Abstract
The structure and ecological roles of the exopolysaccharides (EPSs) from sea ice microorganisms are poorly studied. Here we show that strain SM20310, with an EPS production of 567 mg liter−1, was screened from 110 Arctic sea ice isolates and identified as a Pseudoalteromonas strain. The EPS secreted by SM20310 was purified, and its structural characteristics were studied. The predominant repeating unit of this EPS is a highly complicated α-mannan with a molecular mass greater than 2 × 106 Da. The backbone of the EPS consists of 2-α-, 6-α-mannosyl residues, in which a considerable part of the 6-α-mannosyl residues are branched at the 2 position with either single t-mannosyl residues or two mannosyl residues. The structure of the described EPS is different from the structures of EPSs secreted by other marine bacteria. Analysis of the ecological roles of the identified EPS showed that the EPS could significantly enhance the high-salinity tolerance of SM20310 and improve the survival of SM20310 after freeze-thaw cycles. These results suggest that the EPS secreted by strain SM20310 enables the strain to adapt to the sea ice environment, which is characterized by low temperature, high salinity, and repeated freeze-thaw cycles. In addition to its functions in strain SM20310, this EPS also significantly improved the tolerance of Escherichia coli to freeze-thaw cycles, suggesting that it may have a universal impact on microorganism cryoprotection.
INTRODUCTION
Many bacteria in the marine environment secrete exopolysaccharides (EPSs), which comprise a substantial component of the extracellular polymers surrounding bacterial cells (1). In recent years, the increasing demand for natural polymers for pharmaceutical, food, and other industrial applications has led to a remarkable interest in EPSs produced by marine bacteria (2). The structures and ecological roles of some bacterial EPSs from deep-sea hydrothermal vents and sediments have been reported (3–7). Most EPSs produced by marine bacteria are heteropolysaccharides consisting of 3 or 4 different monosaccharides that may be pentoses, hexoses, amino sugars, or uronic acids and are arranged in groups of 10 or less to form repeating units (8). In the marine environment, bacterial EPSs are essential for the production of aggregates, adhesion to or colonization of surfaces, and the formation of biofilms or sequestration of nutrients; EPSs thus provide protection for bacterial and ecosystem stability (1).
Sea ice is a special marine environment that covers 35 × 106 km2, or 13%, of the world's surface area (9). The sea ice crystal matrix is permeated by a highly connected network of channels and pores, which are filled with brine formed from expelled salts as the ice crystals freeze together (10). It is reported that the brine salinity in situ is approximately 130 to 40‰ from the surface of ice to a depth of 160 cm in March, and more than 1% of the volume of the winter sea ice fills with liquid brine with a salinity above 200‰ (11). In addition, freeze-thaw cycles, which tend to damage living cells and reduce viability, are quite common in the sea ice of the Arctic and the Antarctic (12). Thus, the microorganisms living in the brine channels must have evolved adaptations to tolerate high salinity and repeated freezing and thawing. In recent years, increasing attention has been paid to the EPSs produced by the bacteria in sea ice and their ecological roles (11, 13). Nichols et al. (14) reported the preliminary structural characterization of the EPSs from 6 bacterial strains isolated from Antarctic sea ice, showing that neutral sugars account for nearly half of all 6 EPSs and uronic acid comprises the second most abundant fraction. This group also studied the effects of temperature on EPS production by strain CAM025 from Antarctic sea ice. Strain CAM025 produced 30 times more EPS at both −2°C and 10°C than it did at 20°C, suggesting that EPS production at low temperatures may be a mechanism for adaptation to cold temperature for this strain (15). Therefore, the production of EPS may be a successful evolutionary strategy for bacteria inhabiting extreme sea ice environments. Study of the EPSs produced by sea ice bacteria may reveal important ecological characteristics for the adaptation of bacteria to the constantly changing sea ice ecosystem. However, there are only a few reports on the EPSs from sea ice microorganisms. The structures and the exact ecological roles of these EPSs are mostly unknown.
In this study, EPS-producing bacteria were screened from 110 strains isolated from Arctic sea ice. Strain SM20310, with the highest EPS production, was identified as a Pseudoalteromonas strain. The EPS produced by strain SM20310 was purified, and its structure was characterized. Furthermore, the effects of the EPS on SM20310 tolerance to high salinity and freeze-thaw cycles were studied, and possible ecological roles of the EPS for the adaptation of strain SM20310 to the extreme sea ice environment are hypothesized.
MATERIALS AND METHODS
Screening of EPS-producing isolates.
The 110 isolates used to screen EPS-producing isolates were previously isolated from Arctic sea ice of the Canada Basin (77°30′N to 80°12′N) (16). EPS-producing isolates were screened by using the method of Freeman et al. (17). The isolates were cultured at 15°C for 3 days on Congo red agar plates with marine agar (MA) medium (5 g liter−1 peptone [Oxoid], 1 g liter−1 yeast extract [Oxoid], 1.5 g liter−1 agar, artificial sea water, pH 7.5) supplemented with 3 g liter−1 glucose and 0.8 g liter−1 Congo red. A positive result was indicated by black colonies with a smooth, humid, and mucoid morphology. Then, the screened isolates were cultured at 15°C with shaking at 200 rpm for 3 days in a liquid marine medium composed of 5 g liter−1 peptone, 1 g liter−1 yeast extract, 30 g liter−1 glucose, and artificial seawater (pH 7.5). After cultivation, the broth was centrifuged at 10,000 rpm for 10 min, and the EPS in the supernatant was precipitated using 3 volumes of chilled absolute ethanol. The precipitate was washed twice using cold absolute ethanol and then dissolved in deionized water. The EPS concentration of the solution was determined by the phenol-sulfuric acid method using d-glucose as a standard (18), and the EPS production of each isolate was calculated. Isolate SM20310, which had the highest EPS production, was chosen for further study.
Amplification of the 16S rRNA gene and phylogenetic analysis.
The genomic DNA of strain SM20310 was prepared using a bacterial genomic DNA isolation kit (BioTeke, China). The 16S rRNA gene of strain SM20310 was amplified from the genomic DNA by PCR with the universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-ACGGCTACCTTGTTACGACTT-3′) (19) and sequenced. The 16S rRNA gene sequence obtained was aligned manually with those of related taxa retrieved from the GenBank database using MEGA (version 4.0) software.
EPS purification.
Our preliminary experiment indicated that strain SM20310 had the highest EPS production after it was cultured at 15°C for 3 days in the liquid marine medium as described above (see Fig. S1 in the supplemental material). Therefore, strain SM20310 was cultured at 15°C with shaking at 200 rpm for 3 days for EPS production. The EPS was precipitated from the supernatant of the fermentation broth with 3 volumes of chilled absolute ethanol. The precipitate was dissolved in deionized water, and its proteins were removed using the Sevag method (20). The EPS was precipitated from the deproteinated solution with 3 volumes of chilled absolute ethanol and desiccation in vacuo. The EPS was redissolved in deionized water and purified using anion-exchange chromatography on a column (1.6 by 30 cm) of DEAE-Sepharose Fast Flow (GE Healthcare), which was eluted with a linear gradient of 0 to 1 M NaCl aqueous solution at a flow rate of 60 ml h−1. The EPS fraction was collected and further purified using gel filtration chromatography on a column (1.6 by 100 cm) of Sepharose 4B (GE Healthcare), which was eluted with 0.1 M NaCl aqueous solution at a flow rate of 12 ml h−1. During these processes, the polysaccharide content was assayed using the phenol-sulfuric acid method. The purified EPS was dialyzed against deionized water and lyophilized.
The UV and visible (UV-Vis) absorption spectra of the EPS were recorded on a Jasco V-550 spectrophotometer. Purity analysis of the EPS was performed on a Shimadzu analytical high-pressure liquid chromatography (HPLC) system with a Shimadzu autoinjector using a Waters Ultrahydrogel linear column (7.8 by 300 mm) at 40°C. The sample was examined using a Shimadzu refractive index detector. The molecular mass of the EPS was analyzed using the same HPLC system with T-dextrans (Amersham Pharmacia Biotech, United Kingdom) of different molecular masses (T2000, T500, T70, and T40) as standards.
Structural characterization of the EPS. (i) Glycosyl composition analysis.
Glycosyl composition analysis of the EPS was performed using combined gas chromatography/mass spectrometry (GC/MS) of the per-O-trimethylsilyl (TMS) derivatives of the monosaccharide methyl glycosides produced from the EPS sample by acidic methanolysis. A 1-mg aliquot of the EPS was added to separate tubes with 20 μg of inositol as the internal standard and then lyophilized. Methyl glycosides were prepared from the dry sample following the mild acid treatment by methanolysis in 1 M HCl in methanol at 80°C for 16 h. Re-N-acetylation, which was used to detect amino sugars, was then performed with pyridine and acetic anhydride in methanol. The sample was then per-O-trimethylsilylated by treatment with Tri-Sil (Pierce) at 80°C for 0.5 h (21, 22). GC/MS analysis of the TMS methyl glycosides was performed on a Hewlett Packard 6890 GC interfaced to a 5975b mass selective detector (MSD), using an All Tech EC-1 fused-silica capillary column (30 m by 0.25 mm).
(ii) Glycosyl linkage analysis.
For glycosyl linkage analysis, the EPS was permethylated, depolymerized, reduced, and acetylated. The resultant partially methylated alditol acetates (PMAAs) were analyzed using GC/MS as described by York et al. (22). Initially, 1 mg EPS was suspended in approximately 200 μl of dimethyl sulfoxide (DMSO), placed on a magnetic stirrer for 2 days, and then permethylated by treatment with sodium hydroxide and methyl iodide in dry DMSO (23). The sample was incubated in 1 M NaOH in DMSO for 10 min at room temperature, followed by sequential addition of 200 μl of methyl iodide, 333 μl of 1.6 M NaOH in DMSO, 200 μl of methyl iodide, and 40 μl of 1 M NaOH every 10 min. Then, 40 μl of 1 M NaOH was added, followed by a 40-min incubation. After the incubation period, 100 μl of methyl iodide was added, with the reaction allowed to proceed for another 40 min, before 2 ml water was added, and the methyl iodide was evaporated by bubbling. After the sample preparation, the permethylated material was hydrolyzed using 2 M trifluoroacetic acid at 121°C for 2 h in a sealed tube, reduced with NaBD4, and then acetylated using acetic anhydride-trifluoroacetic acid. The resulting PMAAs were analyzed on a Hewlett Packard 5890 GC interfaced to a 5970 MSD in electron impact ionization mode. Separation was performed on a 30-m Supelco 2330 bonded-phase fused-silica capillary column.
(iii) NMR spectroscopy.
The EPS sample was deuterium exchanged by dissolving the sample in D2O and lyophilizing it, dissolving the sample in 0.27 ml D2O, and placing the sample in a 3-mm nuclear magnetic resonance (NMR) tube. One-dimensional (1-D) proton and two-dimensional (2-D) gradient-selected correlation spectroscopy (gCOSY), total correlation spectroscopy (TOCSY), heteronuclear single-quantum correlation spectroscopy (HSQC), nuclear Overhauser effect spectroscopy (NOESY), gradient-selected heteronuclear multiple quantum coherence (gHMBC), and heteronuclear single quantum coherence-total correlation spectroscopy (HSQC-TOCSY) were performed on Varian Inova 500- and 600-MHz spectrometers at 343 K (70°C) using standard Varian pulse sequences. Chemical shifts (δ's) were measured relative to the δ of internal acetone (δH = 2.225 ppm for 1H spectra, δC = 30.89 ppm for 13C spectra).
Effect of the EPS on high-salinity tolerance of strain SM20310 and Escherichia coli.
2216E media, composed of 5 g liter−1 peptone, 1 g liter−1 yeast extract, and artificial seawater (pH 7.5) with different salinities (10, 30, 70, 110, 120, and 130‰) and EPS concentrations (0, 0.5, and 1.0 mg ml −1), were prepared for strain SM20310. LB media, composed of 10 g liter−1 peptone, 5 g liter−1 yeast extract, and deionized water (pH 7.5) with different salinities (10, 30, 70, 110, 120, and 130‰) and EPS concentrations (0, 0.5, and 1.0 mg ml−1), were prepared for E. coli DH5α. Strain SM20310 was inoculated into the 2216E media and incubated at 200 rpm and 15°C. E. coli DH5α was inoculated into the LB media and incubated at 200 rpm and 37°C. Cell growth (optical density at 600 nm [OD600]) was evaluated every 24 h during fermentation.
Analysis of cryoprotective effect of the EPS.
To investigate the cryoprotective effect of the EPS, strain SM20310 and E. coli DH5α were subjected to freeze-thaw cycles in the presence or absence of the EPS. Strain SM20310 was inoculated into an Erlenmeyer flask (250 ml) containing 50 ml 2216E medium and incubated for 12 h at 15°C and 200 rpm until reaching late logarithmic phase (OD600 = 0.8). The cells were harvested using centrifugation at 6,000 rpm for 10 min at 4°C, washed three times, and then resuspended with sterile artificial seawater. At the same time, E. coli was cultured to late logarithmic phase (OD600 = 0.8), harvested, washed, and suspended with 0.9% (wt/vol) NaCl solution. An equal volume of EPS solutions at different concentrations (0, 0.4, 1, 5, 10, 20, 40, and 60 mg ml−1) was mixed with the suspensions of strain SM20310 or E. coli cells in 1.5-ml sterile tubes. An equal volume of seawater mixed with the suspensions of strain SM20310 or 0.9% (wt/vol) NaCl solution mixed with the suspensions of E. coli was used as a control. The mixtures were frozen at −80°C for 30 min and thawed for 20 min in a water bath at 25°C. The freeze-thaw cycle was repeated 7 consecutive times. At the end of the 3rd, 5th, or 7th thawing, the mixture was properly diluted, and the dilution was spread on an MA plate and then cultured to examine the number of surviving cells.
Nucleotide sequence accession number.
The GenBank accession number for the 16S rRNA gene sequence of strain SM20310 is JN837485.
RESULTS
Screening and identification of strain SM20310.
Thirteen EPS-producing isolates were screened from 110 sea ice isolates using the Congo red agar plate method (see Fig. S2 in the supplemental material). The EPS yields of these isolates under our experimental conditions were in the range of 50 to 567 mg liter−1 (Fig. 1). Isolate SM20310, which contained the highest EPS production (567 mg liter−1 or 90 mg g−1 biomass [dry weight]), was selected for further analysis.
Fig 1.
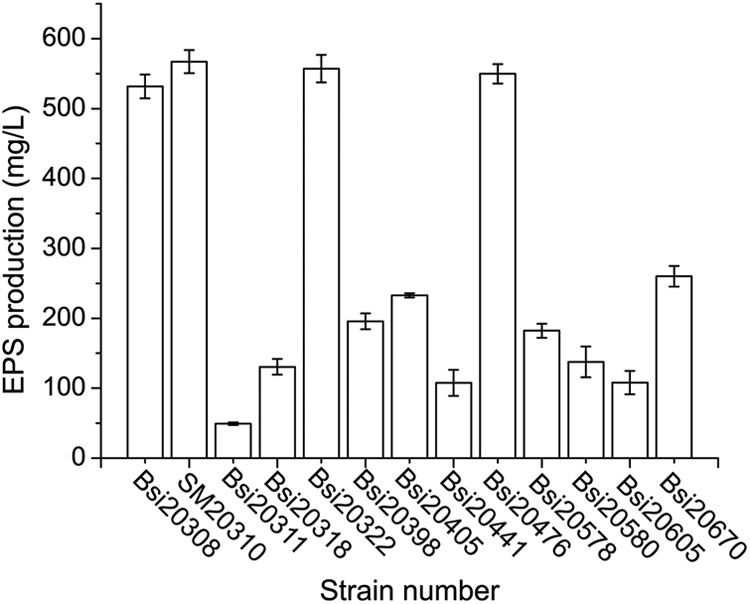
EPS production of 13 bacterial strains. EPS production was determined by quantifying the carbohydrate content as d-glucose equivalents using the phenol-sulfuric acid method. The values shown are means ± SDs from three experimental repeats.
On the basis of the amplified sequence of the nearly complete 16S rRNA gene of strain SM20310, the phylogenetic affiliation of this strain was determined. Strain SM20310 was classified in the genus Pseudoalteromonas, in which many Antarctic strains are found (14). Strain SM20310 is closely related to Pseudoalteromonas issachenkonii and Pseudoalteromonas tetraodonis.
Extraction and purification of the EPS from strain SM20310.
After precipitation from the fermentation broth with absolute ethanol and deproteination using the Sevag method, the EPS produced by strain SM20310 was further purified by anion-exchange chromatography (see Fig. S3 in the supplemental material) and gel filtration chromatography (see Fig. S4 in the supplemental material). Two EPS peaks were eluted from the Sepharose 4B column. The first fraction was too scarce to collect, and the second fraction was collected and analyzed. The purified EPS had no absorption at 280 nm or 260 nm in the UV-Vis absorption spectra, indicating the absence of protein and nucleic acid. Only one symmetrical acute peak was detected from the purified EPS sample on the Shimadzu analytical HPLC system (see Fig. S5 in the supplemental material), which indicated that this EPS sample consisted of a single homogeneous component and could be used for structure analysis. The molecular mass of the EPS analyzed by the same HPLC system was higher than 2 × 106 Da.
Structural characterization of the EPS. (i) Glycosyl composition and glycosyl linkage analysis.
Glycosyl composition analysis of the EPS was performed by GC/MS (see Fig. S6 in the supplemental material). The results of the analysis showed that mannose is the predominant component of the EPS, with peaks for glucose, galactose, and rhamnose and minor peaks for N-acetylgalactosamine, N-acetylglucosamine, and xylose also being found. The percentages of each monosaccharide are shown in Table 1.
Table 1.
Glycosyl composition of the EPS from strain SM20310
| Glycosyl residue | Amta (mol %) |
|---|---|
| Rhamnose (Rha) | 2.1 |
| Xylose (Xyl) | 0.9 |
| Mannose (Man) | 71.7 |
| Galactose (Gal) | 9.0 |
| Glucose (Glc) | 10.7 |
| N-Acetylgalactosamine (GalNAc) | 1.5 |
| N-Acetylglucosamine (GlcNAc) | 4.0 |
Amounts are expressed as the mole percent of total carbohydrates.
Consistent with the glycosyl composition analysis, glycosyl linkage analysis of the EPS sample showed that it is mainly composed of terminally linked mannopyranose, 3-linked glucopyranose, 2,6-linked mannopyranose, 2,4,6- and 2,3,6-linked mannopyranose, and 6-linked mannopyranose, with minor peaks for other residues also being found (Table 2; see Fig. S7 in the supplemental material). Overall, the EPS was a highly branched mannan containing low levels of other sugars.
Table 2.
Glycosyl linkage of the EPS from strain SM20310
| Glycosyl residue | % present |
|---|---|
| Terminal mannopyranose | 19.5 |
| Terminal galactopyranose | 2.0 |
| 3-linked glucopyranose | 19.1 |
| 2-linked mannopyranose | 2.8 |
| 3-linked galactopyranose | 2.8 |
| 4-linked mannopyranose | 3.1 |
| 6-linked mannopyranose | 7.3 |
| 6-linked glucopyranose | 2.8 |
| 4-linked glucopyranose | 2.9 |
| 2,3-linked mannopyranose | 3.4 |
| 3,4-linked mannopyranose | 1.5 |
| 2,3-linked glucopyranose | 2.7 |
| 2,4-linked hexopyranose | 1.7 |
| 4,6-linked mannopyranose | 1.5 |
| 3,6-linked mannopyranose | 4.1 |
| 2,6-linked mannopyranose | 10.5 |
| 3,4,6-linked mannopyranose | 1.0 |
| 2,4,6- and 2,3,6-linked mannopyranose | 8.4 |
| 2,3,4,6-linked mannopyranose | 3.0 |
(ii) Structure analysis of the EPS by NMR.
The 1-D proton spectrum (see Fig. S8 in the supplemental material) showed several anomeric signals (δH = 5.29, 5.11, and 4.91 ppm, and overlapping signals at 5.06 ppm), a number of overlapping peaks in the carbohydrate ring region between 3.4 and 4.2 ppm, and additional, noncarbohydrate signals between 0.8 and 3.0 ppm mostly arising from amino acid contamination. On the basis of the 2-D NMR data (see Fig. S9 to S14 in the supplemental material), major anomeric signals arose from variously linked α-mannosyl residues. The 2-D spectra showed 4 major anomeric signals, although numerous minor anomeric signals were present, and all the major signals were attributed to α-mannose residues. The partial chemical shift assignment of the NMR is shown in Table 3.
Table 3.
Chemical shift of signals in 1H and 13C NMR spectra of the EPS from strain SM20310
| Compound | Residue | Isotope | Chemical shift (ppm) at indicated position |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 6′ | |||
| I | →2,6-α-Man-1→2 | 1H | 5.28 | 4.12 | 3.92 | 3.79 | 3.84 | 4.01 | 3.72 |
| 13C | 101.1 | 79.1 | 71.3 | 67.7 | 71.4 | 66.8 | |||
| II | →2-α-Man-1→6 | 1H | 5.11 | 4.04 | 3.94 | 3.81 | 3.76 | 3.89 | 3.78 |
| 13C | 99.0 | 79.3 | 71.3 | 67.6 | 74.1 | 61.9 | |||
| III | →6-α-Man-1→2 | 1H | 5.06 | 4.08 | 3.84 | 3.70 | |||
| 13C | 102.7 | 70.9 | 71.5 | 67.9 | |||||
| IV | t-α-Man-1→6 | 1H | 4.92 | 4.00 | 3.85 | 3.70 | |||
| 13C | 100.3 | 71.1 | 71.5 | 67.9 | |||||
On the basis of these results, a major structural motif of the heteropolysaccharide was assigned (Fig. 2). It is composed of a backbone of alternating →2)-α-Manp-(1→ and →6)-α-Manp-(1→ residues, in which a considerable part of the →6)-α-Manp-(1→ residues is branched at the O-2 position with either a single t-mannosyl residue or two mannosyl residues.
Fig 2.
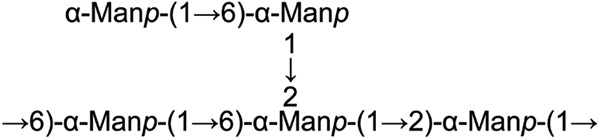
Structure of the predominant repeating units of the EPS from strain SM20310.
Analysis of ecological roles of the EPS. (i) Effect of the EPS on high-salinity tolerance of strain SM20310 and E. coli.
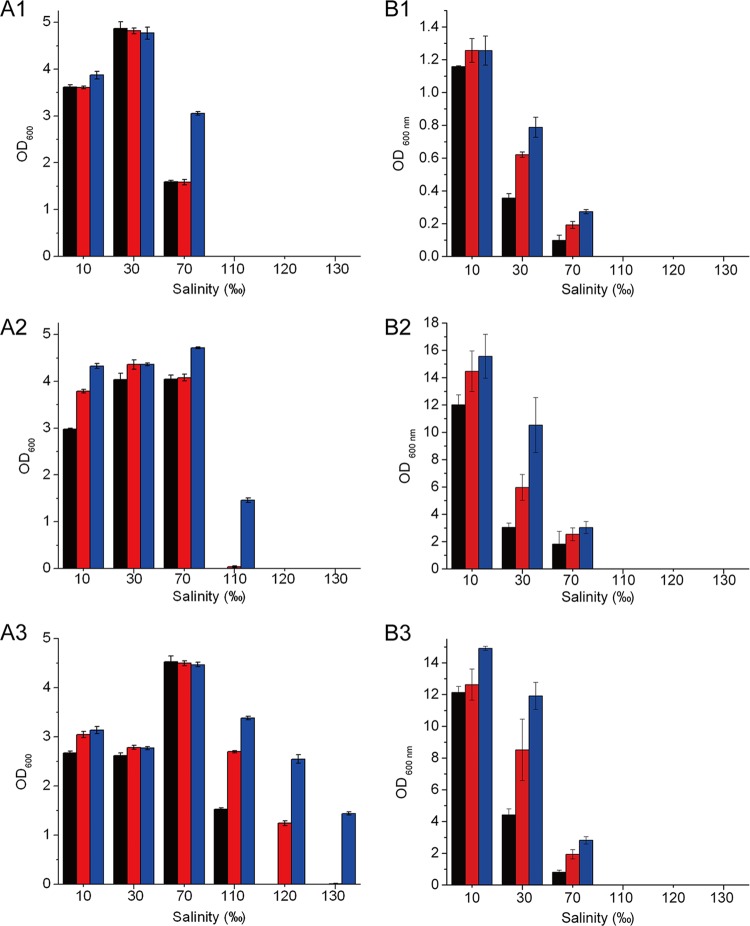
The effect of the EPS on the high-salinity tolerance of strain SM20310 and E. coli was studied by measuring the biomass of strain SM20310 and E. coli cultured in media with different salinities. Without the EPS in the medium, SM20310 could survive at a salinity of only ≤110‰. In contrast, SM20310 could survive at a salinity of 120‰ with 0.5 mg ml−1 EPS in the medium and at a salinity of 130‰ in the presence of 1.0 mg ml−1 EPS (Fig. 3A 1 to 3). However, the EPS did not improve the maximum tolerable salinity of E. coli (Fig. 3B1 to 3). In addition, it was concluded from Fig. 3 that the EPS could increase the growth rate of strain SM20310 when cultured in medium with a salinity of 70 to 120‰ and that E. coli when cultured in medium with a salinity of 30 to 70‰. Therefore, these results indicated that the EPS produced by SM20310 could improve the high-salinity tolerance of strain SM20310.
Fig 3.
Effect of the EPS on the high-salinity tolerance of strain SM20310 (A) and E. coli (B). (A1, B1) Incubation for 24 h; (A2, B2) incubation for 48 h; (A3, B3) incubation for 72 h. The values shown are means ± SDs from three experimental repeats. Black bars, absence of EPS; red bars, 0.5 mg ml−1 EPS; blue bars, 1.0 mg ml−1 EPS.
(ii) Cryoprotective effect of the EPS on strain SM20310 and E. coli.
The effects of the EPS on the survival of strain SM20310 and E. coli cells after freeze-thaw cycles are shown in Fig. 4. With an increase in the EPS concentration from 0 to 30 mg ml−1, the number of surviving cells of both strain SM20310 and E. coli increased after 3, 5, or 7 freeze-thaw cycles, indicating that the EPS had a cryoprotective effect on both strain SM20310 and E. coli. In the presence of 30 mg ml−1 EPS, the number of surviving cells of strain SM20310 after 3 freeze-thaw cycles was 2.65 × 106 ml−1, which was 7 times that of the control (Fig. 4A). The number of surviving cells of E. coli after 3 freeze-thaw cycles was 1.83 × 106 ml−1, which was 18 times that of the control (Fig. 4B). Although the presence of the EPS could increase the survival of strain SM20310 and E. coli after repeated freeze-thaw cycles, the number of surviving cells of both strain SM20310 and E. coli was reduced with an increase in the number of freeze-thaw cycles in the presence or absence of the EPS. In the presence of 30 mg ml−1 EPS, compared with the number of surviving cells after 3 freeze-thaw cycles, the number of surviving cells of strain SM20310 was reduced from 2.65 × 106 ml−1 to 6.95 × 105 ml−1 and 1.60 × 105 ml−1 after 5 and 7 freeze-thaw cycles, respectively, and that of E. coli was reduced from 1.83 × 106 ml−1 to 2.65 × 105 ml−1 and 1.25 × 105 ml−1, respectively.
Fig 4.
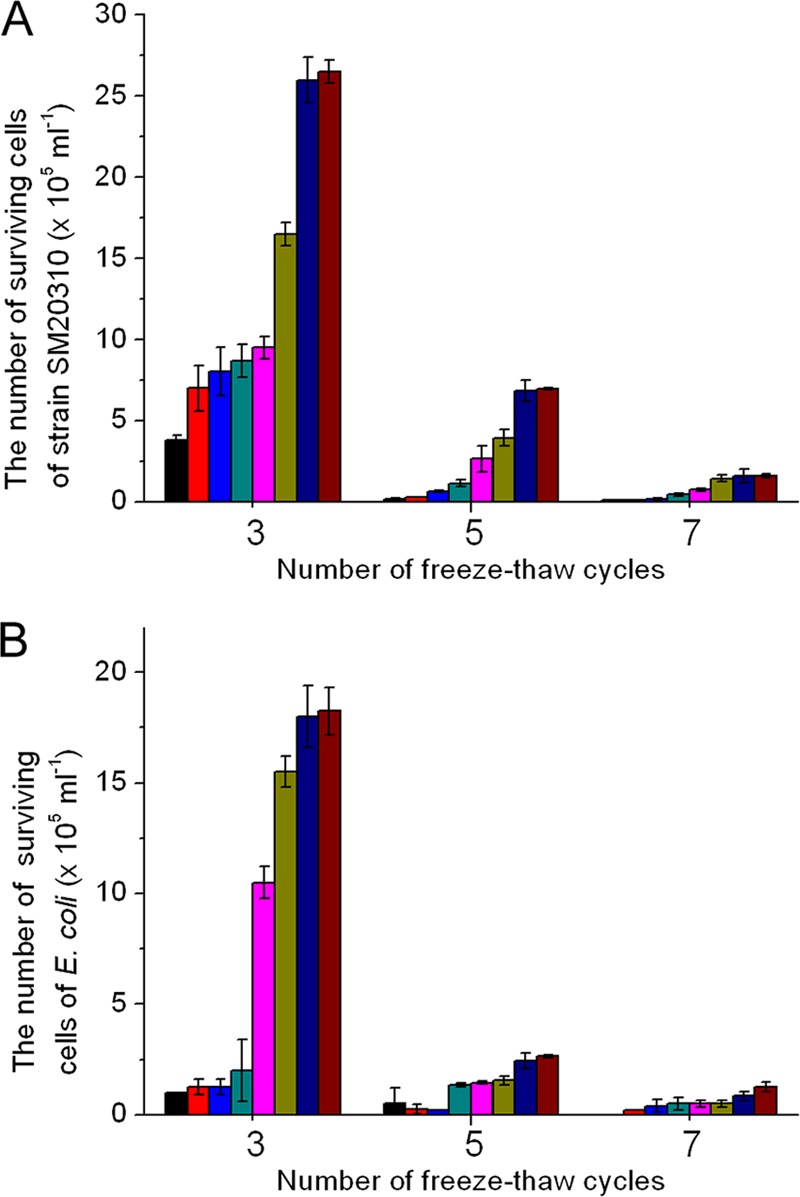
Cryoprotective effect of the EPS on strain SM20310 (A) and E. coli (B) after 3, 5, and 7 freeze-thaw cycles. An equal volume of seawater (A) or 0.9% (wt/vol) NaCl solution (B) in place of EPS was used as the control. The values shown are means ± SDs from three experimental repeats. Black bars, absence of EPS; red bars, 0.2 mg ml−1 EPS; blue bars, 0.5 mg ml−1 EPS; dark cyan bars, 2.5 mg ml−1 EPS; magenta bars, 5.0 mg ml−1 EPS; dark yellow bars, 10 mg ml−1 EPS; navy blue bars, 20 mg ml−1 EPS; wine-colored bars, 30 mg ml−1 EPS.
DISCUSSION
Studies on the diversity of polar sea ice microbial communities show that the Gammaproteobacteria is a dominant taxonomic group generally found in this environment, as determined by cultivation- and non-cultivation-dependent techniques (24–26). Pseudoalteromonas, a genus of the Gammaproteobacteria, is particularly common in sea ice (14). In this study, strain SM20310, which had the highest EPS production, was screened from 110 Arctic sea ice isolates and was identified to be a member of the genus Pseudoalteromonas.
A better understanding of the structure of bacterial EPSs is important for studying their ecological roles and exploring their biotechnological uses. However, only the glycosyl composition of some EPSs produced by sea ice bacteria has been reported (14), and no steric structure of any bacterial EPS from sea ice has been solved. Chemical and structural characterizations of some EPSs produced by Pseudoalteromonas have been reported (4, 14, 27). However, taxonomic relatedness does not necessarily ensure the similarity of EPS structures (1). Some studies found that EPSs produced by closely related Antarctic strains in the genus Pseudoalteromonas varied substantially in their crude chemical compositions (14, 15). The results of this study showed that the EPS produced by SM20310 is a highly complicated α-mannan comprised of more than 70% mannose. The structure of this EPS is different from that of EPSs secreted by other marine bacteria (6, 14, 27, 28). Mannose is a main component of many EPSs from cold marine environment strains. The EPS produced by Pseudoalteromonas haloplanktis TAC 125, isolated from Antarctic seawater, has a phosphomannan structure comprised of a backbone of 6-linked mannose residues which is highly branched at C-2 with mono-, di-, and trisaccharide side chains containing 2- and 3-linked mannose units (27). Nichols et al. (14) isolated 10 EPS-producing strains from the Antarctic sea ice and seawater and found that mannose represented the most abundant neutral sugar in the EPSs. However, the ecological role of mannose in these EPSs is still unknown. In addition, many EPSs secreted by marine bacteria are polyanionic for the presence of uronic acids, ketal-linked pyruvate, or inorganic residues such as phosphate or sulfate as well (2). However, these components were not found in the EPS from strain SM20310.
The ecological roles of EPSs from bacteria are related to the ecological niches and the natural environment from which they have been isolated (2). High salinity is an important characteristic of Arctic and Antarctic sea ice. As ice consolidates, salts from the seawater are expelled to form brines that collect in brine channels and pores. In this case, the ice-trapped organisms have to suffer salinity three times that of seawater (10). There is yet no report on the effect of EPS on bacterial tolerance to high salinity. The EPS produced by strain SM20310 greatly enhanced the high-salinity tolerance of the strain, which is important for the strain to adapt to the high-saline sea ice environment in the winter.
Freeze-thaw cycles are quite common in the cold regions of the Arctic and Antarctic, including sea ice. Selbmann et al. (29) reported that a homosaccharide of glucose produced by the fungus Phoma herbarum CCFEE 5080, isolated from Antarctic soil, could improve mycelial growth after repeated freeze-thaw cycles, suggesting that this EPS plays a cryoprotective role for the strain in the harsh Antarctic environment. There has been no report on the cryoprotective effect of EPSs from sea ice bacteria. Our result showed that the EPS produced by the Arctic sea ice strain SM20310 significantly increased the tolerance of the strain to repeated freeze-thaw cycles, which suggests that the EPS produced by strain SM20310 would be beneficial for the strain to adapt to the freeze-thaw sea ice environment. Kim and Yim (12) showed that the EPS produced by the Antarctic bacterium Pseudoalteromonas arctica KOPRI 21653 could improve the freeze-thaw survival ratio of E. coli and suggested that it may have biotechnological potential as a cryoprotection agent. The EPS from strain SM20310 also significantly improved the freeze-thaw survival ratio of E. coli, thereby suggesting that this EPS may have a universal impact on microorganism cryoprotection.
In summary, SM20310, a Pseudoalteromonas strain screened from Arctic sea ice, produced a highly complex α-mannan. This EPS had a cryoprotective effect on strain SM20310 and could improve the high-salinity tolerance of SM20310, characteristics beneficial for the strain to adapt to the sea ice environment. Further investigation is necessary to elucidate how the EPS from SM20310 may be acting mechanistically as an organic ligand or a protectant against low temperature or high salinity and how the chemical composition and the structure of the EPS are related to its ecological roles.
Supplementary Material
ACKNOWLEDGMENTS
We thank Parastoo Azadi at the Complex Carbohydrate Research Center of the University of Georgia for the NMR analysis.
The work was supported by National Natural Science Foundation of China (grants 31025001, 41106161, 41176130, and 31170055), the Hi-Tech Research and Development program of China (grants 2011AA090703, 2012AA091605, and 2012AA092103), the Natural Science Foundation of Shandong Province, China (grants JQ200910 and ZR2009DZ002), and the Foundation for Young Excellent Scientists in Shandong Province, China (grant BS2010SW015).
Footnotes
Published ahead of print 19 October 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01801-12.
REFERENCES
- 1. Mancuso Nichols CA, Guezennec J, Bowman JP. 2005. Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: a review. Mar. Biotechnol. (NY) 7:253–271 [DOI] [PubMed] [Google Scholar]
- 2. Poli A, Anzelmo G, Nicolaus B. 2010. Bacterial exopolysaccharides from extreme marine habitats: production, characterization and biological activities. Mar. Drugs 8:1779–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cambon-Bonavita MA, Raguenes G, Jean J, Vincent P, Guezennec J. 2002. A novel polymer produced by a bacterium isolated from a deep-sea hydrothermal vent polychaete annelid. J. Appl. Microbiol. 93:310–315 [DOI] [PubMed] [Google Scholar]
- 4. Qin G, Zhu L, Chen X, Wang PG, Zhang Y. 2007. Structural characterization and ecological roles of a novel exopolysaccharide from the deep-sea psychrotolerant bacterium Pseudoalteromonas sp. SM9913. Microbiology 153:1566–1572 [DOI] [PubMed] [Google Scholar]
- 5. Raguenes G, Cambon-Bonavita MA, Lohier JF, Boisset C, Guezennec J. 2003. A novel, highly viscous polysaccharide excreted by an Alteromonas isolated from a deep-sea hydrothermal vent shrimp. Curr. Microbiol. 46:448–452 [DOI] [PubMed] [Google Scholar]
- 6. Rougeaux H, Guezennec J, Carlson RW, Kervarec N, Pichon R, Talaga P. 1999. Structural determination of the exopolysaccharide of Pseudoalteromonas strain HYD 721 isolated from a deep-sea hydrothermal vent. Carbohydr. Res. 315:273–285 [DOI] [PubMed] [Google Scholar]
- 7. Rougeaux H, Kervarec N, Pichon R, Guezennec J. 1999. Structure of the exopolysaccharide of Vibrio diabolicus isolated from a deep-sea hydrothermal vent. Carbohydr. Res. 322:40–45 [DOI] [PubMed] [Google Scholar]
- 8. Decho AW. 1990. Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr. Mar. Biol. Annu. Rev. 28:73–153 [Google Scholar]
- 9. Lizotte MP. 2001. The contributions of sea ice algae to Antarctic marine primary production. Am. Zool. 41:57–73 [Google Scholar]
- 10. Thomas DN, Dieckmann GS. 2002. Antarctic Sea ice—a habitat for extremophiles. Science 295:641–644 [DOI] [PubMed] [Google Scholar]
- 11. Krembs C, Eicken H, Junge K, Deming JW. 2002. High concentrations of exopolymeric substances in Arctic winter sea ice: implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep Sea Res. 49:2163–2181 [Google Scholar]
- 12. Kim SJ, Yim JH. 2007. Cryoprotective properties of exopolysaccharide (P-21653) produced by the Antarctic bacterium, Pseudoalteromonas arctica KOPRI 21653. J. Microbiol. 45:510–514 [PubMed] [Google Scholar]
- 13. Junge K, Eicken H, Deming JW. 2004. Bacterial activity at −2 to −20 degrees C in Arctic wintertime sea ice. Appl. Environ. Microbiol. 70:550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mancuso Nichols CA, Lardiere SG, Bowman JP, Nichols PD, Gibson JAE, Guezennec J. 2005. Chemical characterization of exopolysaccharides from Antarctic marine bacteria. Microb. Ecol. 49:578–589 [DOI] [PubMed] [Google Scholar]
- 15. Mancuso Nichols CA, Garon S, Bowman JP, Raguenes G, Guezennec J. 2004. Production of exopolysaccharides by Antarctic marine bacterial isolates. J. Appl. Microbiol. 96:1057–1066 [DOI] [PubMed] [Google Scholar]
- 16. Yu Y, Li H, Zeng Y, Chen B. 2009. Extracellular enzymes of cold-adapted bacteria from Arctic sea ice, Canada Basin. Polar Biol. 32:1539–1547 [Google Scholar]
- 17. Freeman DJ, Falkiner FR, Keane CT. 1989. New method for detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 42:872–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350–356 [Google Scholar]
- 19. Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–175 In Stackebrandt E, Goodfellow M. (ed), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, United Kingdom [Google Scholar]
- 20. Staub AM. 1965. Removal of proteins: Sevag method. Methods Carbohydr. Chem. 5:5–6 [Google Scholar]
- 21. Merkle RK, Poppe I. 1994. Carbohydrate composition analysis of glycoconjugates by gas-liquid chromatography/mass spectrometry. Methods Enzymol. 230:1–15 [DOI] [PubMed] [Google Scholar]
- 22. York WS, Darvill AG, McNeil M, Stevenson TT, Albersheim P. 1986. Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol. 118:3–40 [Google Scholar]
- 23. Ciucanu I, Kerek F. 1984. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131:209–217 [Google Scholar]
- 24. Bowman JP, McCammon SA, Brown MV, Nichols DS, McMeekin TA. 1997. Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl. Environ. Microbiol. 63:3068–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brinkmeyer R, Knittel K, Jurgens J, Weyland H, Amann R, Helmke E. 2003. Diversity and structure of bacterial communities in Arctic versus Antarctic pack ice. Appl. Environ. Microbiol. 69:6610–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Staley JT, Gosink JJ. 1999. Poles apart: biodiversity and biogeography of sea ice bacteria. Annu. Rev. Microbiol. 53:189–215 [DOI] [PubMed] [Google Scholar]
- 27. Corsaro MM, Lanzetta R, Parrilli E, Parrilli M, Tutino ML, Ummarino S. 2004. Influence of growth temperature on lipid and phosphate contents of surface polysaccharides from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC 125. J. Bacteriol. 186:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bozzi L, Milas M, Rinaudo M. 1996. Characterization and solution properties of a new exopolysaccharide excreted by the bacterium Alteromonas sp. strain 1644. Int. J. Biol. Macromol. 18:9–17 [DOI] [PubMed] [Google Scholar]
- 29. Selbmann L, Onofri S, Fenice M, Federici F, Petruccioli M. 2002. Production and structural characterization of the exopolysaccharide of the Antarctic fungus Phoma herbarum CCFEE 5080. Res. Microbiol. 153:585–592 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.