Abstract
Dietary inclusion of a bacterial meal has recently been shown to efficiently abolish soybean meal-induced enteritis in Atlantic salmon. The objective of this study was to investigate whether inclusion of this bacterial meal in the diet could abrogate disease development in a murine model of epithelial injury and colitis and thus possibly have therapeutic potential in human inflammatory bowel disease. C57BL/6N mice were fed ad libitum a control diet or an experimental diet containing 254 g/kg of body weight BioProtein, a bacterial meal consisting of Methylococcus capsulatus (Bath), together with the heterogenic bacteria Ralstonia sp., Brevibacillus agri, and Aneurinibacillus sp. At day 8, colitis was induced by 3.5% dextran sulfate sodium (DSS) ad libitum in the drinking water for 6 days. Symptoms of DSS treatment were less profound after prophylactic treatment with the diet containing the BioProtein. Colitis-associated parameters such as reduced body weight, colon shortening, and epithelial damage also showed significant improvement. Levels of acute-phase reactants, proteins whose plasma concentrations increase in response to inflammation, and neutrophil infiltration were reduced. On the other, increased epithelial cell proliferation and enhanced mucin 2 (Muc2) transcription indicated improved integrity of the colonic epithelial layer. BioProtein mainly consists of Methylococcus capsulatus (Bath) (88%). The results that we obtained when using a bacterial meal consisting of M. capsulatus (Bath) were similar to those obtained when using BioProtein in the DSS model. Our results show that a bacterial meal of the noncommensal bacterium M. capsulatus (Bath) has the potential to attenuate DSS-induced colitis in mice by enhancing colonic barrier function, as judged by increased epithelial proliferation and increased Muc2 transcription.
INTRODUCTION
Inflammatory bowel disease (IBD) manifests itself by chronic inflammation characterized by acute flares followed by periods of remission. The etiology of IBD remains incompletely understood, but it is generally accepted that a complex interplay between genetic, environmental, and immunological factors contributes to disease initiation and progression (1, 2). Crohn's disease (CD) and ulcerative colitis (UC) are clinically the most serious manifestations of IBD; however, inflammation is frequently present in microscopic colitis, collagen colitis, and even irritable bowel syndrome (IBS), which indicates the central role of inflammation in intestinal homeostasis.
The critical function of the intestinal epithelial cells and the mucus layer is to form a barrier that separates luminal contents from the lamina propria. Loss of epithelial barrier function followed by invasion of bacteria is thought to be an initial event that provokes injury and inflammation in many intestinal disorders, including IBD (3, 4).
The cross talk between intestinal epithelial cells (IECs), immune cells, and intestinal bacteria represents a fundamental feature in maintaining normal intestinal homeostasis and for mounting protective immune responses against pathogens (5, 6). Accumulating evidence indicates that recognition of intestinal bacteria through pattern recognition receptors (PRRs) is important for controlling intestinal homeostasis and that the context of PRR activation is critical for the outcome (7). Basal PRR activation is essential for maintaining the barrier function and the composition of the microbiota in a healthy intestine, while aberrant PRR signaling may be a contributor to the development of IBD (8, 9). The association between the Nod-like receptor nucleotide-binding oligomerization domain-containing protein 2 (NOD2) and CD is well established. It has also been shown that mice lacking specific Toll-like receptors (TLRs) or that are deficient in the shared TLR signaling protein myeloid differentiation primary response gene 88 (Myd88) have increased susceptibility to dextran sulfate sodium (DSS)-induced colitis, characterized by defective tissue repair and increased mortality (10). A possible link between polymorphisms in C-type lectin receptors and IBD has also recently been reported (11). PRRs recognize conserved patterns in diverse microbial molecules, so-called microbe-associated microbial patterns (MAMPs). Such MAMPS bind to PRRs with different avidities, eliciting different intracellular activation events, and combinations of MAMPs may also act in both synergistic and antagonistic ways to modulate cellular responses (12). Traditionally, studies of microbe-host interactions are carried out with different pathogens and natural commensals. Thus, both commensals and probiotic bacteria have been demonstrated to mediate anti-inflammatory effects in different mouse models of IBD (13–15). However, the vast number of noncommensals may represent an unrecognized source of MAMPs for regulation of immune and inflammatory responses. To our knowledge, no one has tried to investigate and exploit the potential of noncommensals in modulating the inflammatory process in IBD.
Romarheim et al. (16) recently reported that dietary inclusion of a bacterial meal (BioProtein) could prevent the development of soybean meal-induced enteritis in Atlantic salmon. BioProtein is a bacterial meal obtained by aerobic fermentation of natural gas by Methylococcus capsulatus (Bath), an obligate methanotroph (17), together with the heterogenic bacteria Ralstonia sp., Brevibacillus agri, and Aneurinibacillus sp., representing minor fractions of the preparation. Thus, this bacterial meal includes a variety of ligands for different PRRs. We wanted to extend the observations made in salmonids to mammals and exploited the well-established model of DSS-induced colitis in mice. Clinical evaluation of DSS-exposed mice fed a standard diet including 25% bacterial meal revealed that the mice had a strikingly enhanced well-being compared to DSS-exposed mice fed the standard diet. These observations were confirmed by morphological data demonstrating a significant reduction of inflammation and colonic tissue damage in mice receiving a bacterial meal-containing diet. We also observed increased epithelial cell proliferation and increased colonic crypt depths in mice fed the bacterial meal. All findings were confirmed in a experiment where C57BL/6N mice were fed a standard diet including 25% Methylococcus capsulatus (Bath) only. These observations suggest that M. capsulatus, a bacterium not recognized to be a gut commensal, may provide new and unique opportunities for studying mechanisms involved in the development and treatment of IBD and how microbe-host interactions can be exploited to manipulate the inflammatory tonus of the intestinal mucosa.
MATERIALS AND METHODS
Animals.
Female C57BL/6NTac mice (age, 5 to 6 weeks; weight, 15 to 18 g) (18) from Taconic (Ry, Denmark) and with conventional microbiological status were used. The animals were divided into four groups with six animals per group. The animals were fed ad libitum a control diet based on AIN-93G (19) or an experimental diet where the casein content (200 g/kg of body weight) and corn starch (54 g/kg) were exchanged with 254 g/kg BioProtein (Norferm AS, Stavanger, Norway) or freeze-dried M. capsulatus (Bath). Two groups of animals were fed the control diet and the other two were fed the experimental diet for 2 weeks. The animals were acclimatized to the diet for 7 days before induction of colitis. At day 8, colitis was induced by 3 to 3.5% DSS (TdB Consultancy AB, Uppsala, Sweden) ad libitum in the drinking water for 6 days. The mice were sacrificed at day 6 following DSS treatment.
Tissue collection.
The colon from the cecum to the rectum was collected, and the length of the colon was measured. The content of the colon was washed out with phosphate-buffered saline before it was opened along the length and prepared in a Swiss roll format. The Swiss roll was immediately frozen in liquid nitrogen and then stored at −80°C. The length of the colon was measured.
RNA extraction and cDNA synthesis.
Twenty to 30 mg of colonic tissue and 600 μl RLT lysis buffer (Qiagen, Germantown, MD) with mercaptoethanol were placed in an M tube (Miltenyi GmBH, Bergisch Gladbach, Germany). The tissue was dissociated by a gentleMACS dissociator (program RNA_02; Miltenyi GmBH, Bergisch Gladbach, Germany). RNA was extracted according to the manual for the Qiagen RNeasy kit (Qiagen, Germantown, MD). DNase treatment was included in the protocol. RNA was eluted in 30 μl RNase-free water and stored at −80°C. The RNA concentration was obtained from A260 measurements using a NanoDrop 2000 spectrophotometer (Thermo Fischer Scientific Inc., Waltham, MA). The RNA integrity number (RIN) was tested by an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA) and ranged from 6.5 to 8. cDNA synthesis was performed by a SuperScript VILO cDNA synthesis kit (Life Technologies Ltd., Paisley, United Kingdom) according to the manufacturer's recommendations (2.3 μg RNA/reaction mixture). The cDNA synthesis program was 25°C for 10 min, 42°C for 120 min, and 85°C for 5 min with a final hold at 4°C (MJ Research thermocycler, Watertown, MA).
Real time-PCR.
PCR was performed using a TaqMan gene expression master mix (Applied Biosystems, Carlsbad, CA), according to the manufacturer's recommendations, with 20 ng cDNA per reaction mixture. All PCR mixtures were amplified by a Rotor Gene 6000 real-time PCR machine (Qiagen, Germantown, MD). The following PCR program was used: 50°C for 2 min, 95°C for 5 min, 95°C for 15 s, and 60°C for 1 min (40 cycles of the two last steps). All primer/probe sets used were TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA). Rpl32 was identified as the most stable reference gene using a mouse geNorm kit (Primerdesign, Southampton, United Kingdom) and geNorm software.
The following TaqMan probes were used in this study: Mm00441203_m1 for serum amyloid A3 (Saa3), Mm00656925_m1 for S100a9, Mm00447886_m1 for myeloperoxidase (MPO), Mm00458293_g1 for mucin 2 (Muc2), Mm00440502_m1 for nitric oxide synthase 2 (NOS2), Mm02528467_g1 for Rpl32, Mm01336189_m1 for interleukin-1β (IL-1β), Mm00446190_m1 for interleukin-6, Mm00439614_m1 for interleukin-10, and Mm00439618_m1 for interleukin-17A.
Histology.
Frozen tissue samples were mounted in Tissue-Tek mounting medium (Sakura, Torrance, CA) in a cryostat at −20°C. Sections (8 μm) were obtained at −20°C, and collected on polylysine-coated glass slides (Thermo Fischer Scientific Inc., Waltham, MA). Sections were briefly dried at room temperature, before they were postfixed in formalin for 1 min. The slides were then stained with hematoxylin-erytrosin (HE).
Histological scoring of the colitis was done blindly by an independent pathologist according to the scoring system presented in Table 1 (20).
Table 1.
Scoring system for inflammation-associated histological changes in the colona
| Score | Histological change |
|---|---|
| 0 | No evidence of inflammation |
| 1 | Low level of inflammation with scattered infiltrating mononuclear cells (1–2 foci) |
| 2 | Moderate inflammation with multiple foci |
| 3 | High level of inflammation with increased vascular density and marked wall thickening |
| 4 | Maximal severity of inflammation with transmural leukocyte infiltration and loss of goblet cells |
Slides were scored by a pathologist and blindly analyzed concerning experimental grouping.
For immunohistochemistry, the sections of paraffin-embedded tissue were incubated for 1 h at room temperature with blocking solution, 5% rabbit serum in Tris-buffered saline–Tween 20 (TBS-T) buffer, before rat Ki67 (clone MM1) antibody solution was added (Monosan). Incubation with primary antibody was performed overnight at 4°C. Primary antibody solution was removed, and sections were washed briefly in TBS-T buffer before rabbit anti-rat fluorescein isothiocyanate antibody solution was added (Jackson ImmunoResearch). Sections were incubated with secondary antibody for 1 h at room temperature in the dark. Sections were again washed in TBS-T buffer, before the nuclear stain Hoechst 3342 was added for 10 min at room temperature (Sigma-Aldrich, St. Louis, MO). Slides were mounted either with immersion oil or ProLong Gold antifade reagent (Life Technologies Ltd., Paisley, United Kingdom) before visualization with a Leica SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany).
Cultivation of bacteria and preparation of M. capsulatus bacterial meal.
Methylococcus capsulatus (Bath) NCIMB 11132 was used to produce single-strain bacterial meal. Nitrate mineral salts (NMS) medium (21) was used for all types of cultivations. M. capsulatus (Bath) culture aliquots were frozen in liquid nitrogen and stored at −80°C. Cultivations on agar plates and in shake flasks were performed at 45°C in an atmosphere of 75% air, 23.25% CH4, and 1.25% CO2. Shake flask cultures were incubated in an orbital shaker incubator at 200 rpm. Continuous cultivation was carried out in a 3-liter bioreactor (ADI Autoclavable bioreactor systems; Applikon, Schiedam, The Netherlands) with a working volume of 2 liters. Cells precultivated in shake flasks were used to inoculate the bioreactor to an optical density at 440 nm (OD440) of 0.1. The temperature was maintained at 45°C, stirring was set to 650 rpm, and a culture pH of 6.8 was maintained by automatic addition of 2.5 M NaOH. A gas mixture of 55% air, 24% methane, 20% O2, and 1% CO2 was sparged into the bioreactor at a constant flow of 0.064 volume of gas per volume of liquid and minute (vvm). The continuous culture was started after an initial batch phase, and the dilution rate was set to 0.01 h−1. The OD440 at steady state was generally sustained at approximately 10. Culture effluent was collected, and cells were harvested by centrifugation. Bacterial cell walls were disrupted by the use of a French press before freeze-drying of the material.
Statistics.
The results were expressed as the means ± standard deviations or with a range with an upper and lower value for exponential values. Experiments were conducted at least in duplicate, and similar results were obtained. The differences between means were tested for statistical significance using one-way analysis of variance (ANOVA). A P value of <0.05 was considered statistically significant in all experiments.
RESULTS
Oral administration of bacterial meal to C57BL/6 mice reduced disease activity and histological inflammation in acute DSS-induced colitis.
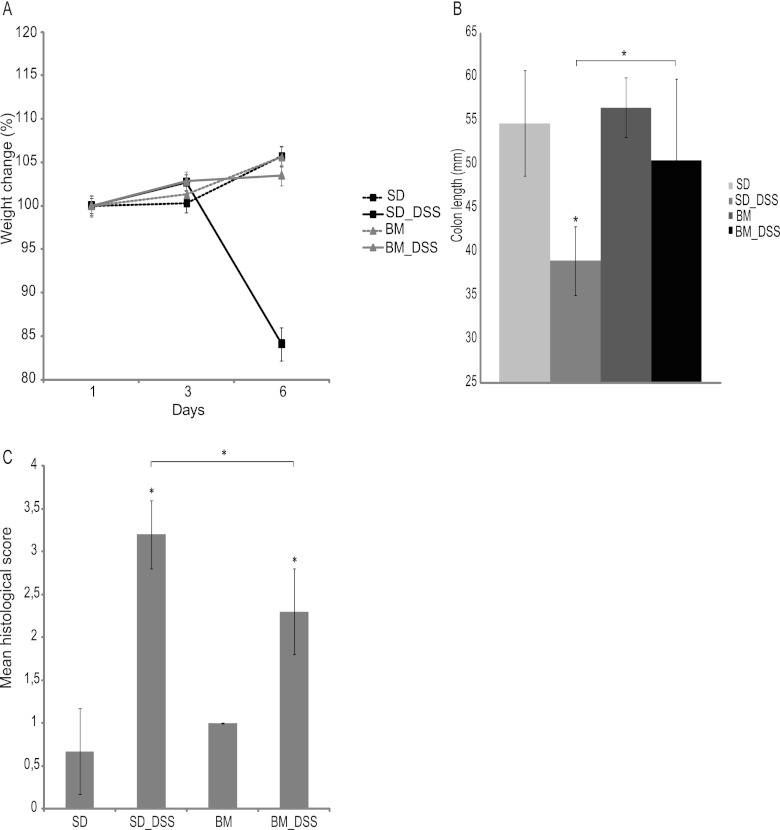
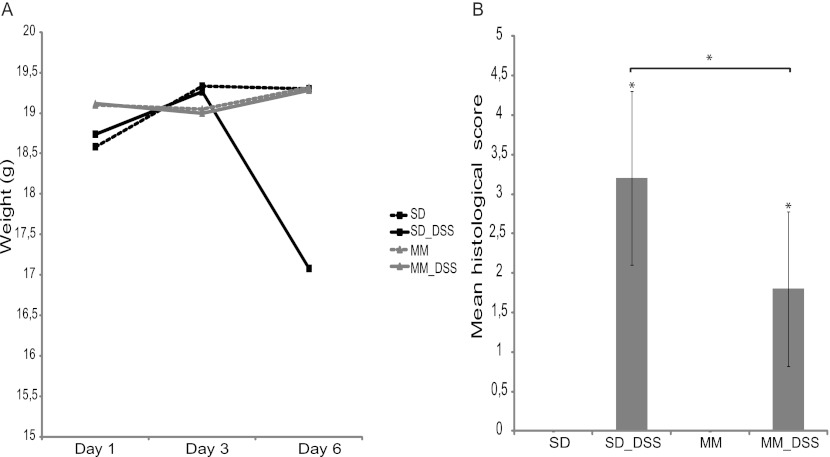
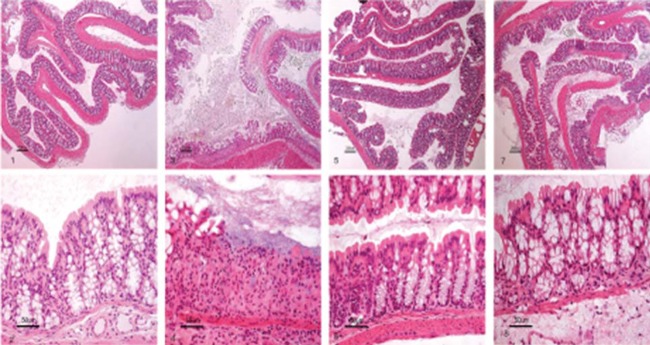
Administration of DSS to C57BL/6 mice induces an acute inflammation of the colon, pronounced weight loss, and bloody diarrhea. In this study, C57BL/6 mice fed the standard diet and given 3.5% DSS in the drinking water developed progressive weight loss (Fig. 1A) with diarrhea and blood in the feces. However, the symptoms of DSS treatment were less profound or minimized after pretreatment with the standard diet containing the bacterial meal. The increase in body weight of DSS-treated mice fed a bacterial meal-containing diet was comparable to that of the control group (5.4% ± 2.6% and 5.6% ± 2.5% mean increases in body weight, respectively), while DSS-treated mice eating the standard diet experienced a significant weight loss (−7.9% ± 11.1%; P < 0.001) In addition, the DSS-treated mice in the standard diet group exhibited a significant shortening of the colon compared to the control group (−1.6 ± 0.3 cm; P < 0.001), while the length of the large intestine of the bacterial meal-pretreated mice did not deviate significantly from that of the large intestine of the control group (−0.4 ± 0.9 cm) (Fig. 1B). On histological examination of colons from mice in the control group, a typical normal structure was found with a mean histological score of 0.7 ± 0.5. In DSS-treated mice receiving the standard diet, the average histological score was 3.2 ± 0.4, which was significantly higher than that for DSS-treated mice receiving a diet containing bacterial meal, which had an average score of 2.3 ± 0.5 (P < 0.05). Mice fed a bacterial meal diet had a mean histological score of 1.0 ± 0, which was comparable to that for the group fed the standard diet (Fig. 1C and Fig. 2).
Fig 1.
(A) Weight of the mice followed during treatment with DSS, presented as change in weight from day 1 (in percent). (B) Colon length measured directly after dissection from the mice before emptying of the contents. (C) Histological scoring. Upon histological examination, the severity of the inflammation was scored on a scale of from 0 to 4, with 4 being the most severe. Data are presented as mean ± standard deviations. Differences between means were tested by one-way ANOVA. A P value of <0.05 was considered statistically significant (*). SD, standard diet; BM, bacterial meal.
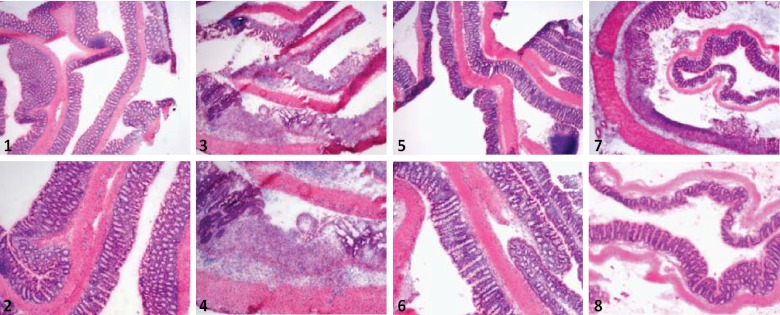
Fig 2.
(1 and 2) HE-stained paraffin-embedded section of colon from a mouse fed a standard diet; (3 and 4) HE-stained paraffin-embedded section of colon from a mouse fed a standard diet and exposed to DSS in the drinking water; (5 and 6) HE-stained paraffin-embedded section of colon from a mouse fed a BioProtein bacterial meal; (7 and 8) HE-stained paraffin-embedded section of colon from a mouse fed a BioProtein bacterial meal and exposed to DSS in the drinking water.
Inclusion of bacterial meal in the diet decreased intestinal inflammatory markers in mice with DSS-induced colitis.
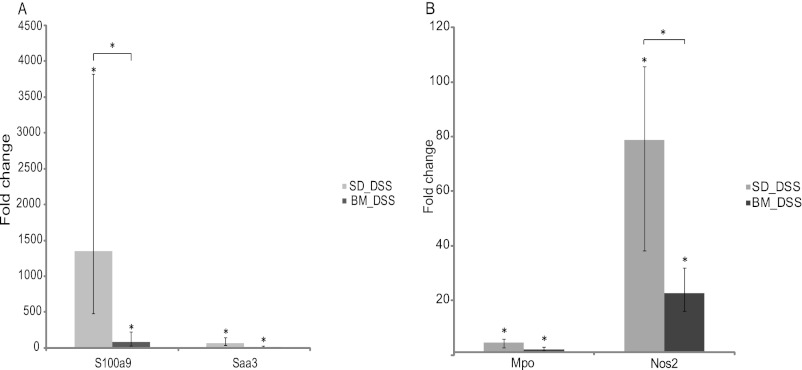
To assess inflammatory activity, expression of the genes for two well-known diagnostic markers of IBD, S100a9 (calprotectin) and serum amyloid A3 (Saa3), in the colon was determined (22, 23). The mRNA levels of both S100a9 and Saa3 were found to be upregulated after treatment with DSS in mice fed the standard diet (1,350.2-fold [range, 478- to 3,822-fold; P < 0.0001] and 64-fold [range, 28- to 147-fold; P < 0.001] for S100a9 and Saa3, respectively). However, DSS-induced expression of the genes for S100a9 and Saa3 was lower in mice fed a diet containing bacterial meal (84-fold [range, 32- to 223-fold; P < 0.001] and 14.9-fold [range, 7- to 32-fold]) than animals in the DSS control group, although not significantly so for Saa3 (Fig. 3A).
Fig 3.
(A) mRNA levels for S100a9 (calprotectin) and Saa in DSS-treated mice given a standard diet or a bacterial meal relative to those in DSS-untreated mice given a standard diet. mRNA levels were determined by real-time PCR. (B) mRNA levels for MPO and NOS2 in DSS-treated mice given a standard diet or a bacterial meal relative to those in DSS-untreated mice given a standard diet. mRNA levels were determined by real-time PCR. Data are presented as the mean fold change ± standard deviation. Differences between means were tested by one-way ANOVA. A P value of <0.05 was considered statistically significant (*).
Neutrophil infiltration and activity were lower in mice fed a bacterial meal.
DSS-induced colitis is characterized by infiltration of immune cells in the mucosa and submucosa, epithelial ulceration, and crypt damage (24, 25). The level of MPO has been shown to be proportional to the number of neutrophils in the inflamed tissue (26). Here, we found that the mRNA level for MPO in the colon was significantly increased in both DSS-treated groups compared to that in the control group (4.6-fold [range, 3.2- to 6.5-fold; P < 0.001] and 2.1-fold [range, 1.5- to 3.0-fold; P < 0.001] for mice fed the standard diet and bacterial meal, respectively). However, MPO mRNA expression decreased by more than 50% in DSS-treated mice receiving the bacterial meal compared to that in the group receiving the standard diet, although the difference was not statistically significant (Fig. 3B). MPO activity has been strongly associated with colonic tissue injury resulting from DSS administration in inducible NOS2−/− mice, suggesting that NOS2 is important for the signaling process in neutrophils (27). After DSS treatment, there was a significant increase in NOS2 mRNA transcription in the colons of both mice fed the standard diet and mice fed the bacterial meal (78.8-fold [range, 51- to 119.4-fold; P < 0.001] and 22.6-fold [range, 16- to 32-fold; P < 0.001], respectively). The NOS2 mRNA transcript level was, however, approximately 70% lower in the colons of DSS-induced mice receiving bacterial meal than the colons of mice fed the standard diet (3.5-fold; range, 2.3- to 5.3-fold; P < 0.01) (Fig. 3B).
In the colons of DSS-treated mice fed a bacterial meal, there was a lower induction of cytokine production.
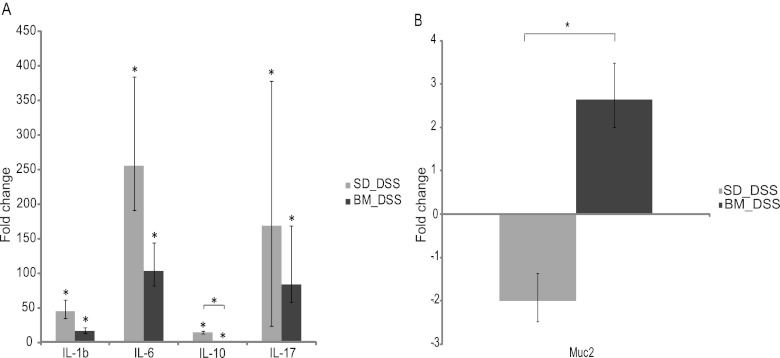
The immunological effects of the bacterial meal-containing diet could be responsible for the clinical improvement seen in the mice. Further insight was provided by analyzing the levels of cytokines in colonic tissue. The mRNA transcript levels of IL-1β, IL-6, IL-10, and IL-17 in the colon were investigated. There was a lower induction of mRNA of all cytokines in DSS-treated mice receiving bacterial meal than in animals fed the standard diet. The proinflammatory cytokine IL-1β was upregulated in both DSS-treated groups (45.3-fold [range, 26- to 78.8-fold; P < 0.001] and 7.2-fold [range, 4.9- to 10.6-fold; P < 0.001]) for mice fed the standard diet and bacterial meal, respectively), but there was a 2.6-fold lower induction of IL-1β in animals receiving bacterial meal. IL-6 was upregulated in both DSS groups (256-fold [range, 128- to 512-fold; P < 0.001] and 104-fold [range, 55.7- to 194-fold; P < 0.001] for mice fed the standard diet and bacterial meal, respectively), but we found a 2.5-fold lower increase in the induction of IL-6 in the group given a bacterial meal than the group given the standard diet. IL-10 was upregulated in both DSS-treated groups (14.9-fold [range, 11.3- to 19.7-fold; P < 0.001] and 2.6-fold [range, 2- to 3.4-fold; P < 0.05] for mice fed the standard diet and bacterial meal, respectively), but we found a 5.7-fold (P < 0.001) lower increase in the induction of IL-10 in the group given a bacterial meal than the group given the standard diet. IL-17 was upregulated in both DSS groups (168.9-fold [range, 48.5- to 588.1-fold; P < 0.01] and 84.4-fold [range, 26- to 274.3-fold; P < 0.01] for mice fed the standard diet and bacterial meal, respectively), but we found a 2-fold lower increase in the induction of IL-17 in the group given a bacterial meal than the group given the standard diet (Fig. 4A). This reduction in cytokines indicates a reduced induction of inflammation in the colonic tissue.
Fig 4.
(A) mRNA levels for the cytokines IL-1β, IL-6, IL-10, and IL-17 in DSS-treated mice given a standard diet or a bacterial meal relative to those in DSS-untreated mice given a standard diet. mRNA levels were determined by real-time PCR. (B) mRNA level for Muc2 in DSS-treated mice given a standard diet or a bacterial meal relative to that in DSS-untreated mice given a standard diet. mRNA levels were determined by real-time PCR. Data are presented as the mean fold change ± standard deviation. Differences between means were tested by one-way ANOVA. A P value of <0.05 was considered statistically significant (*).
The bacterial meal-containing diet led to a slight increase in Muc2 mRNA expression.
An intact inner mucous layer is important in the protection of the colon epithelial cells, as bacteria in contact with the epithelium are likely to trigger an inflammatory response. This mucous layer is formed by the gel-forming mucin Muc2, which is the major secretory mucin synthesized and secreted by goblet cells (28). Gene expression levels of Muc2 were found to be decreased in DSS-treated mice given the control diet (−2-fold [range, −2.6- to −1.5-fold]). On the other hand, DSS-treated mice which received bacterial meal showed a slight increase in Muc2 gene expression (1.3-fold [range, 1- to 1.7-fold]) compared to mice that received the control diet. There was a significant difference in Muc2 mRNA expression between DSS-treated mice receiving the standard diet and those receiving bacterial meal (2.6-fold [range, 2- to 3.5-fold; P < 0.05]) (Fig. 4B). Goblet and absorptive cells also express membrane-bound mucins Muc3 and Muc4, which are components of the glycocalyx, but only slight changes in Muc3 and Muc4 gene expression were found (results not shown).
Mice fed the bacterial meal diet showed increased crypt depths and a higher number of proliferating epithelial cells.
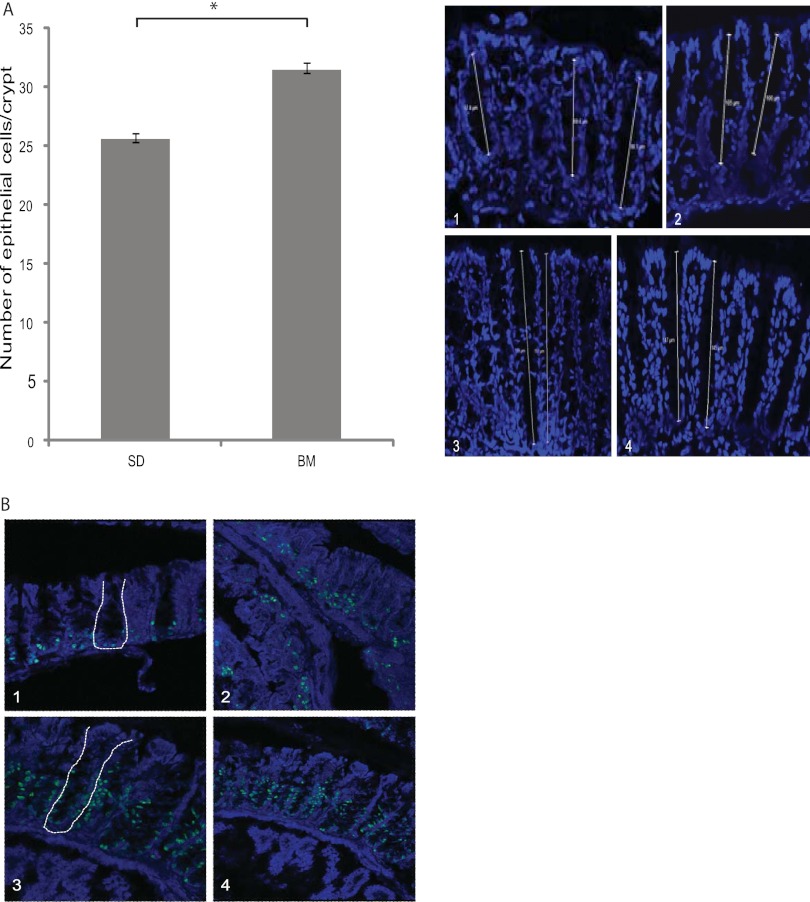
The depth of the colon crypts of mice fed a diet containing the bacterial meal for 14 days was increased compared to that for mice fed the standard diet. Crypt depth reflects the number of epithelial cells present in the crypt. Colon tissue sections were stained with Hoechst 3342 to visualize the nucleus of all cells, and then the number of cells in each crypt was counted. The numbers of epithelial cells in 20 crypts per animal in each of the two groups were counted. The average numbers of cells per crypt were 25.5 ± 0.6 and 31.4 ± 0.18 for mice fed the standard diet and bacterial meal, respectively (P < 0.001) (Fig. 5A).
Fig 5.
(A) Number of cells lining the lumen of the colon crypts in mice fed a standard diet (1 and 2) or a bacterial meal (3 and 4). The number of cells is presented as the mean per crypt ± standard deviation. The cell number was counted in 20 crypts per animal. (B) Paraffin-embedded sections of representative colon crypts from animals fed a standard diet (1 and 2) or a bacterial meal (3 and 4) stained with anti-Ki67 antibodies and Hoechst 3342. Crypt structures are indicated by white lines.
To investigate whether the increase in the number of cells was caused by increased proliferation of the crypt cells, paraffin-embedded tissue was stained with anti-Ki67 antibodies. Antigen Ki67 is a nuclear protein associated with cellular proliferation (29). There was an obvious increase in the number of Ki67-positive cells in the crypts of animals fed a bacterial meal diet compared to that in the crypts of animals fed the standard diet. The Ki67-positive cells were found to be higher up in the crypts in mice fed the bacterial meal than in mice fed the standard diet (Fig. 5B).
Oral administration of a bacterial meal of M. capsulatus (Bath) reduced disease activity and histological inflammation in acute colitis.
M. capsulatus (Bath) is the main constituent of BioProtein, and therefore, an animal study was conducted with a diet containing a bacterial meal of M. capsulatus (Bath). C57BL/6 mice fed the standard diet and exposed to 3.0% DSS in the drinking water developed progressive weight loss (Fig. 6A) with diarrhea and blood in the feces. Animals pretreated with a diet containing M. capsulatus meal showed significantly fewer symptoms after DSS treatment. The body weight of DSS-treated mice fed the M. capsulatus-containing diet was comparable to that of mice in the control group (19.3 ± 0.7 g and 19.3 ± 0.7 g, respectively), while DSS-treated mice eating the standard diet experienced a significant weight loss (17.1 ± 0.9 g; P < 0.001). On histological examination of colons from mice in the control group, a typical normal structure with a mean histological score of 0 was found. In DSS-treated mice receiving the standard diet, the average histological score was 3.2 ± 1.1, which was significantly higher than that for DSS-treated mice fed a diet containing M. capsulatus meal, which had an average histological score of 1.8 ± 1 (P < 0.01). Mice fed an M. capsulatus-containing diet had a mean histological score of 0, which was the same as that for the control group (Fig. 6B and 7). These findings confirm that M. capsulatus (Bath) represents the active component of the bacterial meal BioProtein.
Fig 6.
(A) Weight of the mice fed a diet containing a Methylococcus bacterial meal (MM) followed during the period of treatment with DSS; (B) colon length measured directly after dissection from the mice before emptying of the contents.
Fig 7.
(1 and 2) HE-stained paraffin-embedded section of colon from a mouse fed a standard diet; (3 and 4) HE-stained paraffin-embedded section of colon from a mouse fed a standard diet and exposed to DSS in the drinking water; (5 and 6) HE-stained paraffin-embedded section of colon from a mouse fed a Methylococcus bacterial meal; (7 and 8) HE-stained paraffin-embedded section of colon from a mouse fed a Methylococcus bacterial meal and exposed to DSS in the drinking water.
DISCUSSION
The objective of this study was to investigate whether inclusion of the bacterial meal BioProtein in the diet could abrogate disease in a murine model of epithelial injury and colitis and thus have therapeutic potential in human IBD. This bacterial meal has recently been reported to attenuate soybean meal-induced enteritis in Atlantic salmon (16). Although the mechanism is unknown, it is likely that the enteritis in salmonids is a T-cell- and cytokine-mediated inflammation (30, 31) and thus shows similarities with IBD in mammals.
To evaluate the effect of this bacterial meal, we used the well-established model of DSS-induced colitis in mice (32). DSS-induced colitis exhibits several characteristics resembling human ulcerative colitis, including weight loss, severe diarrhea, rectal bleeding, ulceration and loss of epithelium, and leukocyte infiltration (27, 32, 33). Notably, the bacterial meal-containing diet significantly improved the clinical, colitis-associated findings of reduced body weight, shortening of the colon, and epithelial damage.
The presence of active gut inflammation in IBD is associated with an acute-phase reaction and migration of leukocytes to the gut, which is translated into enhanced expression of acute-phase proteins like Saa3 (23). The level of Saa3 mRNA was significantly increased in both DSS-treated groups but was greatly reduced in animals fed the bacterial meal. This indicates a lower disease activity in animals fed a bacterial meal-containing diet than animals fed a control diet.
It is well accepted that in many inflammatory disorders of the intestine, the combination of epithelial injury, disease activity, and symptoms parallel neutrophil infiltration of the mucosa (34, 35). Neutrophil accumulation also plays an important role in DSS-induced colitis, as the disease can be attenuated by administration of neutrophil antiserum (36), and the level of MPO is reported to be proportional to the number of neutrophils in inflamed tissue (26). We found that the mRNA expression level of the neutrophil marker MPO was reduced by more than 50% in bacterial meal-fed animals, further supporting reduced levels of tissue inflammation. The strong association between MPO expression and tissue injury in DSS-treated NOS2−/− mice (27) indicates that NOS2 is important for neutrophil function. Adding bacterial meal to the diet significantly reduced the level of NOS2 mRNA in DSS-treated animals. Calprotectin (S100a9) represents 60% of the cytosolic proteins in granulocytes. The levels of S100a9 in the lamina propria tend to mirror the level of neutrophil migration to the gastrointestinal tract. S100a9 mRNA levels are also significantly reduced in bacterial meal-fed animals compared to those in mice fed a control diet. Hence, the significant reduction in markers of neutrophil granulocytes clearly shows that inclusion of a bacterial meal in the diet results in lower disease activity and less induction of gastrointestinal inflammation in response to DSS treatment.
The cytokines produced in the gut mucosa tend to greatly influence the immunological outcome. Production of anti-inflammatory cytokines induces mucosal unresponsiveness and tolerance, while production of proinflammatory cytokines indicates a protective immune response and inflammation. Prophylactic treatment with the bacterial meal before DSS exposure decreased the production of proinflammatory cytokines IL-6, IL-1β, and IL-17, as well as the anti-inflammatory cytokine IL-10, in the colon. This indicates that prophylactic feeding of the bacterial meal to the animals strongly reduced the induction of inflammation in the colon, as all inflammatory markers were less induced than they were in the mice fed the control diet. Furthermore, reduced IL-10 mRNA levels also indicate lowered T-cell activation, in general, suggesting enhanced barrier function and less activation of adaptive immunity in bacterial meal-fed mice.
To protect the large intestine from the enormous amounts of commensal bacteria, a mutualistic relationship has evolved where both host and bacteria benefit. An inner, firmly adherent mucous layer and an outer, loose nonadherent mucous layer have been identified (37, 38). Although the molecular compositions of these two layers are similar, consisting mainly of Muc2, the bacterial flora resides in the loose mucus, whereas the inner firm layer of mucus is impervious to bacteria and thereby functions as a protective barrier for the epithelial cell surface (38). DSS treatment resulted in decreased Muc2 mRNA transcription, whereas prophylactic intake of a bacterial meal-containing diet before DSS treatment significantly enhanced Muc2 mRNA transcription. This difference in Muc2 production could be of importance in maintaining epithelial layer integrity in the colons of mice fed a bacterial meal-containing diet.
An important part of the intestinal regenerative response is stimulation of epithelial cell proliferation, as this will supply cells for the epithelium and promote and maintain the crypt and villus development of the regenerating mucosa. Epithelial cell proliferation is increased in crypts adjacent to injured areas with both crypt elongation and crypt fission (39). Mice fed the bacterial meal-containing diet showed a significant increase in the crypt depth of the colon. The number of crypt cells and the number of Ki67-positive proliferating cells were increased in animals eating the bacterial meal-containing diet. This also resulted in a modest increase in the colon length of these animals. Increased epithelial cell proliferation in response to the bacterial meal was also identified in Atlantic salmon by Romarheim et al. (16). Enhanced epithelial cell proliferation and, hence, increased epithelial cell regeneration could be essential for the reduced disease activity seen after inclusion of bacterial meal in the diet. The increased rate of regeneration could make the epithelial cell layer in the intestine more resistant to damage and thereby less susceptible to induction of inflammation.
An animal study with inclusion of a bacterial meal from M. capsulatus (Bath) verifies only that the active component(s) of BioProtein leading to amelioration of colitis is derived from this bacterium.
The Gram-negative bacterium M. capsulatus (Bath) has so far not been reported to be a part of the human microbiota. The bacteria are lysed by high pressure and then spray dried (40), and therefore, the bacterial meal does not contain any live bacteria capable of colonizing the intestine. However, the preparation contains a multitude of bacterial components with potential to interact with cells of the epithelium and lamina propria involved in maintaining mucosal homeostasis. Whether the observed effects are due to one or a combination of microbial components is not known. The analysis and characterization of the component(s) responsible for the observed effects are under scrutiny. This study suggests that even noncommensal bacteria may have potent immunomodulatory properties in mammals and that such bacteria may represent a hitherto untapped and important source of molecules relevant for studying mechanisms involved in homeostatic microbe-host interactions and in intestinal inflammation.
ACKNOWLEDGMENTS
Great thanks go to Rich Boden, University of Plymouth, for sharing his knowledge about cultivation of Methylococcus capsulatus (Bath).
This work was supported by the Ostfold Hospital Trust.
Footnotes
Published ahead of print 12 October 2012
REFERENCES
- 1. Xavier RJ, Podolsky DK. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434 [DOI] [PubMed] [Google Scholar]
- 2. Hill DA, Artis D. 2010. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 28:623–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clayburgh DR, Shen L, Turner JR. 2004. A porous defense: the leaky epithelial barrier in intestinal disease. Lab. Invest. 84:282–291 [DOI] [PubMed] [Google Scholar]
- 4. Dignass AU, Baumgart DC, Sturm A. 2004. Review article: the aetiopathogenesis of inflammatory bowel disease—immunology and repair mechanisms. Aliment. Pharmacol. Ther. 20(Suppl 4):9–17 [DOI] [PubMed] [Google Scholar]
- 5. Artis D. 2008. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 8:411–420 [DOI] [PubMed] [Google Scholar]
- 6. Hooper LV, Macpherson AJ. 2010. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10:159–169 [DOI] [PubMed] [Google Scholar]
- 7. Maloy KJ, Powrie F. 2011. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474:298–306 [DOI] [PubMed] [Google Scholar]
- 8. Abreu MT. 2010. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 10:131–144 [DOI] [PubMed] [Google Scholar]
- 9. Cario E. 2010. Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm. Bowel Dis. 16:1583–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gibson DL, Montero M, Ropeleski MJ, Bergstrom KSB, Ma C, Ghosh S, Merkens H, Huang J, Månsson LE, Sham HP, McNagny KM, Vallance BA. 2010. Interleukin-11 reduces TLR4-induced colitis in TLR2-deficient mice and restores intestinal STAT3 signaling. Gastroenterology 139:1277–1288 [DOI] [PubMed] [Google Scholar]
- 11. Wolfkamp SC, Verstege MI, Vogels EW, Meisner S, Verseijden C, Stokkers PC, Te Velde AA. 2012. Single nucleotide polymorphisms in C-type lectin genes, clustered in the IBD2 and IBD6 susceptibility loci, may play a role in the pathogenesis of inflammatory bowel diseases. Eur. J. Gastroenterol. Hepatol. 24:965–970 [DOI] [PubMed] [Google Scholar]
- 12. Hajishengallis G, Lambris JD. 2011. Microbial manipulation of receptor crosstalk in innate immunity. Nat. Rev. Immunol. 11:187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu YW, Su YW, Ong WK, Cheng TH, Tsai YC. 2011. Oral administration of Lactobacillus plantarum K68 ameliorates DSS-induced ulcerative colitis in BALB/c mice via the anti-inflammatory and immunomodulatory activities. Int. Immunopharmacol. 11:2159–2166 [DOI] [PubMed] [Google Scholar]
- 14. Round JL, Mazmanian SK. 2010. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. U. S. A. 107:12204–12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schultz M, Strauch UG, Linde HJ, Watzl S, Obermeier F, Gottl C, Dunger N, Grunwald N, Scholmerich J, Rath HC. 2004. Preventive effects of Escherichia coli strain Nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin. Diagn. Lab. Immunol. 11:372–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romarheim OH, Overland M, Mydland LT, Skrede A, Landsverk T. 2011. Bacteria grown on natural gas prevent soybean meal-induced enteritis in Atlantic salmon. J. Nutr. 141:124–130 [DOI] [PubMed] [Google Scholar]
- 17. Ward N, Larsen Ø, Sakwa J, Bruseth L, Khouri H, Durkin AS, Dimitrov G, Jiang L, Scanlan D, Kang KH, Lewis M, Nelson KE, Methé B, Wu M, Heidelberg JF, Paulsen IT, Fouts D, Ravel J, Tettelin H, Ren Q, Read T, DeBoy RT, Seshadri R, Salzberg SL, Jensen HB, Birkeland NK, Nelson WC, Dodson RJ, Grindhaug SH, Holt I, Eidhammer I, Jonasen I, Vanaken S, Utterback T, Feldblyum TV, Fraser CM, Lillehaug JR, Eisen JA. 2004. Genomic insights into methanotrophy: the complete genome sequence of Methylococcus capsulatus (Bath). PLoS Biol. 2:e303 doi:10.1371/journal.pbio.0020303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mähler M, Bristol IJ, Leiter EH, Workman AE, Birkenmeier EH, Elson CO, Sundberg JP. 1998. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 274:G544–G551 [DOI] [PubMed] [Google Scholar]
- 19. Reeves PG, Nielsen FH, Fahey GC. 1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 123:1939–1951 [DOI] [PubMed] [Google Scholar]
- 20. Wirtz S, Neufert C, Weigmann B, Neurath MF. 2007. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2:541–546 [DOI] [PubMed] [Google Scholar]
- 21. Whittenbury R, Phillips KC, Wilkinson JF. 1970. Enrichment, isolation and some properties of methane-utilizing bacteria. J. Gen. Microbiol. 61:205–218 [DOI] [PubMed] [Google Scholar]
- 22. Foell D, Roth J. 2004. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 50:3762–3771 [DOI] [PubMed] [Google Scholar]
- 23. Benditt EP, Eriksen N, Hanson RH. 1979. Amyloid protein SAA is an apoprotein of mouse plasma high density lipoprotein. Proc. Natl. Acad. Sci. U. S. A. 76:4092–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sartor RB. 2006. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 3:390–407 [DOI] [PubMed] [Google Scholar]
- 25. Harrison OJ, Maloy KJ. 2011. Innate immune activation in intestinal homeostasis. J. Innate Immun. 3:585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krawisz JE, Sharon P, Stenson WF. 1984. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87:1344–1350 [PubMed] [Google Scholar]
- 27. Krieglstein CF, Cerwinka WH, Laroux FS, Salter JW, Russell JM, Schuermann G, Grisham MB, Ross CR, Granger DN. 2001. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen. J. Exp. Med. 194:1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johansson MEV, Gustafsson JK, Sjöberg KE, Petersson J, Holm L, Sjövall H, Hansson GC. 2010. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One 5:e12238 doi:10.1371/journal.pone.0012238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scholzen T, Gerdes J. 2000. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182:311–322 [DOI] [PubMed] [Google Scholar]
- 30. Lilleeng E, Penn MH, Haugland O, Xu C, Bakke AM, Krogdahl A, Landsverk T, Froystad-Saugen MK. 2009. Decreased expression of TGF-beta, GILT and T-cell markers in the early stages of soybean enteropathy in Atlantic salmon (Salmo salar L.). Fish Shellfish Immunol. 27:65–72 [DOI] [PubMed] [Google Scholar]
- 31. Bakke-McKellep AM, Froystad MK, Lilleeng E, Dapra F, Refstie S, Krogdahl A, Landsverk T. 2007. Response to soy: T-cell-like reactivity in the intestine of Atlantic salmon, Salmo salar L. J. Fish Dis. 30:13–25 [DOI] [PubMed] [Google Scholar]
- 32. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. 1990. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98:694–702 [DOI] [PubMed] [Google Scholar]
- 33. Krieglstein CF, Cerwinka WH, Sprague AG, Laroux FS, Grisham MB, Koteliansky VE, Senninger N, Granger DN, de Fougerolles AR. 2002. Collagen-binding integrin alpha1beta1 regulates intestinal inflammation in experimental colitis. J. Clin. Invest. 110:1773–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koyama S, Rennard SI, Leikauf GD, Shoji S, Von Essen S, Claassen L, Robbins RA. 1991. Endotoxin stimulates bronchial epithelial cells to release chemotactic factors for neutrophils. A potential mechanism for neutrophil recruitment, cytotoxicity, and inhibition of proliferation in bronchial inflammation. J. Immunol. 147:4293–4301 [PubMed] [Google Scholar]
- 35. Nusrat A, Turner JR, Madara JL. 2000. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am. J. Physiol. Gastrointest. Liver Physiol. 279:G851–G857 [DOI] [PubMed] [Google Scholar]
- 36. Domek MJ, Iwata F, Blackman EI, Kao J, Baker M, Vidrich A, Leung FW. 1995. Anti-neutrophil serum attenuates dextran sulfate sodium-induced colonic damage in the rat. Scand. J. Gastroenterol. 30:1089–1094 [DOI] [PubMed] [Google Scholar]
- 37. Atuma C, Strugala V, Allen A, Holm L. 2001. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G922–G929 [DOI] [PubMed] [Google Scholar]
- 38. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. 2008. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U. S. A. 105:15064–15069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson JS, Saxena SK, Sharp JG. 2000. Regulation of intestinal regeneration: new insights. Microsc. Res. Tech. 51:129–137 [DOI] [PubMed] [Google Scholar]
- 40. Øverland M, Tauson A-H, Shearer K, Skrede A. 2010. Evaluation of methane-utilising bacteria products as feed ingredients for monogastric animals. Arch. Anim. Nutr. 64:171–189 [DOI] [PubMed] [Google Scholar]