAg immunization. We observed that Q489A immunization also promoted accumulation of higher numbers of site I-specific T cells (22,159 cells per mouse), suggesting that the Q489A cells may be inherently more immunogenic than WT-TAg cells. Since site I- and site V-specific T cells were co-isolated from individual mice, we were able to directly compare the ratio of site I-and site V-specific CD8+ T cells in naïve mice versus that obtained from mice immunized with WT-TAg or Q489A cells. As shown in figure 7D, the ratio of site I:site V-specific T cells was significantly decreased in Q489A immunized mice compared to mice immunized with WT-TAg cells, indicating that the Q489A substitution results in enhanced in vivo expansion of site V-specific T cells relative to site I-specific T cells. These results suggest that higher accumulation of site V-specific T cells following Q489A cell immunization is not solely due to enhanced immunogenicity of the cell line, but rather that Q489A is more immunogenic. Taken together, our results demonstrate that endogenous site V-specific T cells are present at relatively low levels in naïve mice but expand in-vivo following immunization with WT-TAg cells. T cell accumulation was increased following immunization with Q489A cells at early time points (Fig. 7) and continued to increase at later time points to allow direct ex vivo detection (Fig. 3).
Discussion
Identification of APLs that form stable pMHC-I complexes provides an effective way to enhance determinant-specific immunization for cancer or infectious diseases (18, 20, 51). Although the mechanisms promoting increased immunogenicity of APLs may vary, previous studies indicate that agonist peptides can modify the molecular contacts between the TCR and pMHC, resulting in more effective triggering through the TCR (18, 52). Alternatively, quantitative increases in pMHC on APCs may enhance priming of naïve CD8+ T cells by allowing prolonged T cell/APC interactions (53). The resulting augmented accumulation of T cells is potentially explained by more complete recruitment of the available naïve T cells or through greater expansion and increased survival of primed T cells. In the current study, we provide evidence that APLs with increased pMHC stability both enhance endogenous T cell accumulation and cross-prime a higher fraction of available naïve TCR transgenic CD8+ T cells specific for an immunorecessive determinant. This mechanism may contribute to the overall increase in T cell numbers following immunization, thereby overcoming the immunorecessive phenotype.
A potential consequence of the increased pMHC stability of the site V variants is higher or more durable pMHC presentation on APCs. High-stability-peptides are typically presented in greater numbers than low-stability-peptides (25) due to preferential loading of high-stability peptides by the MHC-I antigen presentation machinery (54). The consequences of increased pMHC presentation for T cell activation have been revealed by intra-vital microscopy studies showing that the number of pMHC presented on pAPCs during priming in the lymph nodes determines the rate at which naïve CD8+ T cells are activated. Specifically, the number of pMHCs presented by DCs inversely correlated with the speed of T cell transition through the three phases of T cell/APC interaction leading to T cell activation (53). Those T cells unable to form prolonged contacts with DCs due to encountering subthreshold antigen levels were unable to transition to the latter stages of T cell activation. Relevant to the current study, the number of pMHCs presented by DCs in vivo was dependent on pMHC stability. Thus, site V analogues that form stable pMHC may trigger a higher proportion of site V-reactive CD8+ T cells due to sustained cross-presentation of increased pMHC complexes.
Differences in pMHC stability can potentially influence both direct presentation by the TAg transformed cells and cross-presentation by APCs. However, the impact on cross-presentation may be more dramatic since antigen capture by DCs is downregulated following initial activation and licensing (55, 56), suggesting that DCs take up a fixed amount of antigen for cross-presentation. This scenario differs from the constant supply of directly presented determinants generated in cells that synthesize the antigen de novo. Shortly following acquisition of antigen, DCs may present a broad range of determinants that later becomes biased toward high-stability pMHC due to rapid loss of low-stability pMHC. Indeed, Yu and colleagues found that pMHC half-life on DCs was directly related to the increased immunogenicity of a self/tumor antigen determinant (51). While the short half-life of site V/pMHC complexes on the cell surface (between 2–3 hours) may be sufficient for immunogenicity, it may limit the proportion of T cells that can achieve sustained signaling through the TCR required for T cell activation (57, 58). This may explain why a sub-population of naïve TCR-V T cells never divides in response to cross-presented TAg.
Changes in pMHC stability or epitope production may influence the number of pMHC complexes available for T cell priming, however increased immunogenicity of the site V variants may additionally be explained by a direct change in TCR/pMHC contacts leading to more efficient triggering through the TCR. For some APLs, amino acid substitutions that increase pMHC stability and immunogenicity do not influence the overall structure of the peptide/MHC complex, indicating that increased immunogenicity was primarily due to formation of more stable pMHC (18, 20). This mechanism may best explain the increased immunogenicity of the Q489A variant, which showed the most dramatic increase in pMHC stability and relative affinity. Alternatively, some APLs with increased pMHC stability alter TCR contacts, resulting in increased affinity of the TCR for the pMHC complex (19). Such a mechanism may explain the increased immunogenicity of the G490A variant that showed a modest increase in pMHC stability without a net increase in the relative affinity for MHC. However, we note that the functional avidity of site V-specific polyclonal T cells and TCR transgenic T cells was not increased for either the Q489A or G490A variant peptides in vitro, suggesting that the variant pMHC do not inherently increase the efficiency of TCR signaling among site V-specific T cells. Further discernment of the contribution of pMHC stability and pMHC conformation toward improved immunogenicity of the site V variants would require structural analysis of the pMHC and pMHC/TCR complex by X-ray crystallography (18, 19).
Increased immunogenicity of the site V variants for the endogenous site V-specific CD8+ T cells could be explained by activation of new clonotypes (59, 60). We found only minor differences in TCRVβ chain family usage among site V-reactive CD8+ T cells induced by wt and Q489A or G490A TAg’s, suggesting that the repertoire is generally restricted to the dominant TCRVβ chains. However, our results leave open the possibility of increased recruitment of T cells utilizing TCRs with unique CDR3 regions within the dominant TCRVβ families. Our data demonstrating that the site V variants prime a higher fraction of naïve TCR transgenic T cells are consistent with the idea that additional site V T cells in the naïve polyclonal repertoire could be recruited.
We previously considered that T cell competition for pAPCs contributes to the immunorecessive phenotype of TAg site V (14). Mice co-immunized with wt-TAg and V-only TAg expressed in separate cells developed T cells specific for both site V and the immunodominant determinants. In contrast, site V-specific T cells were undetectable following immunization with cells co-expressing wt-TAg and V-only Tag. These results suggested that T cells specific for the immunodominant determinants limit priming or accumulation of site V-specific T cells as has been observed in several different systems (61–63). A recent study by Galea and coworkers (64) provides evidence that T cells specific for the dominant TAg site I or site IV determinants compete with T cells specific for site V at the early stages of T cell priming. In addition, they found that modulation of the site IV determinant to reduce the half-life of pMHC complexes alleviated immunodomination over site V when the determinants were co-expressed in tandem in a DNA vaccine. Our observation that enhanced site V/MHC-stability is associated with relieving the immunorecessive phenotype may be explained in part by decreased competition from CD8+ T cells responding to the immunodominant TAg determinants, resulting in more efficient priming or expansion of site V-specific T cells. However, optimal site V-specific responses were best obtained when the G490A variant was expressed in the absence of the dominant determinants, suggesting a continued role for T cell competition.
Although the site V variants were able to produce a detectable endogenous T cell response, site V was not raised to immunodominant status. Our data suggest that if the site V- and site I-specific naïve T cell precursor numbers were equal, site V would remain subdominant due to the limited ability to cross-prime the available precursors. Thus, for site V to move up the immunodominance hierarchy, both an increase in the number of precursors and the proportion of precursors that are triggered would be required. This scenario differs from systems where subdominant CD8+ T cells outnumber their immunodominant counterparts, such that an increase in cross-priming alone might be sufficient to increase immunodominance. For example, La Gruta and colleagues (65) found that the frequency of subdominant Flu determinant-specific CD8+ T cell precursors is significantly higher than that of their immunodominant counterparts. These authors found incomplete recruitment of the available CD8+ T cell clonotypes specific for the subdominant Flu Db-PB1-F262–70 determinant, which has an estimated half-life of ~3 hours (66). In contrast, CD8+ T cells specific for two dominant Db-Flu determinants with half-lives estimated to be ~4 (NP366–374) and 16 (PA224–233) hours (66) underwent more efficient recruitment (65). We hypothesize that naïve T cell recruitment may be nearly complete when pMHC-stability is above a yet undetermined threshold and variably incomplete when pMHC-stability falls below that threshold.
We also show for the first time that site V-specific CD8+ T cells are primed following immunization with wt-TAg-expressing cells. The inability to detect site V-specific T cells in previous studies is likely explained by the limited accumulation of these T cells following immunization, which remain below the limit of detection using standard flow cytometric analysis. Only through MHC tetramer enrichment was this response detected. Considering that T cells were isolated from approximately 1×108 total cells, the frequency of site V-specific T cells detected at 7 days post immunization with WT TAg cells was 1 in 1.4×106. In contrast, the frequency of site V-specific T cells detected following immunization with Q489A cells was approximately 5-fold higher at 1 in 2.9×105. These site V-specific T cells continue to expand over the next several days following immunization with the site V variants while the response in wt-TAg-immunized mice never reaches detectable levels (Fig. 3A). We speculate that site V-specific T cells triggered by wt-TAg may undergo abortive proliferation, while the site V variants induce a more characteristic extended T cell expansion in vivo.
In summary, our results suggest that more complete recruitment of the available naïve T cell population can be achieved through approaches that increase pMHC-stability or bypass cross-presentation. This mechanism may explain the enhanced immunogenicity of some APLs derived from similar subdominant or cryptic determinants and provide a strategy to expand the repertoire of determinants available for control of cancer and infectious diseases.
Supplementary Material
Acknowledgments
The authors thank Jeremy Haley and Melanie Epler for excellent technical assistance and Dr. Satvir S. Tevethia for insightful suggestions. We are grateful to Sandip Savaliya for maintenance of mouse colonies and for the preparation of genomic DNA for sequencing by the Penn State Hershey Molecular Genetics Core. We also thank Nate Scheaffer and Dr. David Stanford from the Penn State Hershey Flow Cytometry Core for their technical expertise and assistance.
Abbreviations used in this paper
- pMHC
peptide-MHC
- MHC-I
MHC class I
- B6
C57BL/6 mouse
- pAPC
professional APC
- TAg
SV40 large T antigen
- TCR-V
site V-specific TCR transgenic
- TCR-I
site I-specific TCR transgenic
- Flu
influenza
- wt
wild type
Footnotes
This work was supported by grant RO1 CA025000 from the National Cancer Institute/National Institutes of Health. A.M.W. was supported by Predoctoral Training Grant T32 CA60395 from the National Cancer Institute/National Institutes of Health.
The authors have no conflicting financial interests.
References
- 1.Ryan CM, Schell TD. Accumulation of CD8+ T cells in advanced-stage tumors and delay of disease progression following secondary immunization against an immunorecessive epitope. J Immunol. 2006;177:255–267. doi: 10.4049/jimmunol.177.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Feltkamp MC, Vreugdenhil GR, Vierboom MP, Ras E, van der Burg SH, ter Schegget J, Melief CJ, Kast WM. Cytotoxic T lymphocytes raised against a subdominant epitope offered as a synthetic peptide eradicate human papillomavirus type 16-induced tumors. Eur J Immunol. 1995;25:2638–2642. doi: 10.1002/eji.1830250935. [DOI] [PubMed] [Google Scholar]
- 3.Frahm N, Kiepiela P, Adams S, Linde CH, Hewitt HS, Sango K, Feeney ME, Addo MM, Lichterfeld M, Lahaie MP, Pae E, Wurcel AG, Roach T, St John MA, Altfeld M, Marincola FM, Moore C, Mallal S, Carrington M, Heckerman D, Allen TM, Mullins JI, Korber BT, Goulder PJR, Walker BD, Brander C. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat Immunol. 2006;7:173–178. doi: 10.1038/ni1281. [DOI] [PubMed] [Google Scholar]
- 4.van der Most RG, Sette A, Oseroff C, Alexander J, Murali-Krishna K, Lau LL, Southwood S, Sidney J, Chesnut RW, Matloubian M, Ahmed R. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J Immunol. 1996;157:5543–5554. [PubMed] [Google Scholar]
- 5.Remakus S, Rubio D, Ma X, Sette A, Sigal LJ. Memory CD8+ T cells specific for a single immunodominant or subdominant determinant induced by peptide-dendritic cell immunization protect from an acute lethal viral disease. J Virol. 2012 doi: 10.1128/JVI.00981-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossmann ME, Davila T, Celis T. Avoiding tolerance against prostatic antigens with subdominant peptide epitopes. J Immunother. 2001;24:237–241. [PubMed] [Google Scholar]
- 7.Schell TD. In vivo expansion of the residual tumor antigen-specific CD8+ T lymphocytes that survive negative selection in simian virus 40 T-antigen-transgenic mice. J Virol. 2004;78:1751–1762. doi: 10.1128/JVI.78.4.1751-1762.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uram JN, Black CM, Flynn E, Huang L, Armstrong TD, Jaffee EM. Nondominant CD8 T cells are active players in the vaccine-induced antitumor immune response. J Immunol. 2011;186:3847–3857. doi: 10.4049/jimmunol.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman RS, Spies AG, Kalos M. Identification of naturally processed CD8 T cell epitopes from prostein, a prostate tissue-specific vaccine candidate. Eur J Immunol. 2004;34:1091–1101. doi: 10.1002/eji.200324768. [DOI] [PubMed] [Google Scholar]
- 10.Tevethia SS, Schell TD. The immune response to SV40, JCV, and BKV. In: Khalili K, Stoner GL, editors. Human Polyomaviruses. John Wiley & Sons, Inc; 2002. pp. 585–610. [Google Scholar]
- 11.Mylin LM, Bonneau RH, Lippolis JD, Tevethia SS. Hierarchy among multiple H-2b-restricted cytotoxic T-lymphocyte epitopes within simian virus 40 T antigen. J Virol. 1995;69:6665–6677. doi: 10.1128/jvi.69.11.6665-6677.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mylin LM, Schell TD, Roberts D, Epler M, Boesteanu A, Collins EJ, Frelinger JA, Joyce S, Tevethia SS. Quantitation of CD8+ T-lymphocyte responses to multiple epitopes from simian virus 40 (SV40) large T antigen in C57BL/6 mice immunized with SV40, SV40 T-antigen-transformed cells, or vaccinia virus recombinants expressing full-length T antigen or epitope minigenes. J Virol. 2000;74:6922–6934. doi: 10.1128/jvi.74.15.6922-6934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu TM, Mylin LM, Schell TD, Bacik I, Russ G, Yewdell JW, Bennink JR, Tevethia SS. An endoplasmic reticulum-targeting signal sequence enhances the immunogenicity of an immunorecessive simian virus 40 large T antigen cytotoxic T-lymphocyte epitope. J Virol. 1998;72:1469–1481. doi: 10.1128/jvi.72.2.1469-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otahal P, Hutchinson SC, Mylin LM, Tevethia MJ, Tevethia SS, Schell TD. Inefficient cross-presentation limits the CD8+ T cell response to a subdominant tumor antigen epitope. J Immunol. 2005;175:700–712. doi: 10.4049/jimmunol.175.2.700. [DOI] [PubMed] [Google Scholar]
- 15.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyall R, Bowne WB, Weber LW, LeMaoult J, Szabo P, Moroi Y, Piskun G, Lewis JJ, Houghton AN, Nikolić-Zugić J. Heteroclitic immunization induces tumor immunity. J Exp Med. 1998;188:1553–1561. doi: 10.1084/jem.188.9.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slansky JE, Rattis FM, Boyd LF, Fahmy T, Jaffee EM, Schneck JP, Margulies DH, Pardoll DM. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000;13:529–538. doi: 10.1016/s1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 18.van Stipdonk MJB, Badia-Martinez D, Sluijter M, Offringa R, van Hall T, Achour A. Design of agonistic altered peptides for the robust induction of CTL directed towards H-2Db in complex with the melanoma-associated epitope gp100. Cancer Res. 2009;69:7784–7792. doi: 10.1158/0008-5472.CAN-09-1724. [DOI] [PubMed] [Google Scholar]
- 19.Chen JL, Stewart-Jones G, Bossi G, Lissin NM, Wooldridge L, Choi EML, Held G, Dunbar PR, Esnouf RM, Sami M, Boulter JM, Rizkallah P, Renner C, Sewell A, van der Merwe PA, Jakobsen BK, Griffiths G, Jones EY, Cerundolo V. Structural and kinetic basis for heightened immunogenicity of T cell vaccines. J Exp Med. 2005;201:1243–1255. doi: 10.1084/jem.20042323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borbulevych OY, Baxter TK, Yu Z, Restifo NP, Baker BM. Increased immunogenicity of an anchor-modified tumor-associated antigen is due to the enhanced stability of the peptide/MHC complex: implications for vaccine design. J Immunol. 2005;174:4812–4820. doi: 10.4049/jimmunol.174.8.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast WM, Melief CJ, Oseroff C, Yuan L, Ruppert J, Sidney J, del Guercio MF, Southwood S, Kubo RT, Chesnut RW, Grey HM, Chisari FV. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 22.Assarsson E, Sidney J, Oseroff C, Pasquetto V, Bui HH, Frahm N, Brander C, Peters B, Grey H, Sette A. A quantitative analysis of the variables affecting the repertoire of T cell specificities recognized after vaccinia virus infection. J Immunol. 2007;178:7890–7901. doi: 10.4049/jimmunol.178.12.7890. [DOI] [PubMed] [Google Scholar]
- 23.van der Burg S, Visseren M, Brandt R, Kast W, Melief C. Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability. J Immunol. 1996;156:3308–3314. [PubMed] [Google Scholar]
- 24.Lipford GB, Bauer S, Wagner H, Heeg K. In vivo CTL induction with point-substituted ovalbumin peptides: immunogenicity correlates with peptide-induced MHC class I stability. Vaccine. 1995;13:313–320. doi: 10.1016/0264-410x(95)93320-9. [DOI] [PubMed] [Google Scholar]
- 25.Levitsky V, Zhang QJ, Levitskaya J, Masucci MG. The life span of major histocompatibility complex-peptide complexes influences the efficiency of presentation and immunogenicity of two class I-restricted cytotoxic T lymphocyte epitopes in the Epstein-Barr virus nuclear antigen 4. J Exp Med. 1996;183:915–926. doi: 10.1084/jem.183.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harndahl M, Rasmussen M, Roder G, Dalgaard Pedersen I, Sørensen M, Nielsen M, Buus S. Peptide-MHC class I stability is a better predictor than peptide affinity of CTL immunogenicity. Eur J Immunol. 2012;42:1405–1416. doi: 10.1002/eji.201141774. [DOI] [PubMed] [Google Scholar]
- 27.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 28.Wolkers MC, Stoetter G, Vyth-Dreese FA, Schumacher TNM. Redundancy of direct priming and cross-priming in tumor-specific CD8+ T cell responses. J Immunol. 2001;167:3577–3584. doi: 10.4049/jimmunol.167.7.3577. [DOI] [PubMed] [Google Scholar]
- 29.Staveley-O’Carroll K, Schell TD, Jimenez M, Mylin LM, Tevethia MJ, Schoenberger SP, Tevethia SS. In vivo ligation of CD40 enhances priming against the endogenous tumor antigen and promotes CD8+ T cell effector function in SV40 T antigen transgenic mice. J Immunol. 2003;171:697–707. doi: 10.4049/jimmunol.171.2.697. [DOI] [PubMed] [Google Scholar]
- 30.Tevethia MJ. Immortalization of primary mouse embryo fibroblasts with SV40 virions, viral DNA, and a subgenomic DNA fragment in a quantitative assay. Virology. 1984;137:414–421. doi: 10.1016/0042-6822(84)90234-4. [DOI] [PubMed] [Google Scholar]
- 31.Cavender JF, Conn A, Epler M, Lacko H, Tevethia MJ. Simian virus 40 large T antigen contains two independent activities that cooperate with a ras oncogene to transform rat embryo fibroblasts. J Virol. 1995;69:923–934. doi: 10.1128/jvi.69.2.923-934.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka Y, Anderson RW, Maloy WL, Tevethia SS. Localization of an immunorecessive epitope on SV40 T antigen by H-2Db-restricted cytotoxic T-lymphocyte clones and a synthetic peptide. Virology. 1989;171:205–213. doi: 10.1016/0042-6822(89)90527-8. [DOI] [PubMed] [Google Scholar]
- 33.Campbell AE, Foley FL, Tevethia SS. Demonstration of multiple antigenic sites of the SV40 transplantation rejection antigen by using cytotoxic T lymphocyte clones. J Immunol. 1983;130:490–492. [PubMed] [Google Scholar]
- 34.Schell TD, Tevethia SS. Control of advanced choroid plexus tumors in SV40 T antigen transgenic mice following priming of donor CD8+ T lymphocytes by the endogenous tumor antigen. J Immunol. 2001;167:6947–6956. doi: 10.4049/jimmunol.167.12.6947. [DOI] [PubMed] [Google Scholar]
- 35.Ljunggren HG, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudrisier D, Mazarguil H, Laval F, Oldstone MB, Gairin JE. Binding of viral antigens to major histocompatibility complex class I H-2Db molecules is controlled by dominant negative elements at peptide non-anchor residues. Implications for peptide selection and presentation. J Biol Chem. 1996;271:17829–17836. doi: 10.1074/jbc.271.30.17829. [DOI] [PubMed] [Google Scholar]
- 37.Yadav R, Yoshimura Y, Boesteanu A, Christianson GJ, Ajayi WU, Shashidharamurthy R, Stanic AK, Roopenian DC, Joyce S. The H4b minor histocompatibility antigen is caused by a combination of genetically determined and posttranslational modifications. J Immunol. 2003;170:5133–5142. doi: 10.4049/jimmunol.170.10.5133. [DOI] [PubMed] [Google Scholar]
- 38.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 39.Deckhut AM, Tevethia SS. Effect of point mutations in the native simian virus 40 tumor antigen, and in synthetic peptides corresponding to the H-2Db-restricted epitopes, on antigen presentation and recognition by cytotoxic T lymphocyte clones. J Immunol. 1992;148:3012–3020. [PubMed] [Google Scholar]
- 40.Schell TD, Lippolis JD, Tevethia SS. Cytotoxic T lymphocytes from HLA-A2.1 transgenic mice define a potential human epitope from simian virus 40 large T antigen. Cancer Res. 2001;61:873–879. [PubMed] [Google Scholar]
- 41.Kotturi MF, Scott I, Wolfe T, Peters B, Sidney J, Cheroutre H, von Herrath MG, Buchmeier MJ, Grey H, Sette A. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol. 2008;181:2124–2133. doi: 10.4049/jimmunol.181.3.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obar JJ, Khanna KM, Lefrançois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazarski CA, Chaves FA, Jenks SA, Wu S, Richards KA, Weaver JM, Sant AJ. The kinetic stability of MHC class II:peptide complexes is a key parameter that dictates immunodominance. Immunity. 2005;23:29–40. doi: 10.1016/j.immuni.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Sigal LJ, Wylie DE. Role of non-anchor residues of Db-restricted peptides in class I binding and TCR triggering. Mol Immunol. 1996;33:1323–1333. doi: 10.1016/s0161-5890(96)00099-5. [DOI] [PubMed] [Google Scholar]
- 46.Sigal LJ, Goebel P, Wylie DE. Db-binding peptides from influenza virus: Effect of non-anchor residues on stability and immunodominance. Mol Immunol. 1995;32:623–632. doi: 10.1016/0161-5890(95)00031-9. [DOI] [PubMed] [Google Scholar]
- 47.Mylin LM, Schell TD, Epler M, Kusuma C, Assis D, Matsko C, Smith A, Allebach A, Tevethia SS. Diversity of escape variant mutations in Simian virus 40 large tumor antigen (SV40 Tag) epitopes selected by cytotoxic T lymphocyte (CTL) clones. Virology. 2007;364:155–168. doi: 10.1016/j.virol.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Velloso LM, Michaëlsson J, Ljunggren HG, Schneider G, Achour A. Determination of structural principles underlying three different modes of lymphocytic choriomeningitis virus escape from CTL recognition. J Immunol. 2004;172:5504–5511. doi: 10.4049/jimmunol.172.9.5504. [DOI] [PubMed] [Google Scholar]
- 49.Meijers R, Lai CC, Yang Y, Liu JH, Zhong W, Wang JH, Reinherz EL. Crystal structures of murine MHC Class I H-2 D(b) and K(b) molecules in complex with CTL epitopes from influenza A virus: implications for TCR repertoire selection and immunodominance. J Mol Biol. 2005;345:1099–1110. doi: 10.1016/j.jmb.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 50.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu Z, Theoret MR, Touloukian CE, Surman DR, Garman SC, Feigenbaum L, Baxter TK, Baker BM, Restifo NP. Poor immunogenicity of a self/tumor antigen derives from peptide MHC-I instability and is independent of tolerance. J Clin Invest. 2004;114:551–559. doi: 10.1172/JCI21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hemmer B, Stefanova I, Vergelli M, Germain RN, Martin R. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signaling events in human T cells. J Immunol. 1998;160:5807–5814. [PubMed] [Google Scholar]
- 53.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, Wong HC, Chakraborty AK, von Andrian UH. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howarth M, Williams A, Tolstrup AB, Elliott T. Tapasin enhances MHC class I peptide presentation according to peptide half-life. Proc Natl Acad Sci U S A. 2004;101:11737–11742. doi: 10.1073/pnas.0306294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West MA, Wallin RPA, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 56.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 58.Mempel TR, Henrickson SE, von Andrian UH. T-cell priming by dendritic cells in lymphnodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 59.Stuge TB, Holmes SP, Saharan S, Tuettenberg A, Roederer M, Weber JS, Lee PP. Diversity and recognition efficiency of T cell responses to cancer. PLoS Medicine. 2004;1:e28. doi: 10.1371/journal.pmed.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baumgartner CK, Ferrante A, Nagaoka M, Gorski J, Malherbe LP. Peptide-MHC class II complex stability governs CD4 T cell clonal selection. J Immunol. 2010;184:573–581. doi: 10.4049/jimmunol.0902107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willis RA, Kappler JW, Marrack PC. CD8 T cell competition for dendritic cells in vivo is an early event in activation. Proc Natl Acad Sci USA. 2006;103:12063–12068. doi: 10.1073/pnas.0605130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grufman P, Wolpert EZ, Sandberg JK, Kärre K. T cell competition for the antigen-presenting cell as a model for immunodominance in the cytotoxic T lymphocyte response against minor histocompatibility antigens. Eur J Immunol. 1999;29:2197–2204. doi: 10.1002/(SICI)1521-4141(199907)29:07<2197::AID-IMMU2197>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 63.Weaver JM, Chaves FA, Sant AJ. Abortive activation of CD4 T cell responses during competitive priming in vivo. Proc Natl Acad Sci USA. 2009;106:8647–8652. doi: 10.1073/pnas.0811584106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galea I, Stasakova J, Dunscombe MS, Ottensmeier CH, Elliott T, Thirdborough SM. CD8+ T-cell cross-competition is governed by peptide MHC class I stability. Eur J Immunol. 2012;42:256–263. doi: 10.1002/eji.201142010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.La Gruta NL, Rothwell WT, Cukalac T, Swan NG, Valkenburg SA, Kedzierska K, Thomas PG, Doherty PC, Turner SJ. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J Clin Invest. 2010;120:1885–1894. doi: 10.1172/JCI41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen W, Pang K, Masterman KA, Kennedy G, Basta S, Dimopoulos N, Hornung F, Smyth M, Bennink JR, Yewdell JW. Reversal in the immunodominance hierarchy in secondary CD8+ T cell responses to influenza A virus: roles for cross-presentation and lysis-independent immunodomination. J Immunol. 2004;173:5021–5027. doi: 10.4049/jimmunol.173.8.5021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.