Abstract
Microorganisms are able to survive and grow in changing environments by activating stress adaptation mechanisms which may enhance bacterial robustness. Stress-induced enhanced robustness complicates the predictability of microbial inactivation. Using psychrotolerant Bacillus weihenstephanensis strain KBAB4 as a model, we investigated the impact of the culturing temperature on mild-oxidative-stress-induced (cross-)protection toward multiple stresses, including severe oxidative, heat, and acid stresses. Culturing at a refrigeration temperature (7°C) compared to the optimal growth temperature (30°C) affected both the robustness level of B. weihenstephanensis and the oxidative stress adaptive response. Scavengers of reactive oxygen species have a crucial role in adaptation to oxidative stresses, and this points to a possible predictive role in mild-oxidative-stress-induced robustness. Therefore, the catalase activity was determined upon mild oxidative stress treatment and was demonstrated to be significantly correlated with the robustness level of mild-stress-treated cells toward severe oxidative and heat stresses but not toward severe acid stress for cells grown at both refrigeration and optimal temperatures. The quantified correlations supported the predictive quality of catalase activity as a biomarker and also underlined that the predictive quality is stress specific. Biomarkers that are able to predict stress-induced enhanced robustness can be used to better understand stress adaptation mechanisms and might allow the design of effective combinations of hurdles to control microbial behavior.
INTRODUCTION
Bacteria are frequently exposed to changing environments in their natural habitats and throughout the human food chain. Dynamic conditions trigger adaptation, and microorganisms can gain robustness upon the activation of stress adaptive mechanisms. Stress-induced enhanced robustness complicates predictions of microbial inactivation, and the early detection of these adaptive traits will allow a better control of stress-adaptive behavior.
Bacillus weihenstephanensis is a psychrotolerant species belonging to the Bacillus cereus group, and due to its abilities to grow at refrigeration temperatures and form heat-resistant spores, this microorganism can proliferate in chilled and minimally processed foods. It has been isolated from egg products (1, 2) and from milk and dairy farm environments (3). In contrast to its closely related species B. cereus, B. weihenstephanensis has not been reported to be involved in food-borne disease. However, virulence in an insect model at a low temperature was recently reported (4), and B. weihenstephanensis strains capable of producing cereulide have been described (5, 6), highlighting the food-borne disease potential of this organism.
The ability of the mesophilic species B. cereus to gain robustness elicited by mild-stress pretreatment has been well documented (7–11), and so has the stress-dependent nature of the protective effect induced by such a treatment. Also, the psychrotolerant species B. weihenstephanensis was shown to become more heat resistant after pretreatment with mild stresses (12), and an overlap in the production of selected stress proteins may contribute to this cross-protective phenomenon (12). Chilling is commonly used in the minimal-processing food chain to control bacterial growth and might influence the adaptive stress response of food-borne microorganisms. As indicated by the limited literature describing the effect of low temperatures on stress adaptation, the adaptive response providing a robustness enhancement indeed depends on the culturing temperature during adaptation (13, 14), and this is especially relevant for further investigations of psychrotolerant species such as B. weihenstephanensis.
Recently, we designed a framework for identifying biomarkers that could predict the robustness level of mild-stress-treated cells (11). Comparisons of genome-wide transcriptome profiles of B. cereus upon exposure to various mild stresses pointed to a rather limited number of candidate biomarkers. Their induction seemed to be stress independent and might therefore be important in adaptation to multiple stresses. The predictive potential of these candidate biomarkers was evaluated by measuring transcript, protein, and activity levels upon mild-stress treatment, after which their induction levels were quantitatively correlated with the robustness level of mildly stressed cells. This revealed that catalase activity could function as a biomarker for mild-stress-induced robustness in B. cereus (11, 15). Scavengers of reactive oxygen species (ROS), such as catalases, are ubiquitous in nature and are known to have crucial roles in stress adaptation and survival in species other than B. cereus, including Bacillus subtilis (16, 17), and also in Saccharomyces cerevisiae (18, 19) and have the potential to function as biomarkers for stress-induced enhanced robustness in psychrotolerant bacteria such as B. weihenstephanensis. A good performance (i.e., predictive quality) of biomarkers in more than one species will strengthen their predictive potential. Therefore, in this study, we investigated the ability of B. weihenstephanensis to gain robustness toward multiple stresses upon pretreatment with mild oxidative stress at optimal and low incubation temperatures and evaluated whether catalase activity could function as a biomarker for mild-stress-induced robustness.
MATERIALS AND METHODS
Bacterial strain and inoculum preparation.
The bacterial strain used in this study was B. weihenstephanensis KBAB4, kindly provided by the Institute National de la Recherche Agronomique (INRA, France). Stock cultures grown in brain heart infusion (BHI; Becton Dickinson) broth were stored at −80°C in 25% (vol/vol) glycerol. Before each experiment, bacteria were inoculated into BHI broth and incubated overnight at 30°C with shaking at 200 rpm (Innova 4335; New Brunswick Scientific) or at 7°C with shaking at 200 rpm (Forma orbital shaker 481; Thermo Electron Corporation) until the stationary phase.
Treatment with mild oxidative stress and subsequent severe H2O2, heat, and acid stress treatment.
The stationary-phase cell suspension was inoculated into fresh BHI broth and incubated at 30°C or 7°C with shaking at 200 rpm until the exponential growth phase (absorbance at 600 nm of 0.4 to 0.5) (Novaspec II spectrophotometer; Pharmacia Biotech). The presence of spores in these exponential-phase cell suspensions was investigated; the number of spores was less than 1 CFU/ml, and the pH of the cultures was not below 7.0. Upon reaching this optical density, cells cultured at 30°C were exposed to 0.05 mM H2O2 for 2, 5, 10, 15, 20, 30, and 60 min with shaking at 200 rpm, whereas cells cultured at 7°C were exposed to 0.05 mM H2O2 for 0.25, 0.5, 1, 1.5, 2.5, 3, and 4 h at this temperature.
Both untreated cells and cells treated with mild oxidative stress were subsequently exposed to three severe stress conditions to determine their robustness level by using an inactivation procedure described previously (10). Based on preliminary experiments, the following inactivation conditions were chosen to ensure inactivation within a rather short time span: cells cultured at 30°C were inactivated by heat treatment at 46°C, pH 3.1 treatment at 30°C, and treatment with 0.2 mM H2O2 at 30°C, and cells cultured at 7°C were inactivated by heat treatment at 47°C, pH 3.7 treatment at 30°C, and treatment with 0.5 mM H2O2 at 30°C. Samples were taken before and after severe stress treatment, and decimal dilutions were made in 0.1% peptone saline (1 g neutralized bacteriological peptone [Oxoid, United Kingdom] supplemented with 8.5 g sodium chloride per liter). After acid inactivation and H2O2 inactivation, samples were decimally diluted in BHI broth to ensure no further inactivation during dilution and plating. Appropriate dilutions were surface plated, in duplicate, onto BHI agar plates (BHI broth supplemented with 15 g agar [Oxoid] per liter) by using a spiral plater (Eddy Jet; IUL Instruments), and the plates were incubated at 30°C for at least 24 h. The inactivation experiments were replicated 2 to 4 times on different days. The robustness of both untreated cells and cells treated with mild oxidative stress was determined as the number of microorganisms surviving the inactivation treatment, N(t), compared to the initial number of microorganisms before the inactivation treatment at time zero, N(0).
Determination of catalase activity upon treatment with mild oxidative stress.
Catalase activity was determined in untreated and mild-oxidative-stress-treated cells cultured at 30°C and 7°C was determined according to procedures described previously (10, 20). Briefly, cells were washed in phosphate-buffered saline and subsequently exposed to H2O2, and the decrease in the absorbance at 240 nm was measured over time at 30°C by using a spectrophotometer (Spectramax Plus 384; Molecular Devices). One unit of catalase activity was defined as a decrease in the absorbance at 240 nm of 1 unit per min. The rate of the decrease for each sample was corrected for the amount of cells used in the assay mixture and standardized to an absorbance value of 0.5 at 600 nm. For all experimental conditions, three catalase activity experiments were performed on different days.
Catalase activity was also visualized on polyacrylamide gels for untreated cells and cells treated with mild oxidative stress. For that, crude cell-free protein extracts were obtained according to a similar procedure described previously (20), and 50 μg was separated on a native 10% Tris HCl-polyacrylamide gel (Criterion; Bio-Rad Laboratories). Catalase activity was visualized as previously described (21), using a 2% ferric chloride potassium ferricyanide solution for staining.
Correlation of catalase activity and mild-oxidative-stress-induced robustness.
To evaluate the correlation between the induction of catalase activity and the induction of robustness upon pretreatment with mild oxidative stress, the survival of cells treated with mild oxidative stress (s) was expressed relative to that of untreated cultures (un), log10 transformed, , and then averaged for the replicates. Also, the induction of catalase activity upon treatment with mild oxidative stress was expressed relative to that of untreated cultures, log10 transformed, and averaged for the replicates. The induction of catalase activity was then correlated to the induction of mild-stress-induced robustness, and the Pearson correlation coefficient, r, was calculated and tested for significance by using SPSS Statistics.
RESULTS
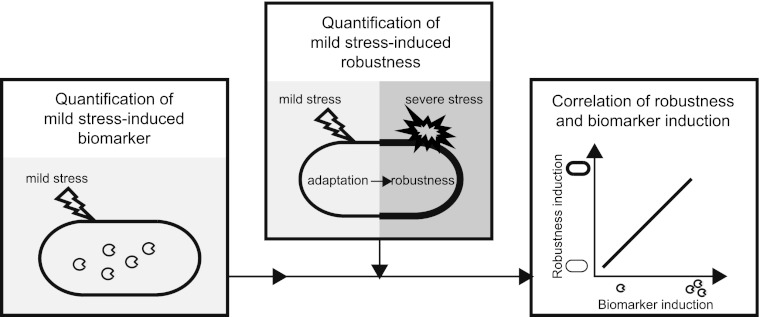
To assess whether catalase activity could function as a biomarker for mild-stress-induced robustness for B. weihenstephanensis, a previously designed (10) stepwise approach was followed (Fig. 1). First, the induction pattern of catalase activity upon mild-stress treatment was determined. Previous findings pointed to the promising predictive potential of catalase activity (11, 15), and therefore, catalase activity was selected as a candidate biomarker in this study. The ability of B. weihenstephanensis to gain a robustness advantage upon mild-stress pretreatment was then investigated and quantified. To evaluate whether catalase activity could predict the robustness advantage elicited by mild-stress pretreatment and, therefore, could function as a biomarker, the induction of catalase activity upon mild-stress treatment was correlated to mild-stress-induced robustness, and this correlation was tested for significance.
Fig 1.
Schematic representation of the approach followed to evaluate the predictive potential of catalase activity as a biomarker for mild-stress-induced robustness. The induction of catalase activity ( ) was quantified upon mild-stress treatment. Also, cells were exposed to conditions of mild stress and subsequently exposed to severe stress to quantify the robustness enhancement elicited by mild-stress pretreatment. To evaluate whether the catalase activity could function as a biomarker for mild-stress-induced robustness, the induction of catalase activity upon mild-stress treatment was correlated to mild-stress-induced robustness, and this correlation was tested for significance (Adapted from reference 15 [published by BioMed Central].)
) was quantified upon mild-stress treatment. Also, cells were exposed to conditions of mild stress and subsequently exposed to severe stress to quantify the robustness enhancement elicited by mild-stress pretreatment. To evaluate whether the catalase activity could function as a biomarker for mild-stress-induced robustness, the induction of catalase activity upon mild-stress treatment was correlated to mild-stress-induced robustness, and this correlation was tested for significance (Adapted from reference 15 [published by BioMed Central].)
Induction of catalase activity upon mild-stress treatment.
The catalase activity was measured in exponentially growing B. weihenstephanensis cells precultured at 30°C and upon treatment with mild oxidative stress (Fig. 2). As expected, mild-stress treatment resulted in increased catalase activity over time. Real-time reverse transcription-PCR analysis of the genes encoding catalases, namely, BcerKBAB4_1058, BcerKBAB4_2820, and BcerKBAB4_4605, demonstrated that only BcerKBAB4_1058, which encodes the main vegetative catalase, was significantly induced and showed a transient induction pattern upon treatment with mild oxidative stress (data not shown). These observations are in line with previous findings for B. cereus (11) and highlight that transient gene expression can be linked to stable functional enzyme levels.
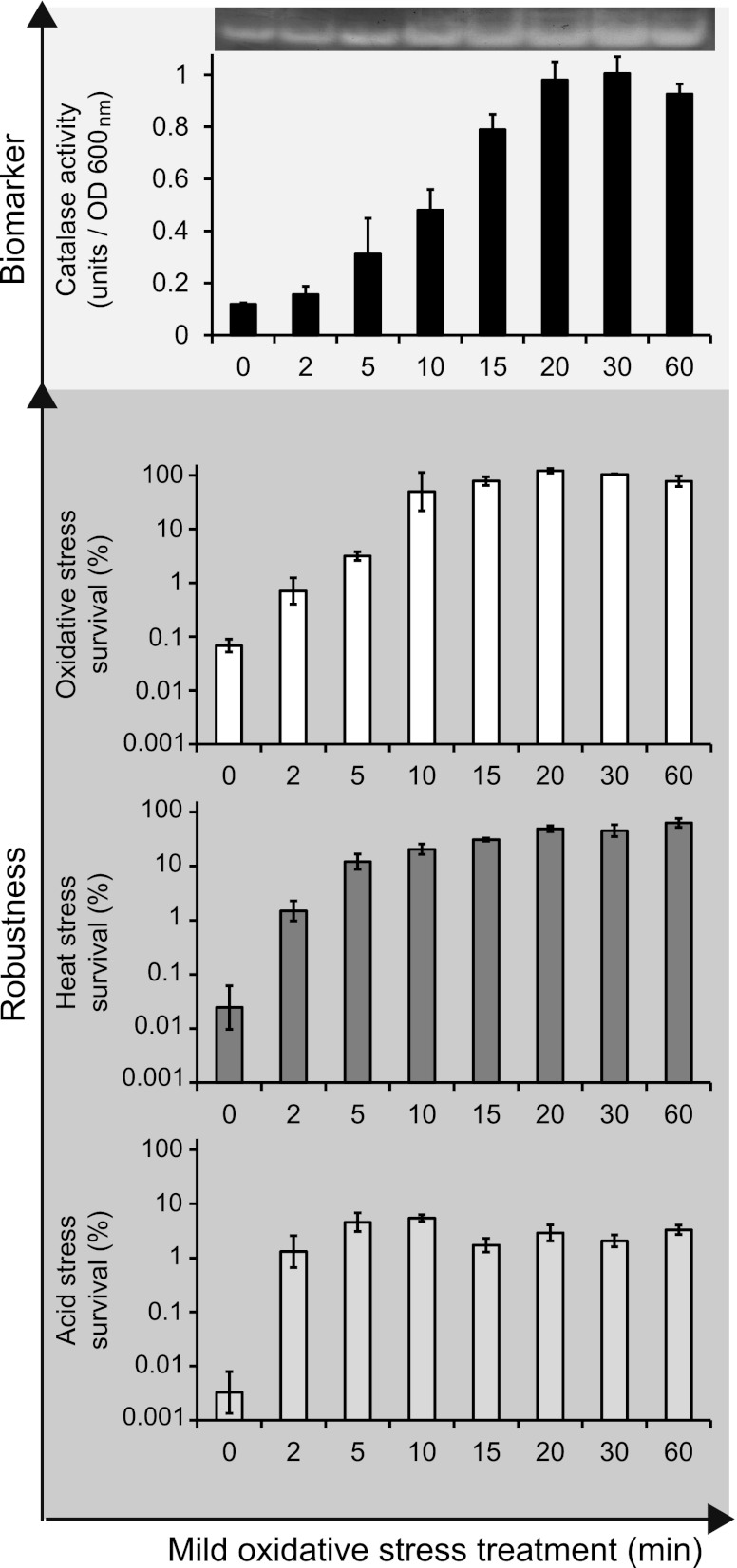
Fig 2.
Catalase activity upon treatment with mild oxidative stress and robustness toward severe stresses for Bacillus weihenstephanensis cells precultured at 30°C. Cells were exposed to mild oxidative stress (0.05 mM H2O2), and catalase activity was determined before (0 min) and after treatment with mild oxidative stress for 2 to 60 min. Also, untreated cells and mild-oxidative-stress-treated cells were inactivated by exposure to severe oxidative stress (0.2 mM H2O2 at 30°C for 3.5 min), severe heat stress (46°C for 5 min), and severe acid stress (pH 3.1 at 30°C for 3.5 min) to quantify the effect of mild-stress treatment on robustness. The columns mark the number of cells that survived the severe-stress treatment compared to the initial number of cells (percent). Error bars represent standard errors of the replicates. OD, optical density.
Catalase activity was also measured in cells cultured at 7°C and upon treatment with mild oxidative stress at this temperature, and a rather slow but sure increased catalase activity was shown over time (Fig. 3). At the transcription level, an increased gene expression level of BcerKBAB4_1058 was also observed in cells cultured at 7°C upon treatment with mild oxidative stress. The expression level of this gene in untreated cells cultured at 7°C was higher than that at 30°C (data not shown), which coincided with a slightly higher level of catalase activity in exponentially growing cells cultured at 7°C.
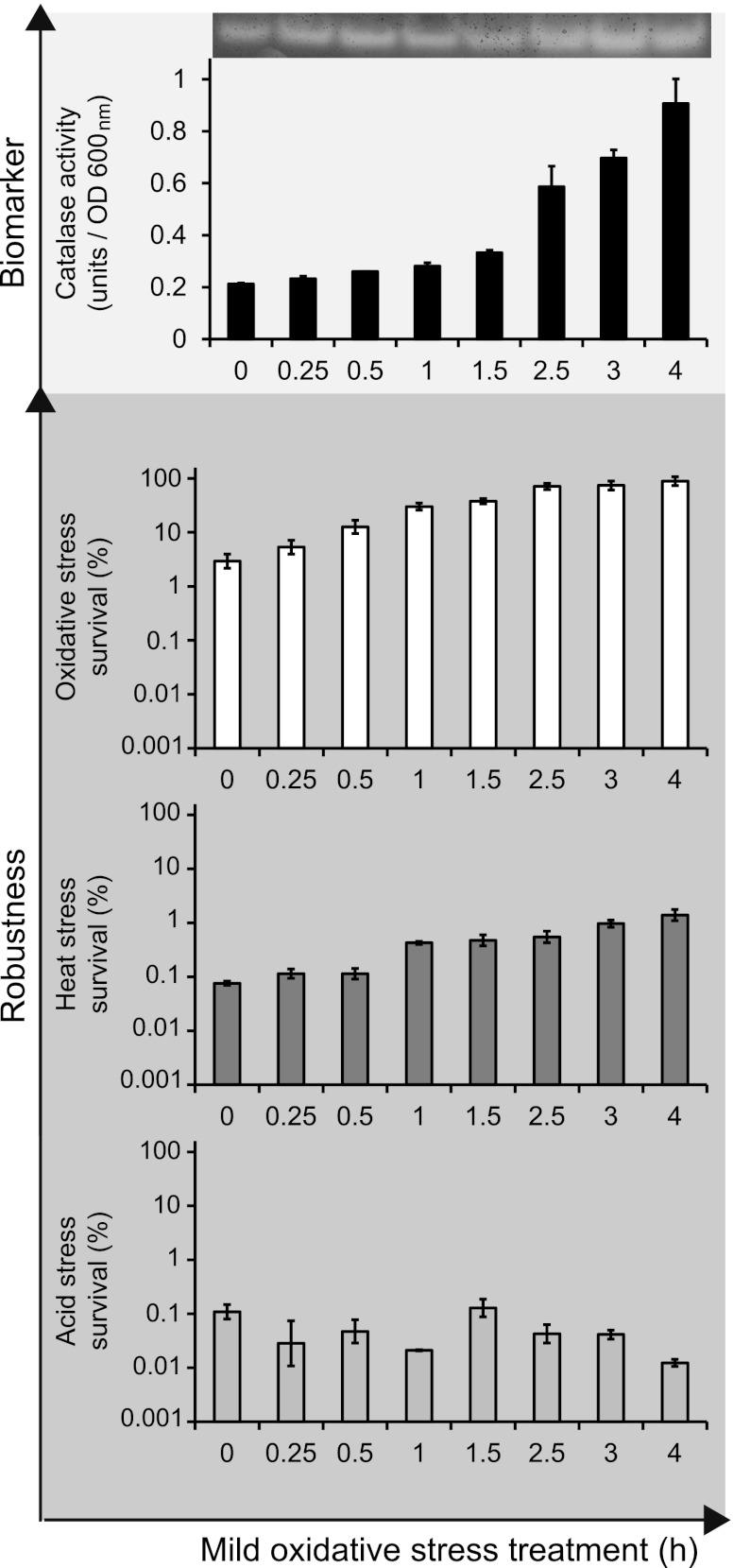
Fig 3.
Catalase activity upon treatment with mild oxidative stress and robustness toward severe stresses for Bacillus weihenstephanensis cells precultured at 7°C. Cells were exposed to mild oxidative stress (0.05 mM H2O2), and catalase activity was determined before (0 min) and after treatment with mild oxidative stress for 0.25 to 4 h. Also, untreated cells and cells treated with mild oxidative stress were inactivated by exposure to severe oxidative stress (0.5 mM H2O2 at 30°C for 3.5 min), severe heat stress (47°C for 3 min), and severe acid stress (pH 3.7 at 30°C for 3.5 min) to quantify the effect of mild-stress treatment on robustness. The columns mark the numbers of cells that survived the severe-stress treatment compared to the initial number of cells (percent). Error bars represent standard errors of the replicates. OD, optical density.
Mild-stress-induced robustness toward severe stresses.
Next, the impact of treatment with mild oxidative stress on the robustness level of B. weihenstephanensis was quantified. For that, B. weihenstephanensis cells precultured at 30°C were exposed to mild oxidative stress (0.05 mM H2O2), after which they were exposed to three severe stresses, namely, severe oxidative, heat, and acid stresses. Mild-stress pretreatment provided B. weihenstephanensis a robustness advantage toward severe oxidative stress and also conferred cross-protection against severe heat and acid stresses (Fig. 2), with the (cross-)protection level being increased with increasing mild-stress pretreatment time. This was in agreement with previous findings with regard to the mesophilic species B. cereus (11) and highlighted that these two species, which are closely related, have similar adaptive traits.
Effect of refrigeration temperature on robustness and the adaptive stress response.
The psychrotolerant characteristics of B. weihenstephanensis make it interesting to also quantify its robustness level and adaptive stress response at a low temperature. The temperature during culturing and upon treatment with mild oxidative stress did have a significant effect on the robustness level of B. weihenstephanensis. Exponentially growing cells cultured at 7°C were more resistant to severe oxidative stress than cells cultured at 30°C but were more sensitive to severe acid stress (Fig. 3). The adaptive stress response elicited by treatment with mild oxidative stress was also affected by the incubation temperature. The robustness enhancement provided by pretreatment with mild oxidative stress was significant only for severe oxidative and heat stresses, whereas cells did not gain enhanced robustness toward severe acid stress upon pretreatment with mild oxidative stress when precultured at a low temperature. Also, the level of induced protection against severe oxidative and heat stresses was influenced by temperature, since the enhancement of robustness toward these stresses elicited by pretreatment with mild oxidative stress was, although significant, much lower than that observed at 30°C (Fig. 2).
Evaluation of the biomarker potential of catalase activity.
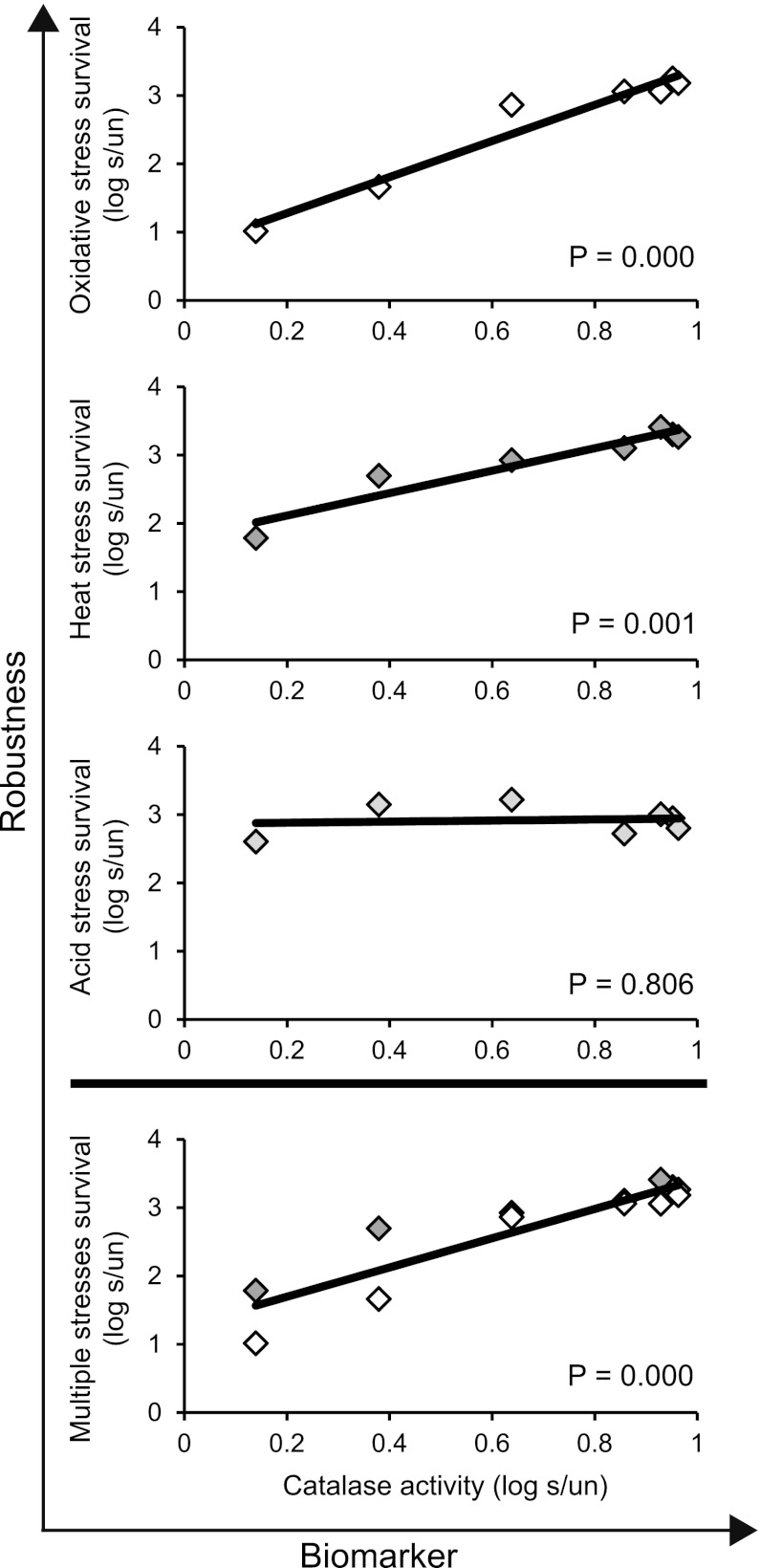
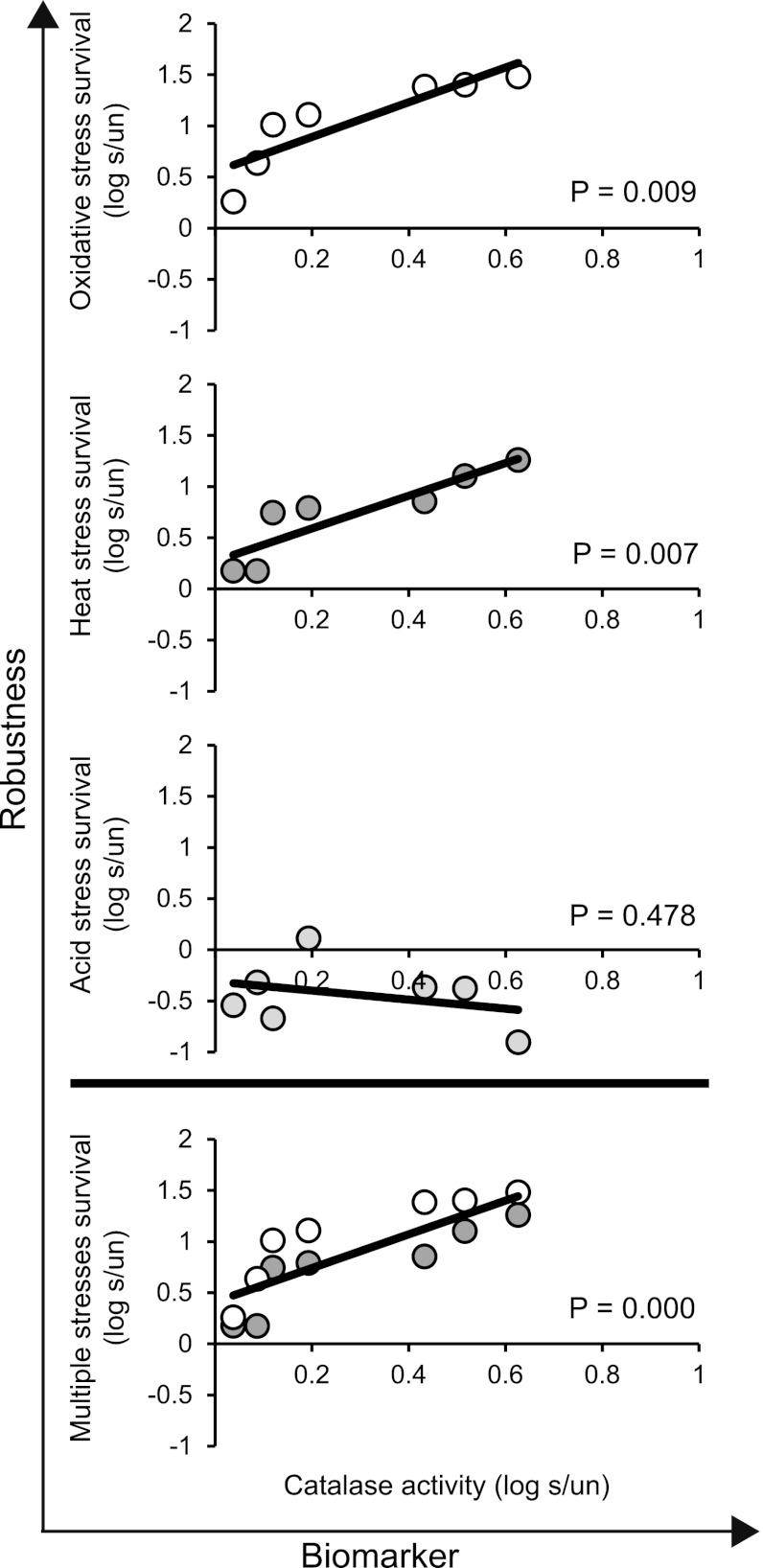
To evaluate whether catalase activity could predict the robustness level of cells treated with mild oxidative stress, the induction of catalase activity upon mild stress treatment was correlated to mild-stress-induced robustness. The correlation between both responses was significant for severe oxidative stress and heat stresses (P < 0.05) but not for severe acid stress (Fig. 4). Because catalase activity was suitable to predict the enhanced robustness level toward both severe oxidative and heat stresses, these induction patterns were combined, and this correlation was shown to be significant (Fig. 4). Catalase activity was therefore defined as a divergent biomarker (11), as it could predict the enhanced level of robustness toward multiple stresses.
Fig 4.
Catalase activity as a potential biomarker for mild-stress-induced robustness for Bacillus weihenstephanensis cells precultured at 30°C. Cells were exposed to mild oxidative stress (0.05 mM H2O2) for 2 to 60 min, and the induction of catalase activity and the induction of robustness for mild-stress-treated cells (s) were expressed relative to those of untreated cells (un) (log s/un). The robustness of untreated and mild-stress-treated cells was defined as the number of cells that survived the severe-stress treatment compared to the initial number of cells.
Also, for cold-grown cells, catalase activity emerged as a biomarker for mild-oxidative-stress-induced robustness toward severe oxidative and heat stresses, although the P values were slightly higher than those for cells precultured at 30°C, and the correlation between the induction of catalase activity and enhanced robustness was not significant for severe acid stress (Fig. 5). When the significant induction patterns for severe oxidative and heat stresses were combined, catalase activity was suitable to predict the enhancement of robustness toward both stresses originating from pretreatment with mild oxidative stress and, therefore, also emerged as a divergent biomarker for cold-grown cells.
Fig 5.
Catalase activity as a potential biomarker for mild-stress-induced robustness for Bacillus weihenstephanensis cells precultured at 7°C. Cells were exposed to mild oxidative stress (0.05 mM H2O2) for 0.25 to 4 h, and the induction of catalase activity and the induction of robustness for mild-stress-treated cells (s) were expressed relative to those of untreated cells (un) (log s/un). The robustness of untreated and mild-stress-treated cells was defined as the number of cells that survived the severe-stress treatment compared to the initial number of cells.
DISCUSSION
Changing environments trigger microbial adaptation and the activation of protective components that equip microorganisms against subsequent stresses and may provide enhanced robustness. Scavengers of reactive oxygen species (ROS) have crucial roles in controlling and adapting to oxidative stresses that microorganisms encounter during disinfection treatments and atmospheric modifications and as part of the host defense response (22, 23). The significant role of ROS scavengers could point to a potential role as a biomarker for stress-induced robustness. During processing, distribution, and storage and upon the ingestion of foods, the temperature might vary significantly. Thus, investigations of the impact of temperature on the adaptive stress response and the predictive quality of potential biomarkers are of great interest. Predictions of stress-induced enhanced robustness using biomarkers will allow a better understanding of stress adaptation mechanisms and predictions of stress-adaptive traits in microorganisms.
Recently, we demonstrated that treatment with mild oxidative stress triggered enhanced robustness toward multiple stresses, including severe oxidative, heat, and acid stresses, in the mesophilic species B. cereus (11), and similar adaptive patterns were observed for the psychrotolerant species B. weihenstephanensis in this study. These overlapping effects pointed to possibly overlapping mechanisms of cell death. Indeed, some studies showed that different severe stresses elicited a common secondary oxidative stress. Severe stresses, including heat and acid stress treatments, caused radical formation in B. cereus (24–26), and a phenotypic screening of B. subtilis mutants showed that many of the genes displaying multiple severe stress management effects appeared to be involved in protection against oxidative damage (16, 27). Also, in B. weihenstephanensis, a secondary oxidative stress response may be a common component of multiple severe stress stimuli, and it is conceivable that pretreatment with mild oxidative stress provides bacterial cells with a better ability to survive these conditions.
Chilling is widely used in minimally processed foods, and this study showed that it affected the robustness level of B. weihenstephanensis. Lowered incubation temperatures increased the robustness toward severe oxidative stress. Recently, it was shown for B. cereus that RNA helicases, which are critical in the RNA secondary structure resolution, were required for optimal growth under conditions of both low temperatures and oxidative stress (28). Also, increased protein expression levels of catalase and superoxide dismutase were observed previously for Listeria monocytogenes at low temperatures (29), and these findings indicate an overlap in the adaptation to both stresses. We observed increased transcriptional expression levels of catalases and higher enzyme activities in cold-grown B. weihenstephanensis cells, which might have contributed to the observed cross-protective effect. In contrast, culturing at low temperatures reduced the robustness level of B. weihenstephanensis cells toward acid stress compared to that of cells cultured at the optimal temperature, and this is in line with previously reported observations for other bacteria, such as Escherichia coli O157:H7 (30), L. monocytogenes (31), and Salmonella enterica serovar Typhimurium (32), while underlying mechanisms remain to be elucidated.
Interestingly, temperature also affected the level of induced cross-protection elicited by pretreatment with mild oxidative stress in B. weihenstephanensis, and the robustness advantage decreased when the culturing temperature decreased. These results agree with data from previous studies of B. cereus (13) and L. monocytogenes (14) and indicate that a limited adaptive stress response might be expected at refrigeration temperatures. This underlines that the culturing temperature needs to be considered when adaptive stress responses are quantified to model microbial behavior throughout the chilled-food chain.
The canonical role of catalases in controlling and adapting to oxidative stresses pointed to a potential predictive role in oxidative-stress-induced enhanced robustness, and this was indeed demonstrated in this study. The quantified correlations supported the predictive quality of catalase activity but also underlined that predictive quality was stress specific. Catalase activity was shown to have a divergent predictive potential, as it could predict the robustness enhancement toward multiple stresses. Interestingly, catalase activity also emerged as a divergent biomarker in B. cereus (11), revealing similar adaptive traits in two species of the B. cereus group. This pointed to an important role in stress adaptation and underlined the predictive potential of catalase activity as a biomarker.
In conclusion, we demonstrated that catalase activity could serve as a biomarker for stress-adaptive behavior in the psychrotolerant species B. weihenstephanensis but also highlighted that the predictive quality is stress specific. Similar findings for the mesophilic species B. cereus strengthen its predictive potential. Such findings must be validated for several species and may allow for the selection of robust biomarkers that can provide a better understanding of stress adaptation and may contribute to predict adaptive traits in microorganisms.
ACKNOWLEDGMENT
We gratefully acknowledge Wilma Hazeleger (Wageningen University, Laboratory of Food Microbiology) for critical reading of the manuscript.
Footnotes
Published ahead of print 12 October 2012
REFERENCES
- 1. Baron F, Cochet M-F, Grosset N, Madec M-N, Briandet R, Dessaigne S, Chevalier S, Gautier M, Jan S. 2007. Isolation and characterization of a psychrotolerant toxin producer, Bacillus weihenstephanensis, in liquid egg products. J. Food Prot. 70:2782–2791 [DOI] [PubMed] [Google Scholar]
- 2. Jan S, Brunet N, Techer C, Le Maréchal C, Koné AZ, Grosset N, Cochet MF, Gillard A, Gautier M, Puterflam J, Baron F. 2011. Biodiversity of psychrotrophic bacteria of the Bacillus cereus group collected on farm and in egg product industry. Food Microbiol. 28:261–265 [DOI] [PubMed] [Google Scholar]
- 3. Ivy RA, Ranieri ML, Martin NH, den Bakker HC, Xavier BM, Wiedmann M, Boor KJ. 2012. Identification and characterization of psychrotolerant sporeformers associated with fluid milk production and processing. Appl. Environ. Microbiol. 78:1853–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stenfors Arnesen L, Granum PE, Buisson C, Bohlin J, Nielsen-LeRoux C. 2011. Using an insect model to assess correlation between temperature and virulence in Bacillus weihenstephanensis and Bacillus cereus. FEMS Microbiol. Lett. 317:196–202 [DOI] [PubMed] [Google Scholar]
- 5. Hoton FM, Fornelos N, N′Guessan E, Hu X, Swiecicka I, Dierick K, Jääskeläinen E, Salkinoja-Salonen M, Mahillon J. 2009. Family portrait of Bacillus cereus and Bacillus weihenstephanensis cereulide-producing strains. Environ. Microbiol. Rep. 1:177–183 [DOI] [PubMed] [Google Scholar]
- 6. Thorsen L, Hansen BM, Nielsen KF, Hendriksen NB, Phipps RK, Budde BB. 2006. Characterization of emetic Bacillus weihenstephanensis, a new cereulide-producing bacterium. Appl. Environ. Microbiol. 72:5118–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Browne N, Dowds BCA. 2001. Heat and salt stress in the food pathogen Bacillus cereus. J. Appl. Microbiol. 91:1085–1094 [DOI] [PubMed] [Google Scholar]
- 8. Browne N, Dowds BCA. 2002. Acid stress in the food pathogen Bacillus cereus. J. Appl. Microbiol. 92:404–414 [DOI] [PubMed] [Google Scholar]
- 9. Den Besten HMW, Mataragas M, Moezelaar R, Abee T, Zwietering MH. 2006. Quantification of the effects of salt stress and physiological state on thermotolerance of Bacillus cereus ATCC 10987 and ATCC 14579. Appl. Environ. Microbiol. 72:5884–5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. den Besten HMW, Mols M, Moezelaar R, Zwietering MH, Abee T. 2009. Phenotypic and transcriptomic analyses of mildly and severely salt-stressed Bacillus cereus ATCC 14579 cells. Appl. Environ. Microbiol. 75:4111–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. den Besten HMW, Arvind A, Gaballo HMS, Moezelaar R, Zwietering MH, Abee T. 2010. Short- and long-term biomarkers for bacterial robustness: a framework for quantifying correlations between cellular indicators and adaptive behavior. PLoS One 5:e13746 doi:10.1371/journal.pone.0013746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Periago PM, Abee T, Wouters JA. 2002. Analysis of the heat-adaptive response of psychrotrophic Bacillus weihenstephanensis. Int. J. Food Microbiol. 79:17–26 [DOI] [PubMed] [Google Scholar]
- 13. Den Besten HMW, van der Mark E-J, Hensen L, Abee T, Zwietering MH. 2010. Quantification of the effect of culturing temperature on salt-induced heat resistance of Bacillus species. Appl. Environ. Microbiol. 76:4286–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koutsoumanis KP, Kendall PA, Sofos JN. 2003. Effect of food processing-related stresses on acid tolerance of Listeria monocytogenes. Appl. Environ. Microbiol. 69:7514–7516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abee T, Wels M, de Been M, den Besten H. 2011. From transcriptional landscapes to the identification of biomarkers for robustness. Microb. Cell Fact. 10:S9 doi:10.1186/1475-2859-10-S1-S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Höper D, Völker U, Hecker M. 2005. Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis. J. Bacteriol. 187:2810–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersohn A, Brigulla M, Haas S, Hoheisel JD, Völker U, Hecker M. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Causton HC, Ren B, Koh SS, Harbison CT, Kanin E, Jennings EG, Lee TI, True HL, Lander ES, Young RA. 2001. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12:323–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Schaik W, Zwietering MH, de Vos WM, Abee T. 2005. Deletion of the sigB gene in Bacillus cereus ATCC 14579 leads to hydrogen peroxide hyperresistance. Appl. Environ. Microbiol. 71:6427–6430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woodbury W, Spencer AK, Stahmann MA. 1971. An improved procedure using ferricyanide for detecting catalase isozymes. Anal. Biochem. 44:301–305 [DOI] [PubMed] [Google Scholar]
- 22. Block SS. 2000. Disinfection, sterilization, and preservation, 5th ed Lippincott Williams & Wilkins, Baltimore, MD [Google Scholar]
- 23. Fang FC. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2:820–832 [DOI] [PubMed] [Google Scholar]
- 24. Mols M, Pier I, Zwietering MH, Abee T. 2009. The impact of oxygen availability on stress survival and radical formation ofBacillus cereus. Int. J. Food Microbiol. 135:303–311 [DOI] [PubMed] [Google Scholar]
- 25. Mols M, Van Kranenburg R, Van Melis CCJ, Moezelaar R, Abee T. 2010. Analysis of acid-stressed Bacillus cereus reveals a major oxidative response and inactivation-associated radical formation. Environ. Microbiol. 12:873–885 [DOI] [PubMed] [Google Scholar]
- 26. Mols M, Abee T. 2011. Primary and secondary oxidative stress in Bacillus. Environ. Microbiol. 13:1387–1394 [DOI] [PubMed] [Google Scholar]
- 27. Reder A, Höper D, Gerth U, Hecker M. 2012. The contribution of individual σB-dependent general stress genes to oxidative stress resistance of Bacillus subtilis. J. Bacteriol. 194:3601–3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pandiani F, Chamot S, Brillard J, Carlin F, Nguyen-the C, Broussolle V. 2011. Role of the five RNA helicases in the adaptive response of Bacillus cereus ATCC 14579 cells to temperature, pH, and oxidative stresses. Appl. Environ. Microbiol. 77:5604–5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cacace G, Mazzeo MF, Sorrentino A, Spada V, Malorni A, Siciliano RA. 2010. Proteomics for the elucidation of cold adaptation mechanisms in Listeria monocytogenes. J. Proteomics 73:2021–2030 [DOI] [PubMed] [Google Scholar]
- 30. Cheng CM, Kaspar CW. 1998. Growth and processing conditions affecting acid tolerance in Escherichia coli O157:H7. Food Microbiol. 15:157–166 [Google Scholar]
- 31. Patchett RA, Watson N, Fernandez PS, Kroll RG. 1996. The effect of temperature and growth rate on the susceptibility of Listeria monocytogenes to environmental stress conditions. Lett. Appl. Microbiol. 22:121–124 [DOI] [PubMed] [Google Scholar]
- 32. Álvarez-Ordóñez A, Fernández A, Bernardo A, López M. 2010. Acid tolerance in Salmonella typhimurium induced by culturing in the presence of organic acids at different growth temperatures. Food Microbiol. 27:44–49 [DOI] [PubMed] [Google Scholar]